The Numerical Simulation of the Injection Filling of the Fluidity Probe Die with Pattern Waxes
Abstract
1. Introduction
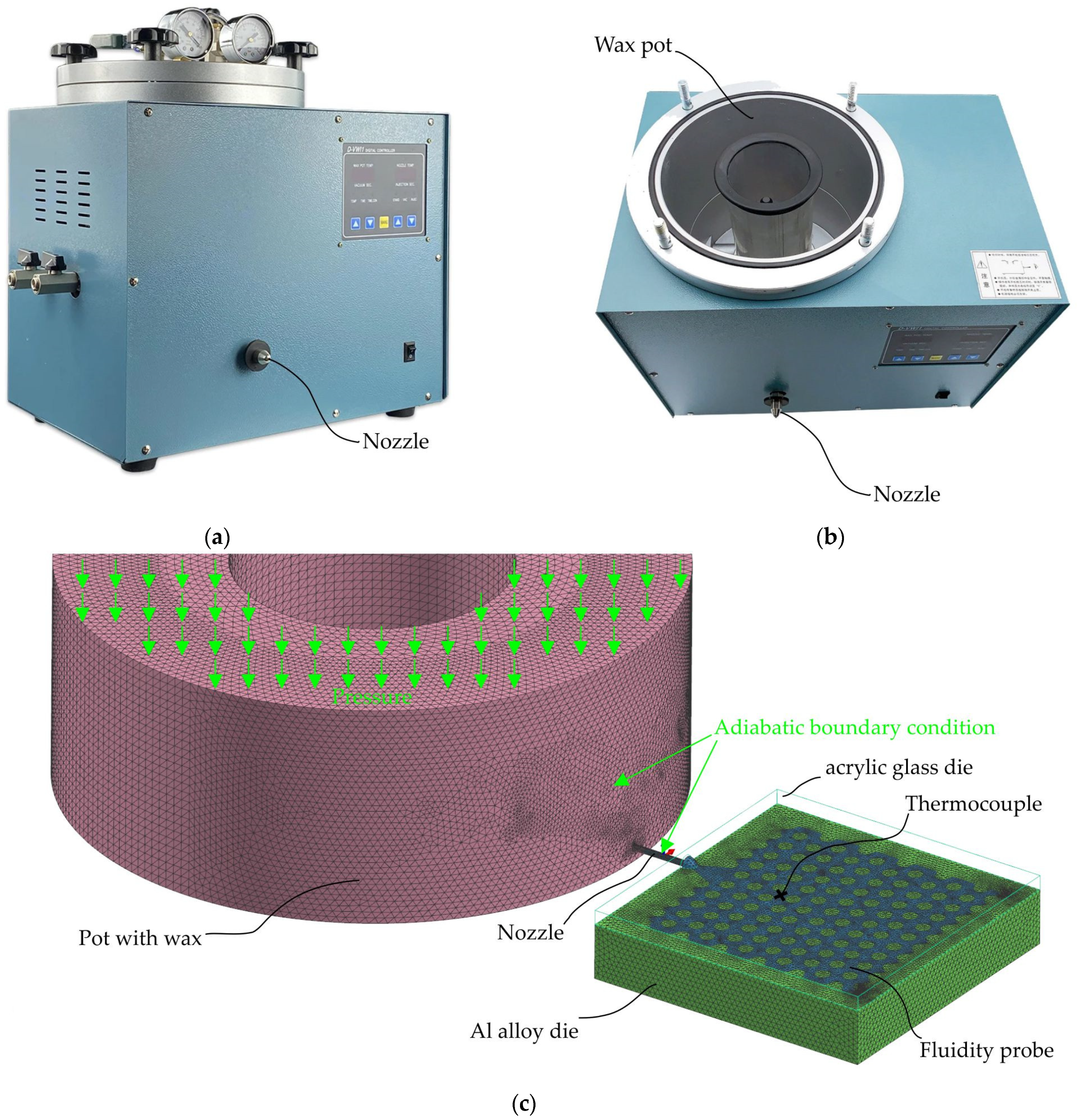
2. Materials and Methods
3. Simulation Procedure and Wax Properties Determining
4. Results
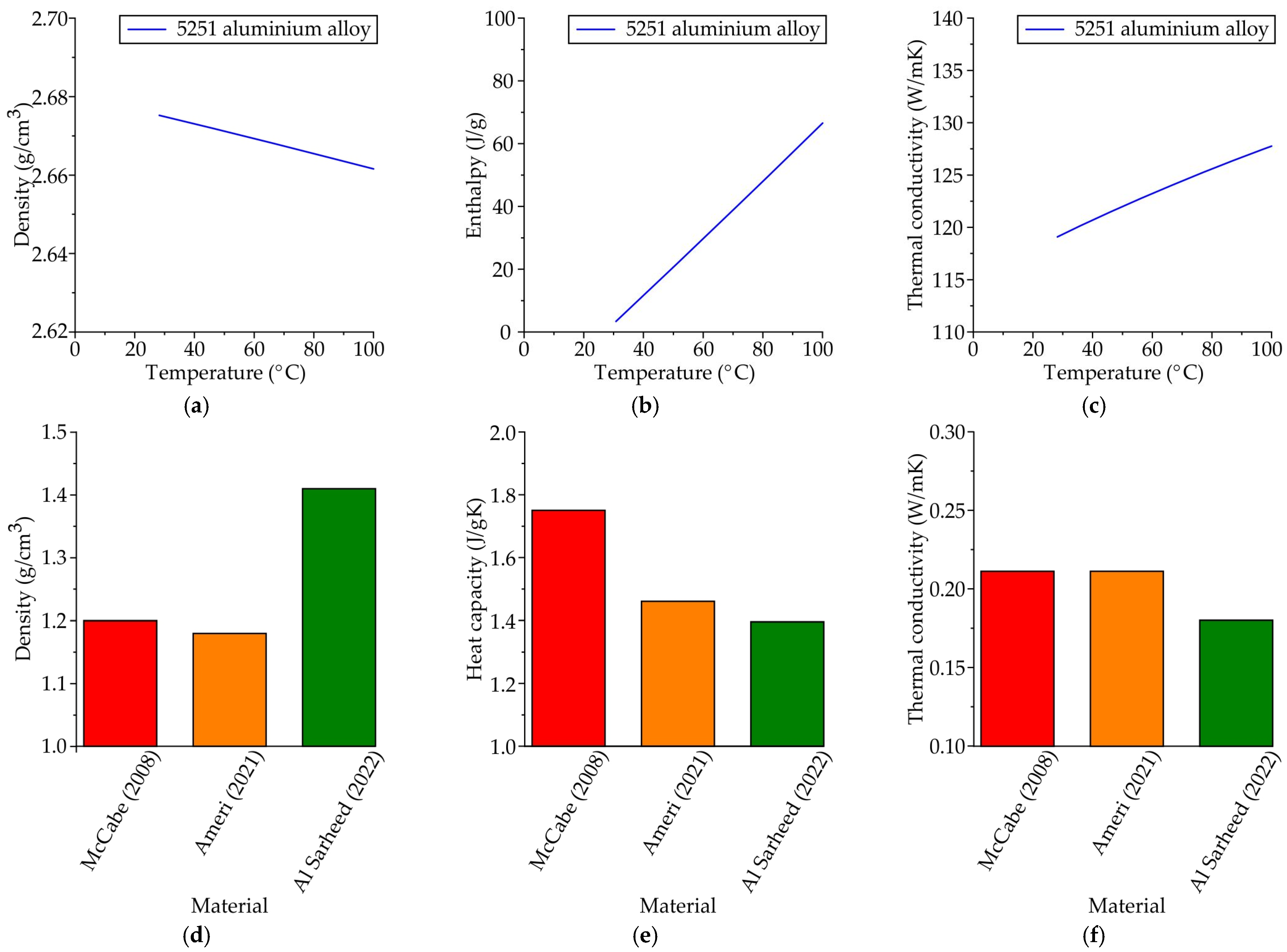
4.1. The Waxes Thermal Properties
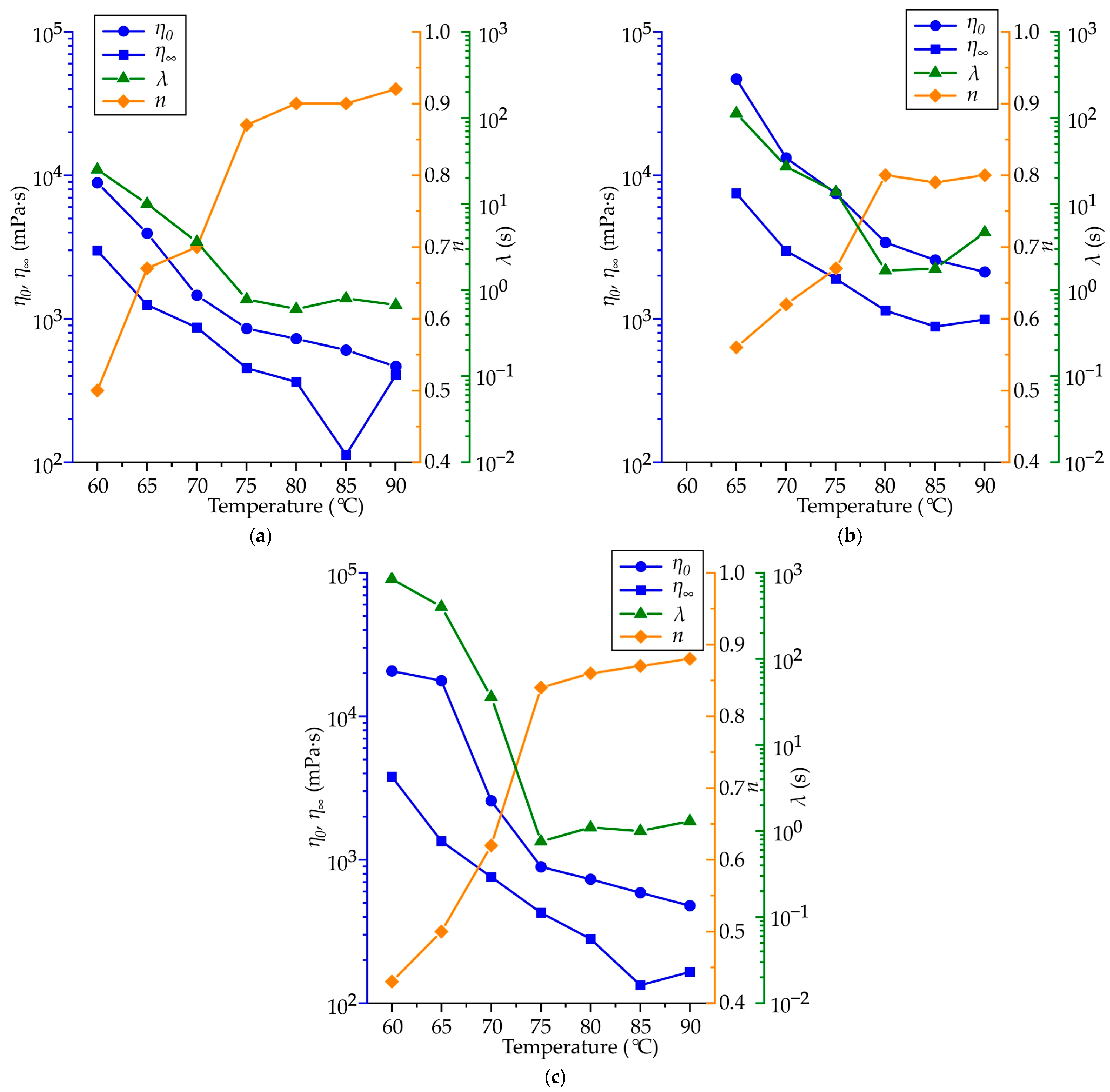
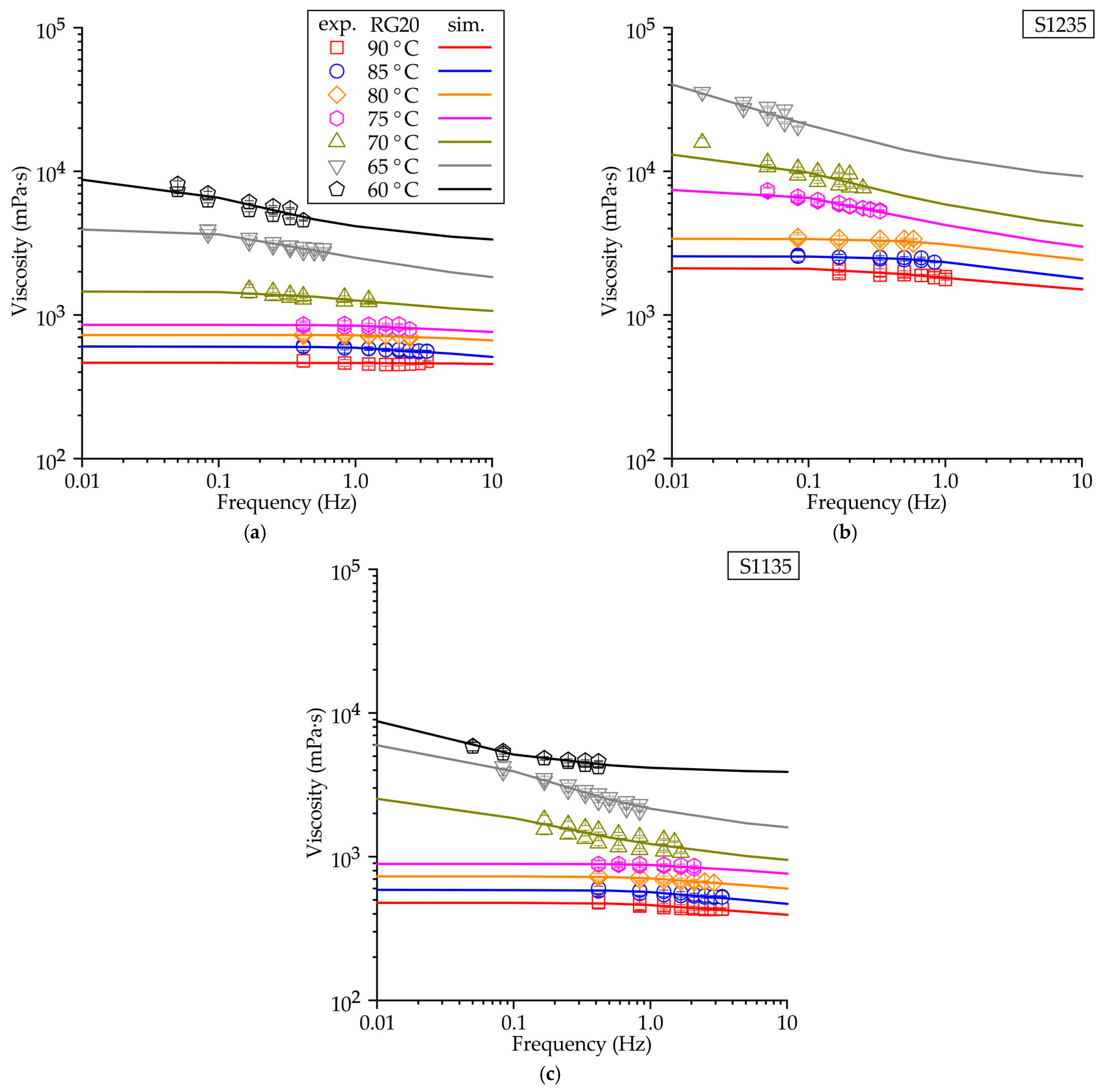
4.2. The Waxes Rheological Properties
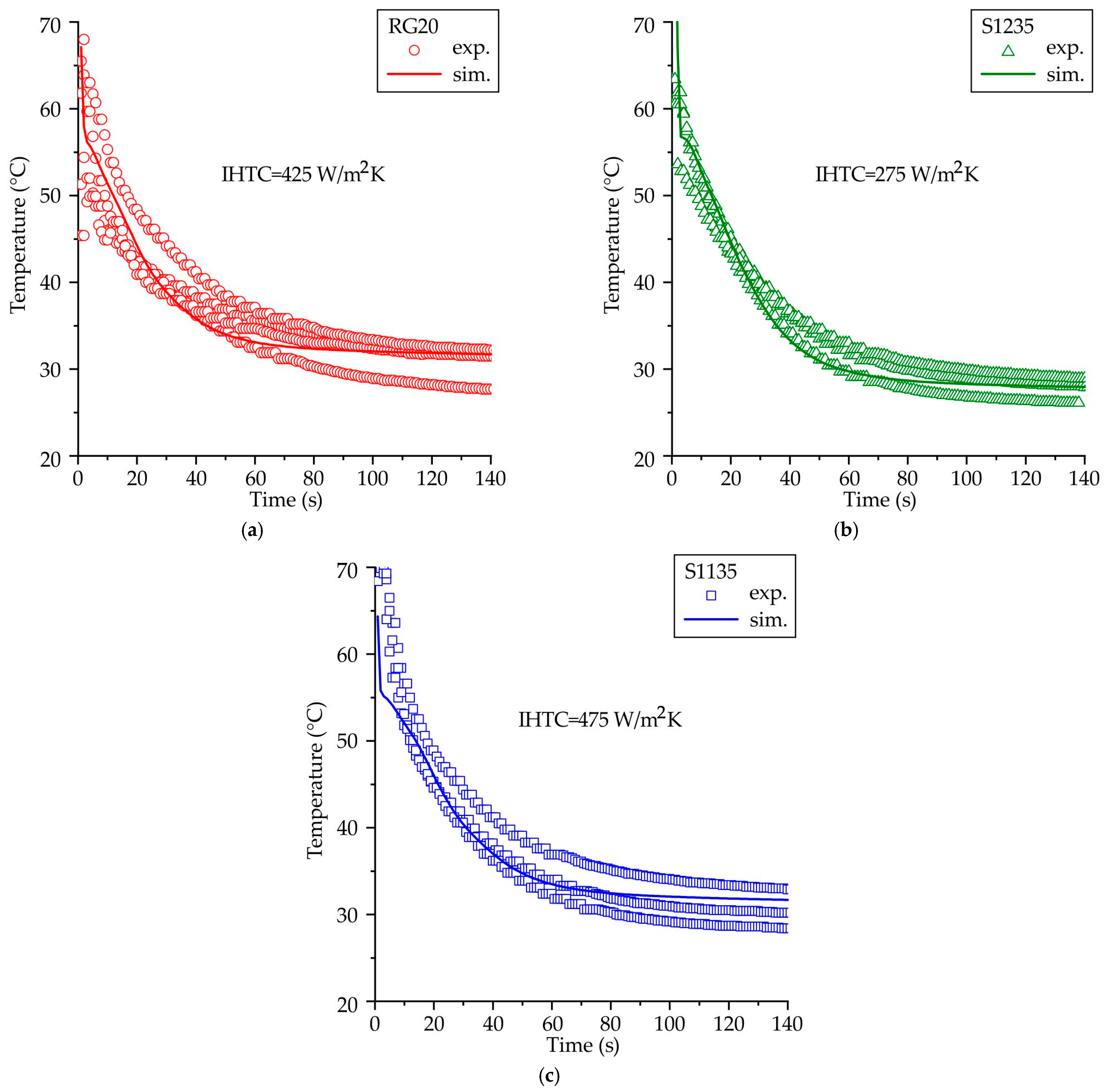
4.3. The IHTC between the Waxes and Die
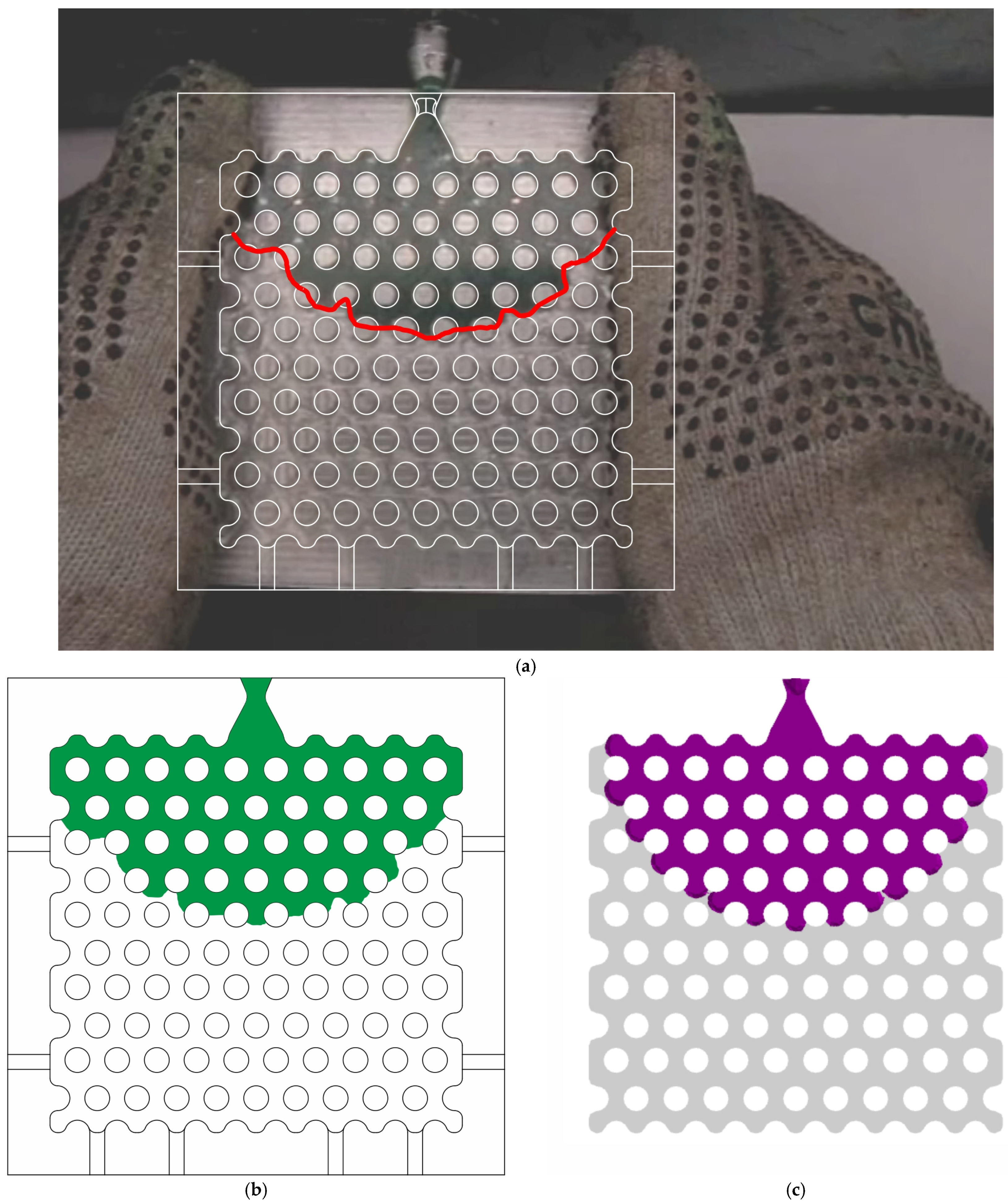
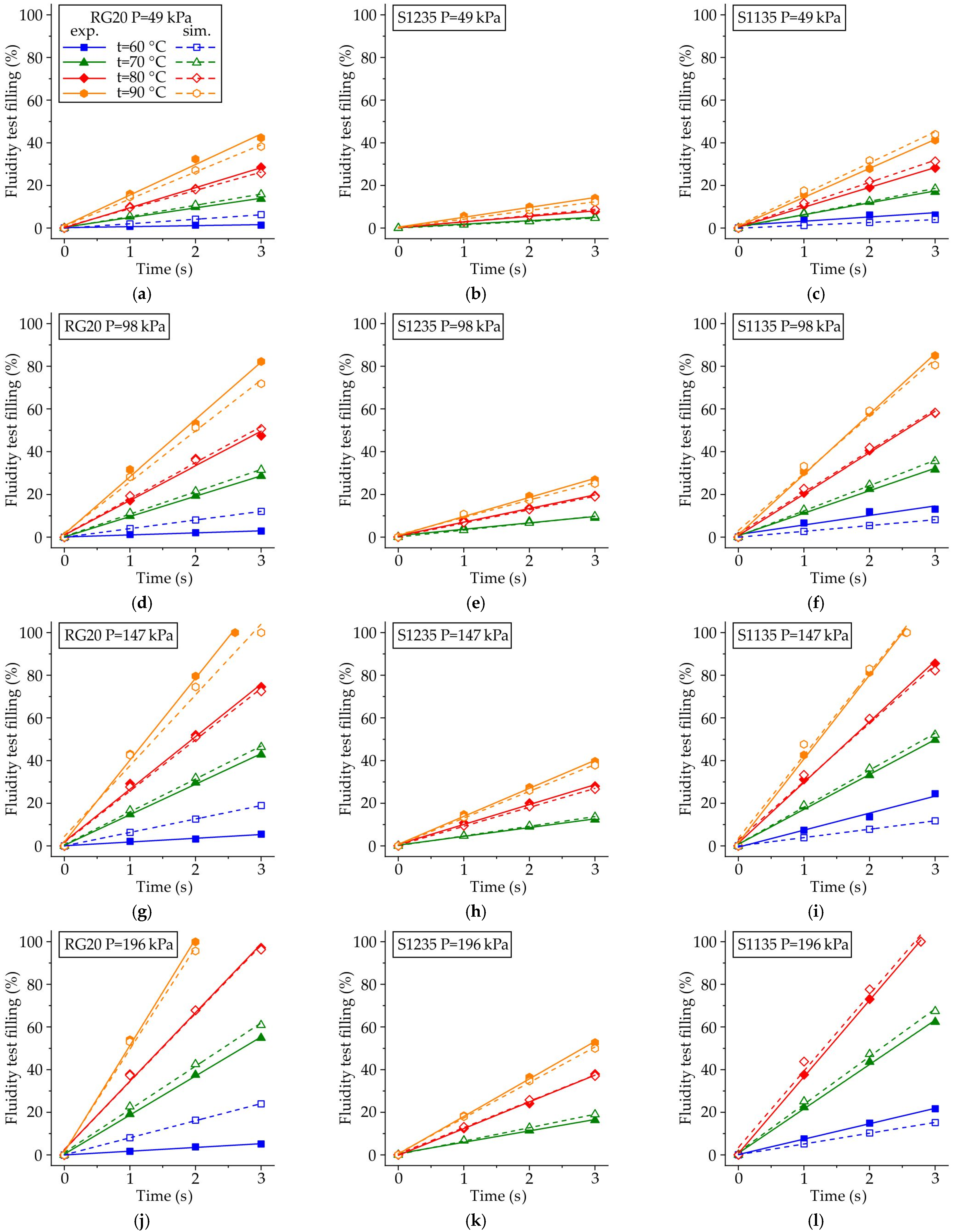
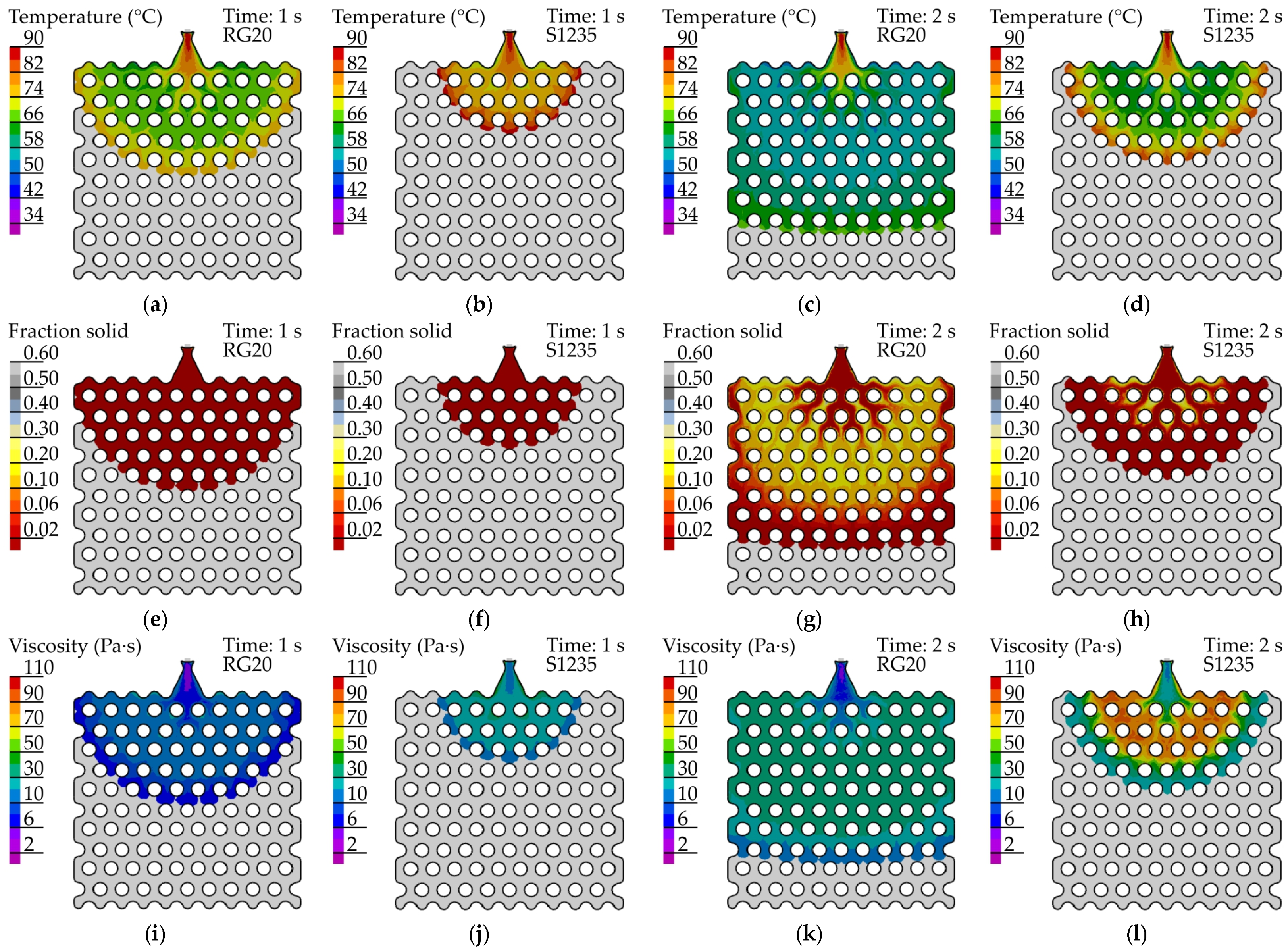
4.4. The Comparison of Experimental and Simulated Fluidity Probe Filling
5. Discussion
6. Conclusions
- i.
- The thermal properties of RG20, S1235, and S1135 pattern waxes were determined. At room temperature, the waxes were found to have a thermal conductivity of 0.18–0.26 W/mK, a heat capacity of 1.36–1.64 J/gK, and a density of 0.97–1.00 g/cm3.
- ii.
- The following parameters, namely , , n, and λ, from the Carrea viscosity equation were determined as functions of temperature. These parameters enabled us to predict the viscosity of the RG20, S1235, and S1135 pattern waxes both at varying shear rates and temperatures. The sensitivity of the viscosity to the shear rate (n) corresponds well to the values of the drop melt point of waxes.
- iii.
- The IHTC for the pattern wax/die pairs was determined. The fitted IHTC values for RG20, S1235, and S1135 waxes are 425, 275, and 475 W/m2K, respectively. The simulated temperatures obtained using the determined IHTC values in this study exhibited a satisfactory correlation with the experimental temperatures.
- iv.
- The filling fraction of the fluidity probe was determined through experimental analysis and simulated in ProCast software for the RG20, S1235, and S1135 pattern waxes. At varying injection temperatures and pressures, a satisfactory comparison was obtained between the experimental and simulation results, confirming the high accuracy of the waxes’ thermal and rheological properties, as well as the wax/die IHTC values.
- v.
- It was established that the primary factor influencing the fluidity of the wax is its viscosity, which exhibits a significant increase at both decreasing temperatures and shear rates. The lowest fluidity of S1235 wax is associated with its initial high viscosity, which results in a reduced flow magnitude and thereby leads to further increases in viscosity due to low shear rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pradyumna, R.; Sridhar, S.; Satyanarayana, A.; Chauhan, A.S.; Baig, M.A.H. Wax Patterns for Integrally Cast Rotors/Stators of Aeroengine Gas Turbines. Mater. Today Proc. 2015, 2, 1714–1722. [Google Scholar] [CrossRef]
- Bemblage, O.; Karunakar, D.B. A Study on the Blended Wax Patterns in Investment Casting Process. In Proceedings of the World Congress on Engineering (WCE 2011), London, UK, 6–8 July 2011; Volume 1, pp. 1–7. [Google Scholar]
- Singh, S.; Singh, R. Precision investment casting: A state of art review and future trends. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2015, 230, 2143–2164. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, P.; Mishra, B.K. Investigations of the effect of injection parameters on the dimensional accuracy of wax patterns used in ceramic shell investment casting. Int. J. Manuf. Technol. Manag. 2010, 21, 148–159. [Google Scholar] [CrossRef]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Developments in investment casting process—A review. J. Mater. Process. Technol. 2012, 212, 2332–2348. [Google Scholar] [CrossRef]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Multi-characteristic optimization of wax patterns in the investment casting process using grey–fuzzy logic. Int. J. Adv. Manuf. Technol. 2013, 67, 1577–1587. [Google Scholar] [CrossRef]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. A prediction model for the lost wax process through fuzzy-based artificial neural network. J. Mech. Eng. Sci. 2013, 228, 1259–1271. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, P.; Mishra, B.K. Experimental investigation of wax blends in investment casting process. Indian Foundry J. 2006, 52, 29–36. [Google Scholar]
- Gebelin, J.-C.; Jolly, M.R.; Cendrowicz, A.M.; Blackburn, S.; Cirre, J. Simulation of die filling for the wax injection process: Part II. Numerical simulation. Metall. Mater. Trans. B 2004, 35, 761–768. [Google Scholar] [CrossRef]
- Piwonka, T.S.; Woodbury, K.A.; Wiest, J.M. Modeling casting dimensions: Effect of wax rheology and interfacial heat transfer. Mater. Des. 2000, 21, 365–372. [Google Scholar] [CrossRef]
- Gebelin, J.-C.; Cendrowicz, A.M.; Jolly, M.R. Modelling of the wax injection process for the investment casting process: Prediction of defects. In Proceedings of the Third International Conference on CFD in the Minerals and Process Industries CSIRO, Melbourne, Australia, 10–12 December 2003; pp. 415–420. [Google Scholar]
- Gebelin, J.-C.; Jolly, M.R.; Cendrowicz, A.M.; Blackburn, S.; Cirre, J. Simulation of die filling for the wax injection process: Part I. Models for material behavior. Metall. Mater. Trans. B 2004, 35, 755–759. [Google Scholar] [CrossRef]
- Gebelin, J.-C.; Jolly, M.R. Modelling of the investment casting process. J. Mater. Process. Technol. 2003, 135, 291–300. [Google Scholar] [CrossRef]
- Wang, D.; He, B.; Li, F.; Sun, B. Numerical Simulation of the Wax Injection Process for Investment Casting. Mater. Manuf. Process. 2013, 28, 220–224. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, H.W.; Li, K.W.; Wang, G.; Li, F. Numerical Simulation of Mold Filling Process for Wax Pattern of the Impeller in Investment Casting. Appl. Mech. Mater. 2011, 80–81, 965–968. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Sannikov, A.V.; Kovyshkina, E.P.; Koltygin, A.V.; Bazlov, A.I.; Belov, V.D.; Dmitriev, D.N. The Influence of Injection Temperature and Pressure on Pattern Wax Fluidity. J. Manuf. Mater. Process. 2023, 7, 141. [Google Scholar] [CrossRef]
- Grzeskowiak, K.; Czarnecka-Komorowska, D.; Sytek, K.; Wojciechowski, M. Influence of waxes remelting used in investment casting on their thermal properties and linear shrinkage. Metalurgija 2015, 54, 350–352. [Google Scholar]
- ESI Group. ProCAST 2010.0 User’s Manual (ESI Group, 2010). Available online: https://myesi.esi-group.com/downloads/software-documentation/procast-2021.0-user-guide-visual-cast-procast-rev-b-online-online-online-online (accessed on 1 June 2024).
- Yang, L.; Chai, L.H.; Liang, Y.F.; Zhang, Y.W.; Bao, C.L.; Liu, S.B.; Lin, J.P. Numerical simulation and experimental verification of gravity and centrifugal investment casting low pressure turbine blades for high Nb–TiAl alloy. Intermetallics 2015, 66, 149–155. [Google Scholar] [CrossRef]
- Lu, S.-L.; Xiao, F.-R.; Guo, Z.-H.; Wang, L.-J.; Li, H.-Y.; Liao, B. Numerical simulation of multilayered multiple metal cast rolls in compound casting process. Appl. Therm. Eng. 2016, 93, 518–528. [Google Scholar] [CrossRef]
- Dantzig, J.A.; Rappaz, M. Solidification; EPFL Press: Lausanne, Switzerland, 2009; pp. 105–151. [Google Scholar]
- Carreau, P.J. Rheological Equations from Molecular Network Theories. Trans. Soc. Rheol. 1972, 16, 99–127. [Google Scholar] [CrossRef]
- Ameri, M.; Farzan, H.; Nobari, M. Evaluation of Different Glazing Materials, Strategies, and Configurations in Flat Plate Collectors Using Glass and Acrylic Covers: An Experimental Assessment. Iran. J. Energy Environ. 2021, 12, 297–306. [Google Scholar] [CrossRef]
- McCabe, J.F.; Walls, A.W.G. Applied Dental Materials, 9th ed.; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 21–209. [Google Scholar]
- Al Sarheed, M.; Sedaghat, A.; Malayer, M.A.; Salem, H.; Oloomi, S.A.A.; Hussam, W.K.; Al Anazi, A.A.; Sharifpur, M. Manufacturing Wood-Plastic Composites and Their Thermal Performance in Building Envelope (Version 1). Preprint 2022. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Tselovalnik, Y.V.; Koltygin, A.V.; Belov, V.D. Investigation of the interfacial heat transfer coefficient at the metal–mold interface during casting of an A356 aluminum alloy and AZ81 magnesium alloy into steel and graphite molds. Int. J. Met. 2021, 15, 625–637. [Google Scholar] [CrossRef]
- Bonilla, W.; Masood, S.H.; Iovenitti, P. An Investigation of Wax Patterns for Accuracy Improvement in Investment Cast Parts. Int. J. Adv. Manuf. Technol. 2001, 18, 348–356. [Google Scholar] [CrossRef]
- Sabau, A.S.; Viswanathan, S. Material properties for predicting wax pattern dimensions in investment casting. Mater. Sci. Eng. A 2003, 362, 125–134. [Google Scholar] [CrossRef]
- Sabau, A.S.; Viswanathan, S. Prediction of Wax Pattern Dimensions in Investment Casting. AFS Trans. 2002, 110, 733–746. [Google Scholar]
- Burlaga, B.; Kroma, A.; Poszwa, P.; Kłosowiak, R.; Popielarski, P.; Stręk, T. Heat Transfer Analysis of 3D Printed Wax Injection Mold Used in Investment Casting. Materials 2022, 15, 6545. [Google Scholar] [CrossRef] [PubMed]
- Ukrainczyk, N.; Kurajica, S.; Šipušić, J. Thermophysical Comparison of Five Commercial Paraffin Waxes as Latent Heat Storage Materials. Iranian (Iranica) Chemical and Biochemical Engineering Quarterly. Chem. Biochem. Eng. Q. 2010, 24, 129–137. [Google Scholar]
- Battezzati, L.; Greer, A.L. The viscosity of liquid metals and alloys. Acta Metall. 1989, 37, 1791–1802. [Google Scholar] [CrossRef]
- Taşcıoğlu, S.; İnem, B.; Akar, N. Conversion of an investment casting sprue wax to a pattern wax by the modification of its properties. Mater. Des. 2004, 25, 499–505. [Google Scholar] [CrossRef]
- Taşcıoğlu, S.; Akar, N. A novel alternative to the additives in investment casting pattern wax compositions. Mater. Des. 2003, 24, 693–698. [Google Scholar] [CrossRef]
- Chakravorty, S. The Properties of Waxes Used in the Investment Casting Industry: Final Report; NPL Report No. CMMT(A)245; National Physical Laboratory: Middlesex, UK, 1999; pp. 2–8. [Google Scholar]



| Wax | RG20 | S1235 | S1135 |
|---|---|---|---|
| Additives | Cross-linked polystyrene particles | Cross-linked polystyrene particles | Cross-linked polystyrene particles/terephthalic acid |
| Additives content (%) | 18 | 35 | 22/13 |
| Drop melt point (°C) | 71 | 77 | 75 |
| Viscosity at 100 °C (mPa·s) | 310 | 400 | 350 |
| Linear shrinkage (%) | 0.65 | 0.65 | 0.7 |
| Ash content (%) | 0.02 | 0.04 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhenov, V.E.; Ovsyannikov, A.S.; Kovyshkina, E.P.; Stepashkin, A.A.; Nikitina, A.A.; Koltygin, A.V.; Belov, V.D.; Dmitriev, D.N. The Numerical Simulation of the Injection Filling of the Fluidity Probe Die with Pattern Waxes. J. Manuf. Mater. Process. 2024, 8, 213. https://doi.org/10.3390/jmmp8050213
Bazhenov VE, Ovsyannikov AS, Kovyshkina EP, Stepashkin AA, Nikitina AA, Koltygin AV, Belov VD, Dmitriev DN. The Numerical Simulation of the Injection Filling of the Fluidity Probe Die with Pattern Waxes. Journal of Manufacturing and Materials Processing. 2024; 8(5):213. https://doi.org/10.3390/jmmp8050213
Chicago/Turabian StyleBazhenov, Viacheslav E., Arseniy S. Ovsyannikov, Elena P. Kovyshkina, Andrey A. Stepashkin, Anna A. Nikitina, Andrey V. Koltygin, Vladimir D. Belov, and Dmitry N. Dmitriev. 2024. "The Numerical Simulation of the Injection Filling of the Fluidity Probe Die with Pattern Waxes" Journal of Manufacturing and Materials Processing 8, no. 5: 213. https://doi.org/10.3390/jmmp8050213
APA StyleBazhenov, V. E., Ovsyannikov, A. S., Kovyshkina, E. P., Stepashkin, A. A., Nikitina, A. A., Koltygin, A. V., Belov, V. D., & Dmitriev, D. N. (2024). The Numerical Simulation of the Injection Filling of the Fluidity Probe Die with Pattern Waxes. Journal of Manufacturing and Materials Processing, 8(5), 213. https://doi.org/10.3390/jmmp8050213






