Processing of the Ti25Ta25Nb3Sn Experimental Alloy Using ECAP Process for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khrunyk, Y.Y.; Ehnert, S.; Grib, S.V.; Illarionov, A.G.; Stepanov, S.I.; Popov, A.A.; Ryzhkov, M.A.; Belikov, S.V.; Xu, Z.; Rupp, F.; et al. Synthesis and characterization of a novel biocompatible alloy, ti-nb-zr-ta-sn. Int. J. Mol. Sci. 2021, 22, 10611–10630. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, B.; Luo, L.; Wang, L.; Su, Y.; Xu, Y.; Wang, F.; Han, B.; Huang, H.; Guo, J.; et al. Effect of zirconium content on the microstructure and corrosion behavior of as-cast Ti-Al-Nb-Zr-Mo alloy. J. Mater. Res. Technol. 2019, 15, 4896–4913. [Google Scholar] [CrossRef]
- Carobolante, J.P.A.; Pereira Júnior, A.; Bortolini Junior, C.; Barboza da Silva, K.; Sabino, R.M.; Popat, K.C.; Claro, A.P.R.A. Processing and Characterization of a New Quaternary Alloy Ti10Mo8Nb6Zr for Potential Biomedical Applications. Materials 2022, 15, 8636–8653. [Google Scholar] [CrossRef]
- Cardoso, G.C.; de Almeida, G.S.; Corrêa, D.O.G.; Zambuzzi, W.F.; Buzalaf, M.A.R.; Correa, D.R.N.; Grandini, C.R. Preparation and characterization of novel as-cast Ti-Mo-Nb alloys for biomedical applications. Sci. Rep. 2022, 12, 11874. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Carobolante, J.P.A.; Pereira, C.A.; Dias-Netipanyj, M.F.; Popat, K.C.; Claro, A.P.R. Cell and bacteria-Baterial interactions on the Ti10Mo8Nb alloy after surface modification. Mater. Res. 2018, 21, e20170508. [Google Scholar] [CrossRef]
- Carobolante, J.P.A.; da Silva, K.B.; Chaves, J.A.M.; Dias Netipanyj, M.F.; Popat, K.C.; Alves Claro, A.P.R. Nanoporous layer formation on the Ti10Mo8Nb alloy surface using anodic oxidation. Surf. Coat. Technol. 2020, 386, 125467–125476. [Google Scholar] [CrossRef]
- Churakova, A.A.; Gunderov, D.V.; Raab, G.I.; Prokoshkin, S.D.; Sheremetyev, V.A.; Filho, P.N.L.; Pedro, J.; Claro, A.P.R.A. Influence of ECAP on the structure and properties of Ti18Zr15Nb and Ti10Mo8Nb6Zr alloys for medical application. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1014, 012006–012010. [Google Scholar] [CrossRef]
- Takahashi, M.; Kikuchi, M.; Takada, Y. Mechanical properties and microstructures of dental cast Ti-6Nb-4Cu, Ti-18Nb-2Cu, and Ti-24Nb-1Cu alloys. Dent. Mater. J. 2016, 35, 564–570. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.; Li, Y.; Zhang, Y.; Munir, K.; Jiang, H.; Wen, C. Deformation mechanism and mechanical properties of a thermomechanically processed β Ti–28Nb–35.4 Zr alloy. J. Mech. Behav. Biomed. Mater. 2018, 78, 224–234. [Google Scholar] [CrossRef]
- Yamako, G.; Janssen, D.; Hanada, S.; Anijs, T.; Ochiai, K.; Totoribe, K.; Chosa, E.; Verdonschot, N. Improving stress shielding following total hip arthroplasty by using a femoral stem made of β type Ti-33.6Nb-4Sn with a Young’s modulus gradation. J. Biomech. 2017, 63, 135–143. [Google Scholar] [CrossRef]
- Keshtta, A.; Gepreel, M.A.H. Superelasticity evaluation of the biocompatible Ti-17Nb-6Ta alloy. J. Healthc. Eng. 2019, 2019, 8353409. [Google Scholar] [CrossRef]
- Seixas, M.R.; Bortolini, C., Jr.; Konatu, R.T.; Pereira, A., Jr.; Rosifini Alves Claro, A.P. Mechanical and microstructural characterization of the Ti-25Ta-25Nb alloy for dental applications. Mater. Sci. Forum 2016, 869, 935–939. [Google Scholar] [CrossRef]
- Zhan, H.; Zeng, W.; Wang, G.; Kent, D.; Dargusch, M. On the deformation mechanisms and strain rate sensitivity of a metastable β Ti–Nb alloy. Scr. Mater. 2015, 107, 34–37. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Zhao, X.; Sheng, Y.; Li, W.; Chang, C.-L.; Zhang, Q.; Yu, Z.; Wang, X. Microstructure, mechanical properties and corrosion behaviors of biomedical Ti-Zr-Mo-xMn alloys for dental application. Corros. Sci. 2019, 161, 108195. [Google Scholar] [CrossRef]
- Seixas, M.R.; Bortolini, C.; Pereira, A.; Nakazato, R.Z.; Popat, K.C.; Alves Claro, A.P.R. Development of a new quaternary alloy Ti–25Ta–25Nb–3Sn for biomedical applications. Mater. Res. Express 2018, 5, 025402. [Google Scholar] [CrossRef]
- Silva, K.B.D.; Carobolante, J.P.A.; Rajan, S.S.; Júnior, C.B.; Sabino, R.M.; Seixas, M.R.; Nakazato, R.Z.; Popat, K.C.; Claro, A.P.R.A. Mechanical Properties, Corrosion Behavior, and In Vitro Cell Studies of the New Ti-25Ta-25Nb-5Sn Alloy. Materials 2023, 16, 1970. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2004, 371, 283–290. [Google Scholar] [CrossRef]
- Wang, C.H.; Liu, M.; Hu, P.F.; Peng, J.C.; Wang, J.A.; Ren, Z.M.; Cao, G.H. The effects of α″ and ω phases on the superelasticity and shape memory effect of binary Ti-Mo alloys. J. Alloys Compd. 2017, 720, 488–496. [Google Scholar] [CrossRef]
- Bertrand, E.; Gloriant, T.; Gordin, D.M.; Vasilescu, E.; Drob, P.; Vasilescu, C.; Drob, S.I. Synthesis and characterisation of a new superelastic Ti-25Ta-25Nb biomedical alloy. J. Mech. Behav. Biomed. Mater. 2010, 3, 559–564. [Google Scholar] [CrossRef]
- Bertrand, E.; Castany, P.; Gloriant, T. Investigation of the martensitic transformation and the damping behavior of a superelastic Ti–Ta–Nb alloy. Acta Mater. 2013, 61, 511–518. [Google Scholar] [CrossRef]
- Li, S.J.; Cui, T.C.; Hao, Y.L.; Yang, R. Fatigue properties of a metastable β-type titanium alloy with reversible phase transformation. Acta Biomater. 2008, 4, 305–317. [Google Scholar] [CrossRef]
- Ma, B.; Ju, D.; Liu, Q. Design, Simulation and Performance Research of New Biomaterial Mg30Zn30Sn30Sr5Bi5. Coatings 2022, 12, 531. [Google Scholar] [CrossRef]
- Da Silva Dias, C.; Rossi, M.C.; Apolonio, E.V.P.; dos Santos Rosa, G.; Pfeifer, J.P.H.; Hussni, C.A.; Watanabe, M.J.; Alves, A.L.G. Low Mg content on Ti-Nb-Sn alloy when in contact with eBMMSCs promotes improvement of its biological functions. J. Mater. Sci. Mater. Med. 2021, 32, 144. [Google Scholar] [CrossRef]
- Çakmak, Ö.; Kaya, M. Effect of sintering procedure on microstructure and mechanical properties of biomedical TiNbSn alloy produced via powder metallurgy. Appl. Phys. A 2021, 127, 561. [Google Scholar] [CrossRef]
- Edalati, K.; Bachmaier, A.; Beloshenko, V.A.; Beygelzimer, Y.; Blank, V.D.; Botta, W.J.; Bryła, K.; Čížek, J.; Divinski, S.; Enikeev, N.A.; et al. Nanomaterials by severe plastic deformation: Review of historical developments and recent advances. Mater. Res. Lett. 2022, 10, 163–256. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Polyakov, A.V.; Semenova, I.P.; Raab, G.I.; Churakova, A.A.; Gimaltdinova, E.I.; Sabirov, I.; Segurado, J.; Sitdikov, V.D.; Alexandrov, I.V.; et al. Evolution of microstructure, macrotexture and mechanical properties of commercially pure Ti during ECAP-conform processing and drawing. Mater. Sci. Eng. A 2013, 562, 128–136. [Google Scholar] [CrossRef]
- Polyakov, A.V.; Semenova, I.P.; Huang, Y.; Valiev, R.Z.; Langdon, T.G. Fatigue Life and Failure Characteristics of an Ultrafine-Grained Ti–6Al–4V Alloy Processed by ECAP and Extrusion. Adv. Eng. Mater. 2014, 16, 1038–1043. [Google Scholar] [CrossRef]
- Terynková, A.; Bartha, K.; Stráský, J.; Janeček, M.; Landa, M.; Polyakova, V.; Semenova, I. Microstructure and mechanical properties of Ti15Mo alloy prepared by ECAP. Acta Phys. Pol. A 2018, 134, 787–789. [Google Scholar] [CrossRef]
- Semenova, I.P.; Polyakov, A.V.; Polyakova, V.V.; Grishina, Y.F.; Huang, Y.; Valiev, R.Z.; Langdon, T.G. Mechanical behavior and impact toughness of the ultrafine-grained Grade 5 Ti alloy processed by ECAP. Mater. Sci. Eng. A 2017, 696, 166–173. [Google Scholar] [CrossRef]
- Xu, H.Q.; Wei, K.X.; Wei, W.; Dzugan, J.; Alexandrov, I.V.; An, X.L.; Wang, D.D.; Liu, X.K.; Daniel, M.; Hradil, D.; et al. Microstructure and mechanical properties evolution of Ti-13Nb-13Zr alloy processed by ECAP-Conform and rotary swaging. J. Alloys Compd. 2023, 969, 172351. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.; Wright, P.F.; Ozan, S.; Li, Y.; Wen, C. Mechanical, corrosion, nanotribological, and biocompatibility properties of equal channel angular pressed Ti-28Nb-35.4 Zr alloys for biomedical applications. Acta Biomater. 2022, 149, 387–398. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Prokofiev, E.A.; Kazarinov, N.A.; Raab, G.I.; Minasov, T.B.; Stráský, J. Developing nanostructured Ti alloys for innovative implantable medical devices. Materials 2020, 13, 967–983. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Wu, Y.; Huang, X.; Zhang, Y.; Su, Y.; Qiao, L. Corrosion and tribocorrosion behavior of equiatomic refractory medium entropy TiZr (Hf, Ta, Nb) alloys in chloride solutions. Corros. Sci. 2022, 199, 110166. [Google Scholar] [CrossRef]
- Khan, H.M.; Özer, G.; Yilmaz, M.S.; Koc, E. Corrosion of additively manufactured metallic components: A review. Arab. J. Sci. Eng. 2022, 47, 5465–5490. [Google Scholar] [CrossRef]
- Motyka, M. Martensite formation and decomposition during traditional and AM processing of two-phase titanium alloys—An overview. Metals 2021, 11, 481–498. [Google Scholar] [CrossRef]
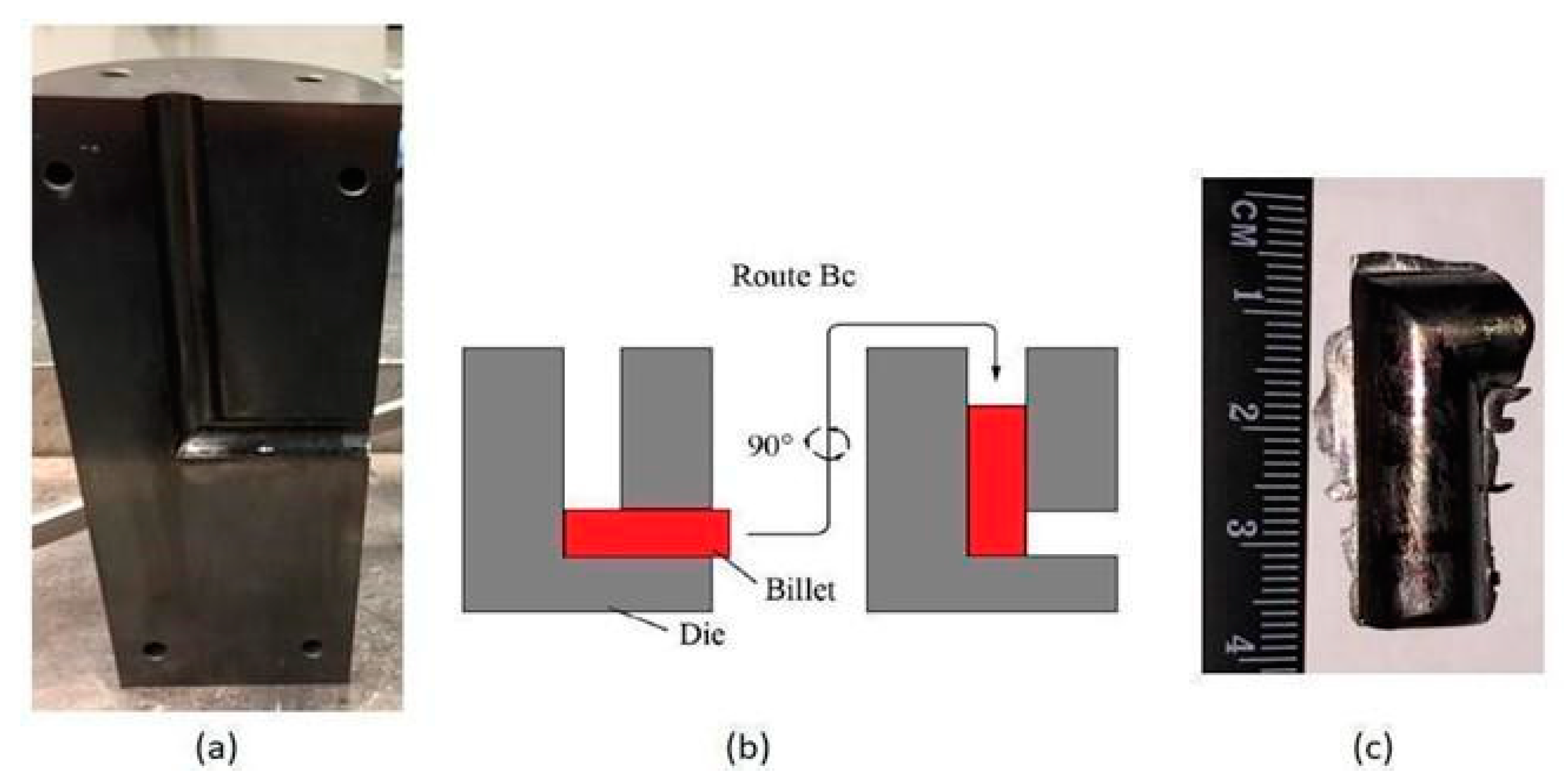


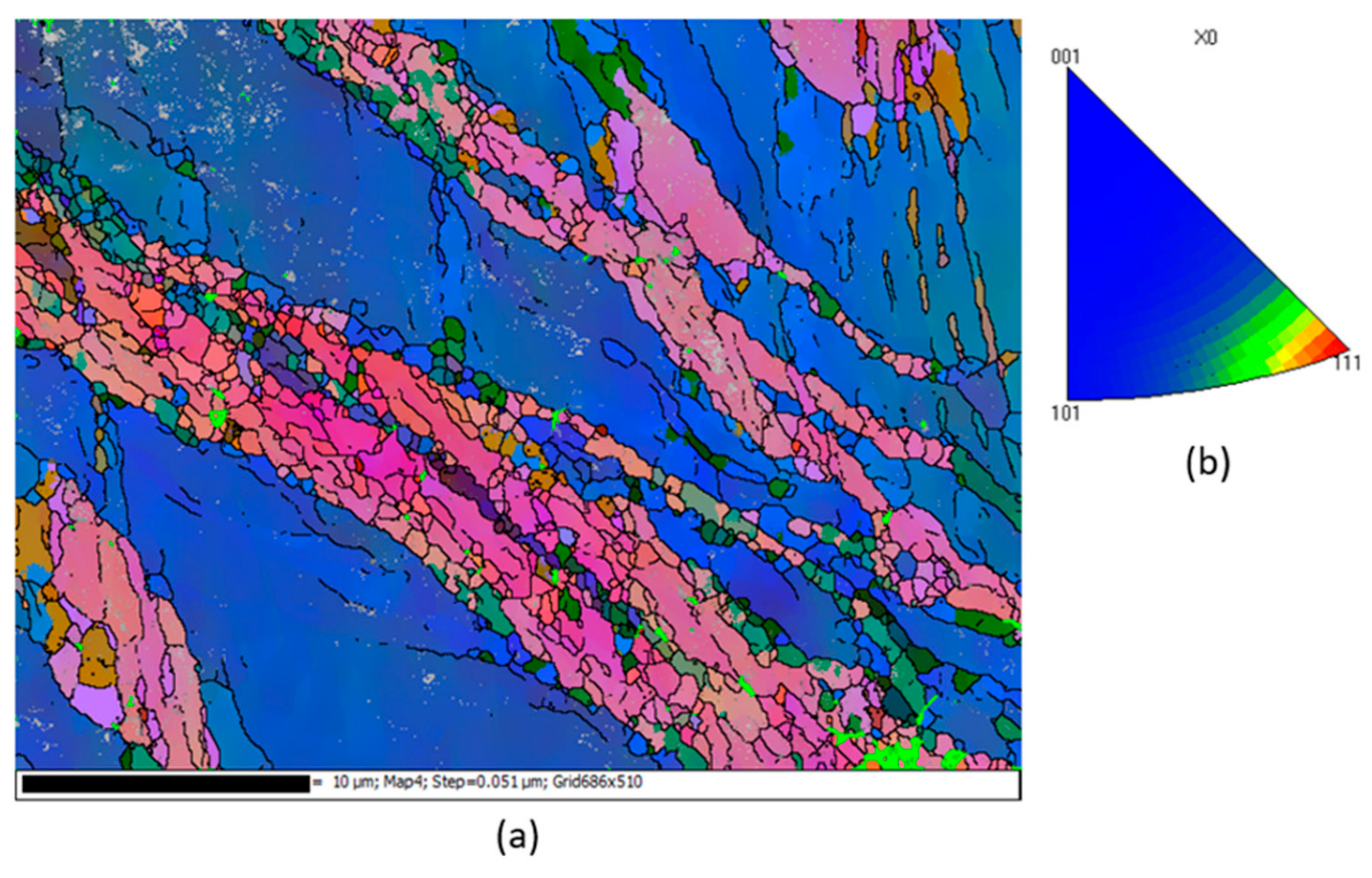
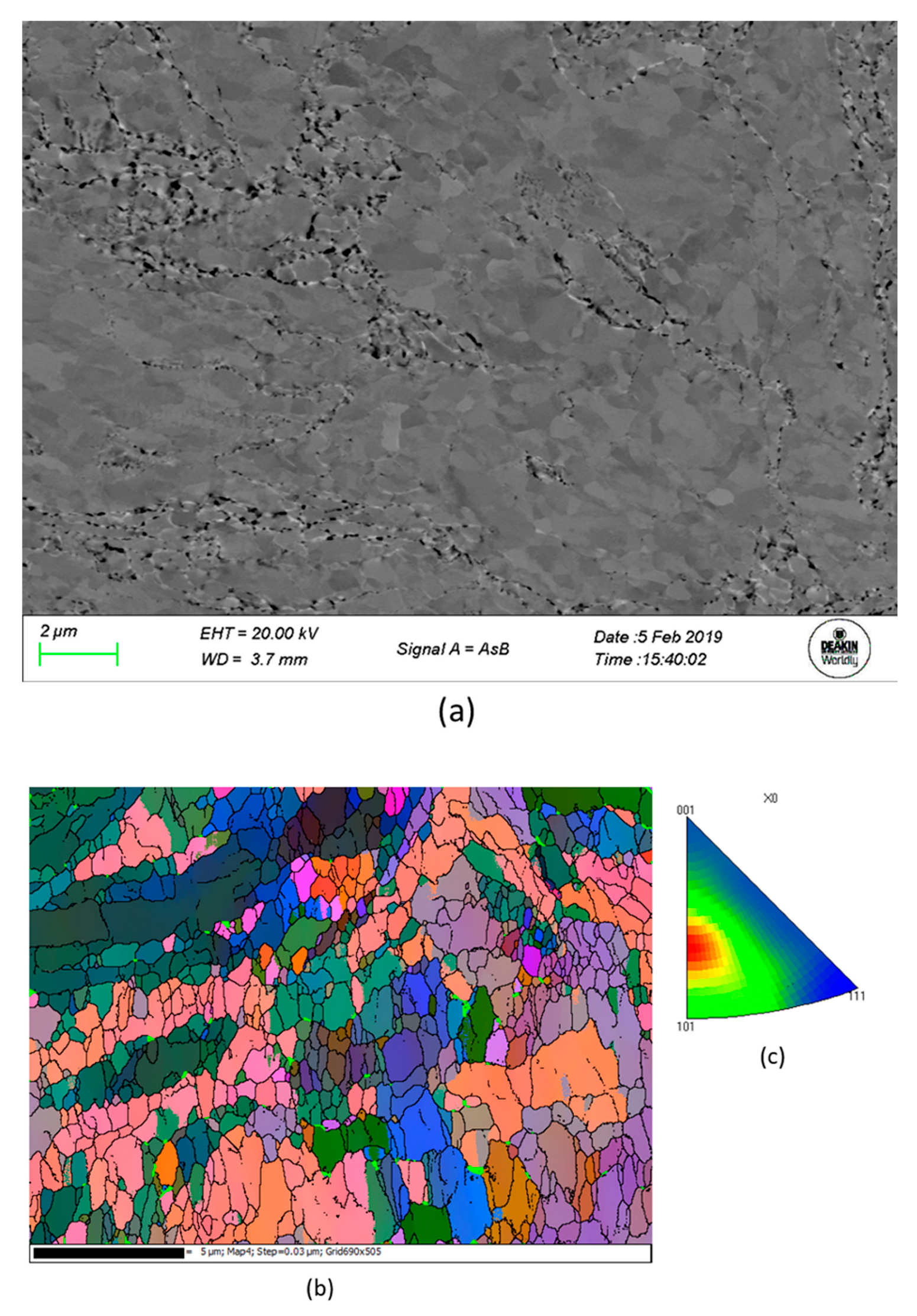
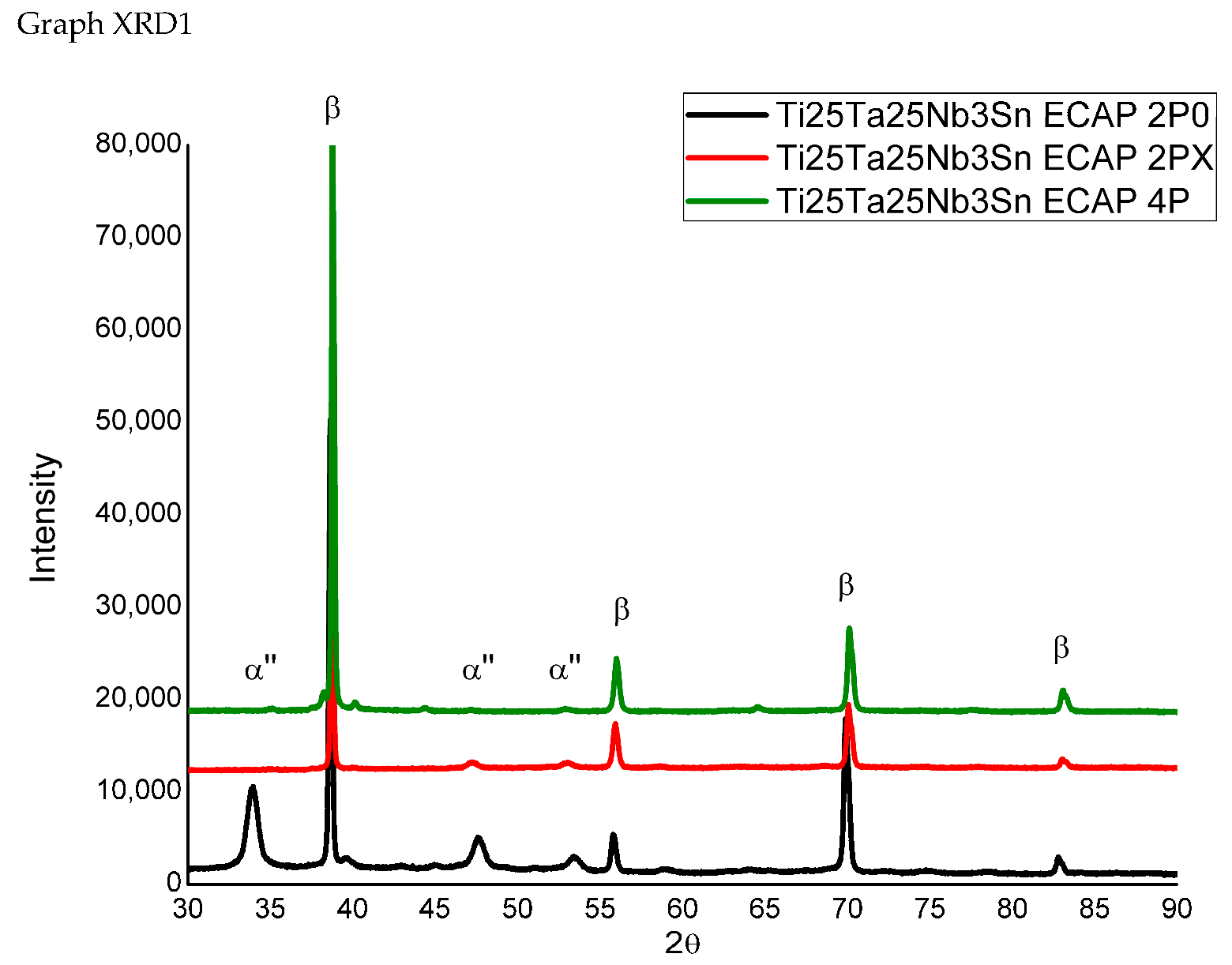

| Sample | Microhardness (HV) |
|---|---|
| As fabricated | 193 (3) |
| ECAP—2 Passes (cross-section) | 201 (5) |
| ECAP—2 Passes (longitudinal section) | 201 (4) |
| ECAP—4 Passes | 212 (4) |
| Sample | EOC (mV) | Ecorr (mV) | Jcorr (nA∙cm−2) | Jp (μA∙cm−2) |
|---|---|---|---|---|
| Ti25Ta25Nb3Sn | −277 (64.4) | −283 (60.4) | 12.2 (1.8) | 10.9 (1.9) |
| Ti25Ta25Nb3Sn ECAP-2 | −178 (67.9) | −206 (28.0) | 6.7 (2.2) | 14.5 |
| Ti25Ta25Nb3Sn ECAP-4 | −337 (14.4) | −353 (4.0) | 3.7 (1.1) | 9.28 (0.5) |
| Ti (grade 02) * | −383 * | −376 * | 37.4 * | 28.0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortolini, C., Jr.; Carobolante, J.P.A.; Timokhina, I.; Caporalli Filho, A.; Rosifini Alves, A.P. Processing of the Ti25Ta25Nb3Sn Experimental Alloy Using ECAP Process for Biomedical Applications. J. Manuf. Mater. Process. 2023, 7, 201. https://doi.org/10.3390/jmmp7060201
Bortolini C Jr., Carobolante JPA, Timokhina I, Caporalli Filho A, Rosifini Alves AP. Processing of the Ti25Ta25Nb3Sn Experimental Alloy Using ECAP Process for Biomedical Applications. Journal of Manufacturing and Materials Processing. 2023; 7(6):201. https://doi.org/10.3390/jmmp7060201
Chicago/Turabian StyleBortolini, Celso, Jr., João Pedro Aquiles Carobolante, Ilana Timokhina, Angelo Caporalli Filho, and Ana Paula Rosifini Alves. 2023. "Processing of the Ti25Ta25Nb3Sn Experimental Alloy Using ECAP Process for Biomedical Applications" Journal of Manufacturing and Materials Processing 7, no. 6: 201. https://doi.org/10.3390/jmmp7060201
APA StyleBortolini, C., Jr., Carobolante, J. P. A., Timokhina, I., Caporalli Filho, A., & Rosifini Alves, A. P. (2023). Processing of the Ti25Ta25Nb3Sn Experimental Alloy Using ECAP Process for Biomedical Applications. Journal of Manufacturing and Materials Processing, 7(6), 201. https://doi.org/10.3390/jmmp7060201







