Finite Element Modelling and Validation of Thermomechanical Behaviour for Layered Aluminium Parts Made by Composite Metal Foil Manufacturing
Abstract
:1. Introduction
2. Experimental Procedure
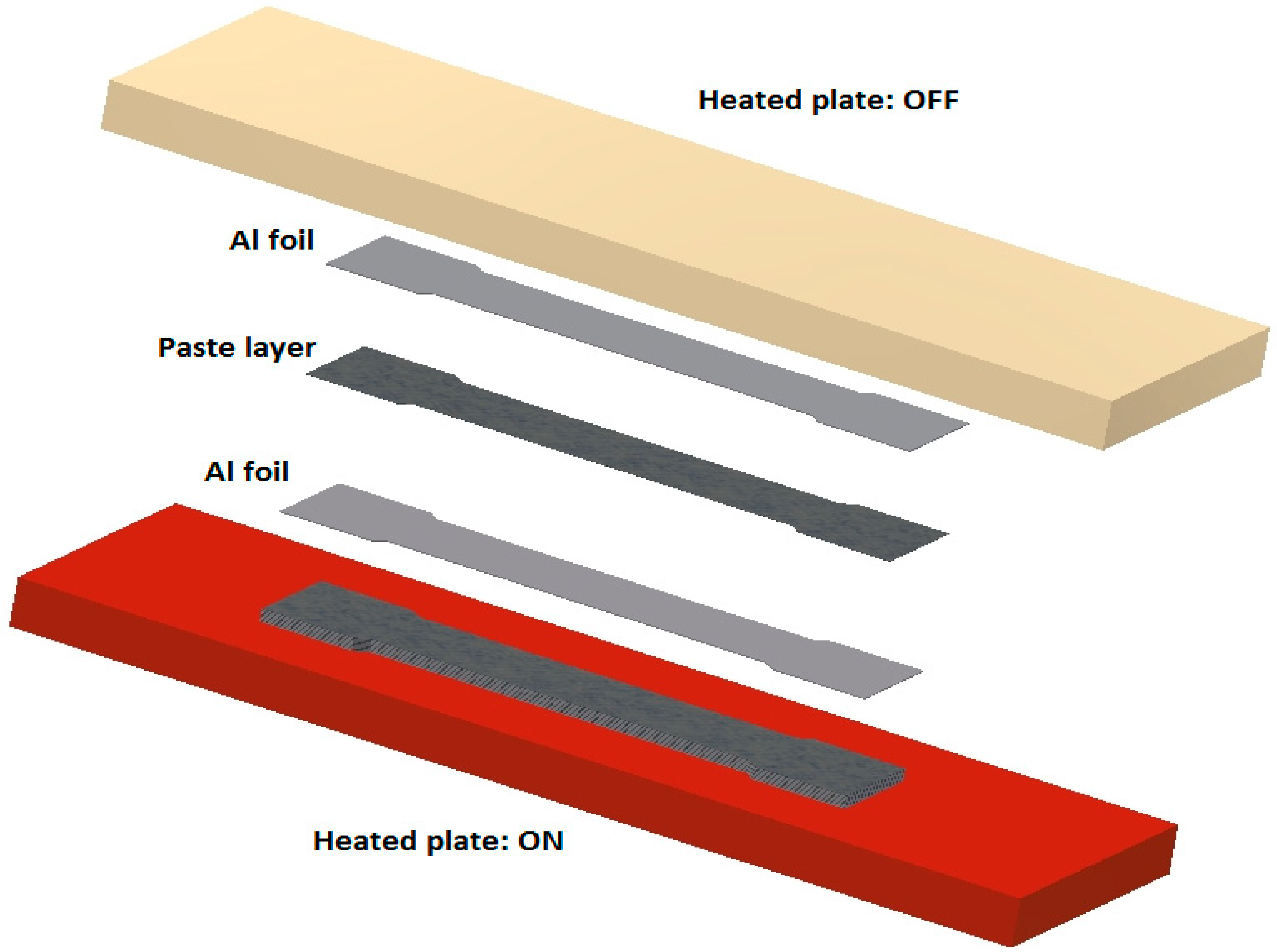
2.1. Modelling for Numerical Analysis
2.2. Experiment for Heating Time
2.3. Tensile Test for Dog Bone Specimens
2.4. Corrosion Test of Dog Bone Specimens
2.5. Microstructural Analysis
3. Results and Discussions
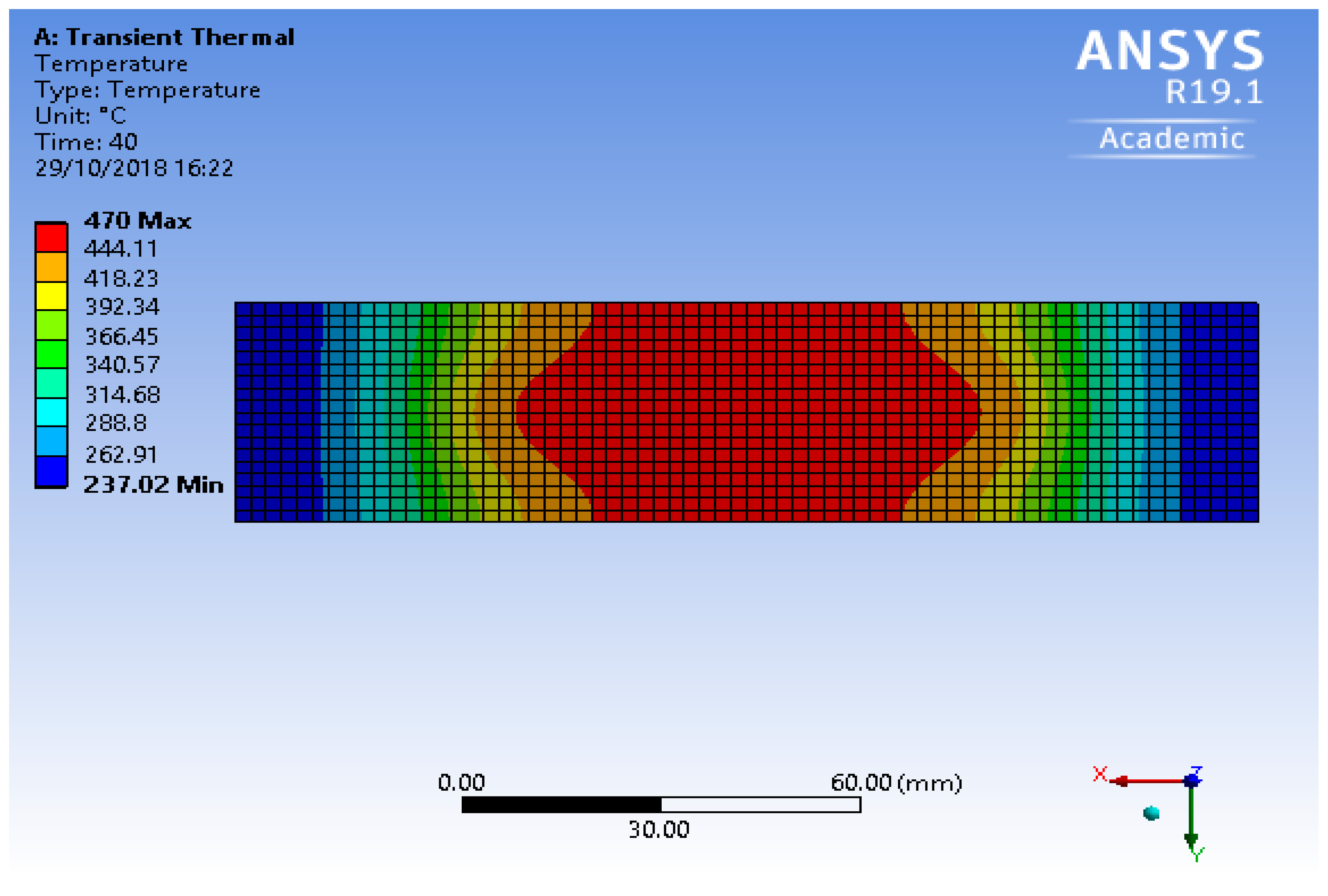
3.1. Distribution of Temperature
- The flux in the paste could have burned off, and it would not have been able to make a strong bond;
- Overheating would have resulted in pitting of the foils and increased thermal stress, as well as strain; and
- A change in material properties could have made the products useless for any engineering application.
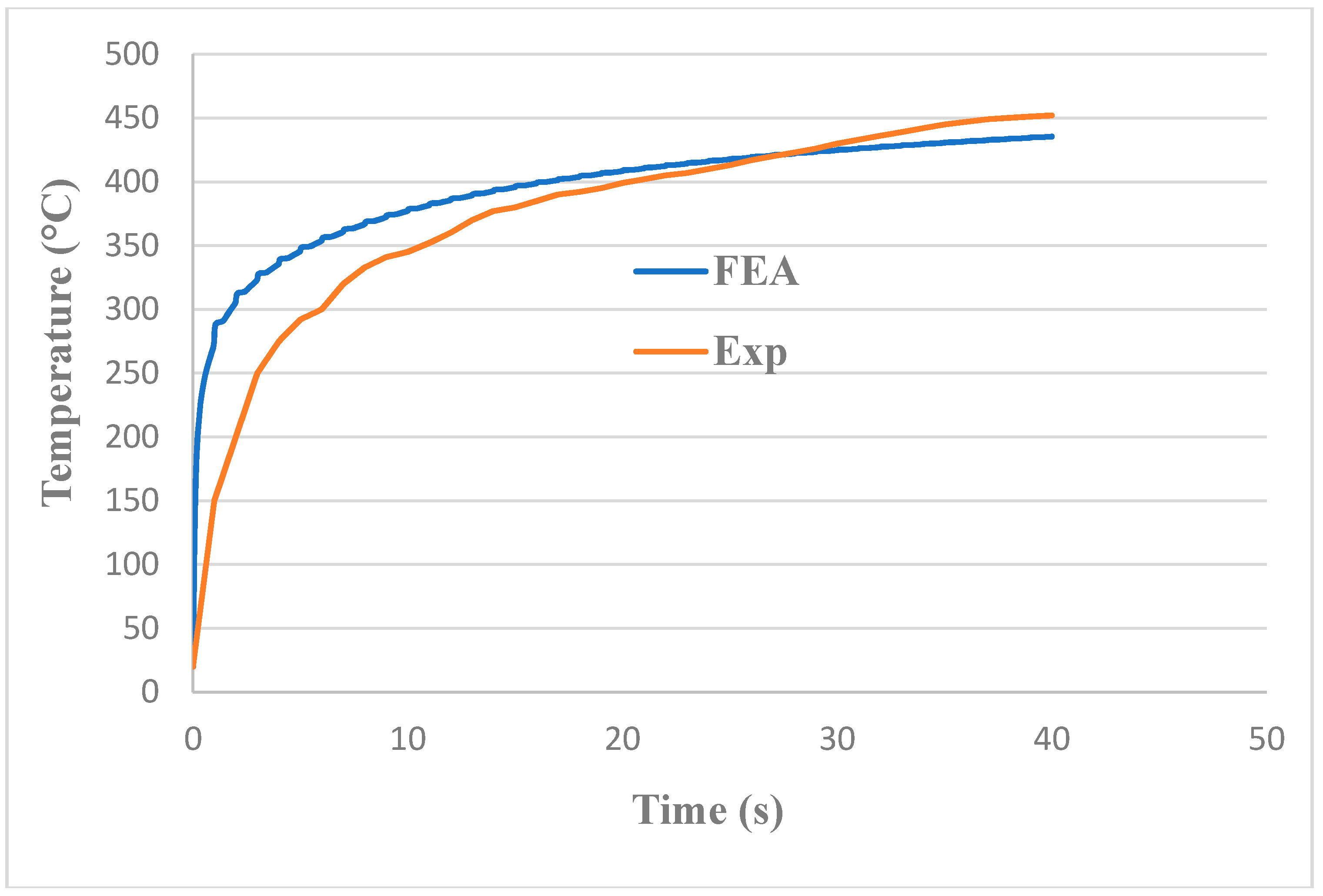
3.2. Validation for Heating Time
3.2.1 Thermal Stress and Strain
3.3. Results from the Tensile Test of the Dog-Bone Specimens
3.4. Results from Corrosion Test of Dog Bone Specimens
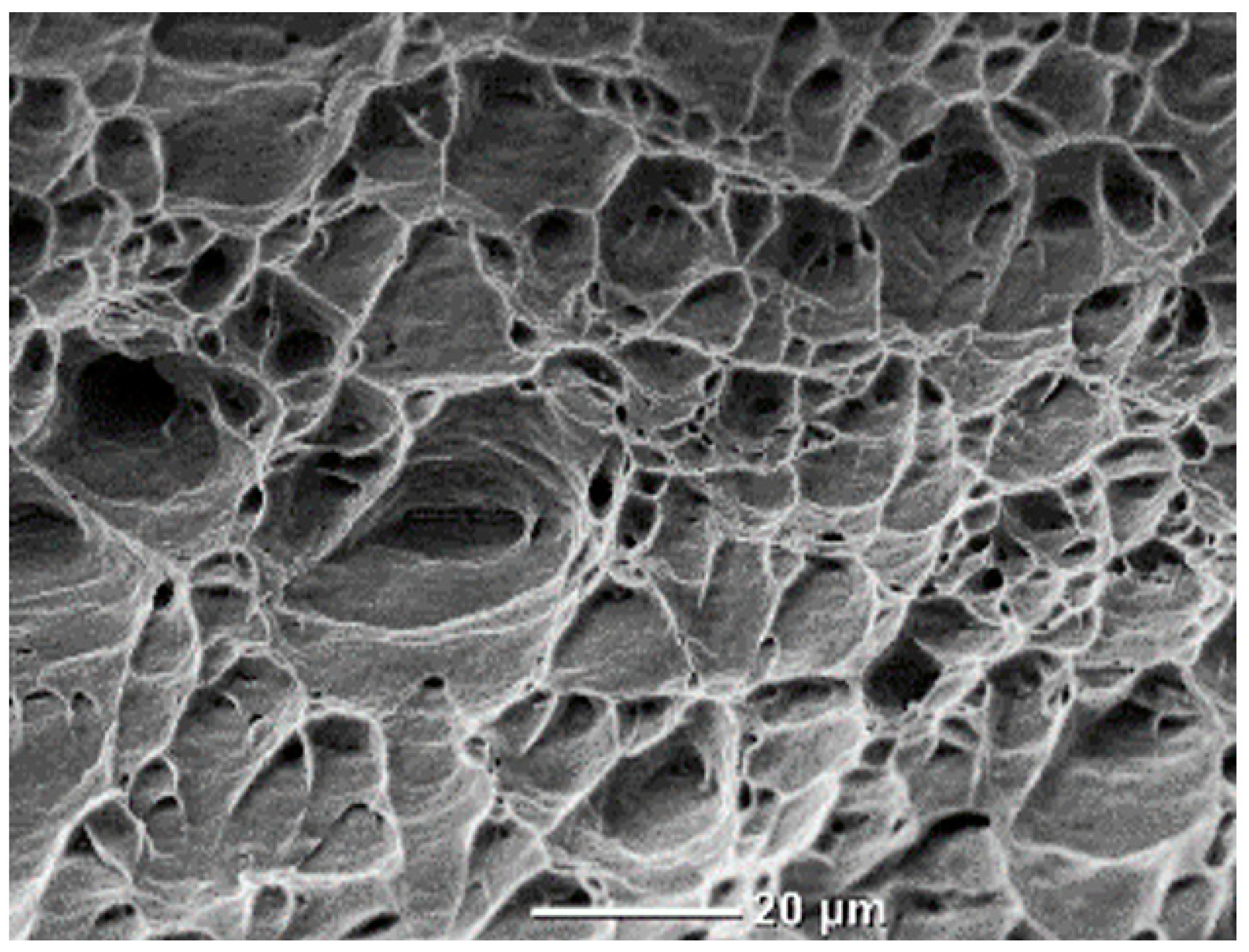
3.5. Results from Microstructural Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoozbashizadeh, M.; Yavari, P.; Khoshnevis, B. Novel method for additive manufacturing of metal-matrix composite by thermal decomposition of salts. Addit. Manuf. 2018, 24, 173–182. [Google Scholar] [CrossRef]
- Tang, S.H.; Cheng, C.W.; Yeh, R.Y.; Hsu, R.Q. Direct joining of 3D-printed thermoplastic parts to SLM-fabricated metal cellular structures by ultrasonic welding. Int. J. Adv. Manuf. Technol. 2018, 99, 729–736. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Horikawa, S.; Kakehi, K. Effects of build direction and heat treatment on creep properties of Ni-base superalloy built up by additive manufacturing. Scr. Mater. 2017, 129, 74–78. [Google Scholar] [CrossRef]
- Levy, A.; Miriyev, A.; Elliott, A.; Babu, S.S.; Frage, N. Additive manufacturing of complex-shaped graded TiC/steel composites. Mater. Des. 2017, 118, 198–203. [Google Scholar] [CrossRef]
- Butt, J.; Shirvani, H. Experimental analysis of metal/plastic composites made by a new hybrid method. Addit. Manuf. 2018, 22, 216–222. [Google Scholar] [CrossRef]
- Kazantseva, N.; Krakhmalev, P.; Yadroitsev, I.; Fefelov, A.; Merkushev, A.; Ilyinikh, M.; Vinogradova, N.; Ezhov, I.; Kurennykh, T. Oxygen and nitrogen concentrations in the Ti-6Al-4V alloy manufactured by direct metal laser sintering (DMLS) process. Mater. Lett. 2017, 209, 311–314. [Google Scholar] [CrossRef]
- Hadadzadeh, A.; Baxter, C.; Amirkhiz, B.S.; Mohammadi, M. Strengthening mechanisms in direct metal laser sintered AlSi10Mg: Comparison between virgin and recycled powders. Addit. Manuf. 2018, 23, 108–120. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, H.L.; Li, S.J.; Wang, S.G.; Wang, W.J.; Hou, W.T.; Hao, Y.L.; Yang, R.; Zhang, L.C. Compressive and fatigue behavior of beta-type titanium porous structures fabricated by electron beam melting. Acta Mater. 2017, 126, 58–66. [Google Scholar] [CrossRef]
- Arrieta, E.; Haque, M.; Mireles, J.; Stewart, C.; Carrasco, C.; Wicker, R.B. Mechanical Behavior of Differently Oriented Electron Beam Melting Ti–6Al–4V Components Using Digital Image Correlation. J. Eng. Mater. Technol. 2019, 141, 011004. [Google Scholar] [CrossRef]
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. Wire-feed additive manufacturing of metal components: Technologies, developments and future interests. Int. J. Adv. Manuf. Technol. 2015, 81, 465–481. [Google Scholar] [CrossRef]
- Silva, C.C.; Miranda, E.C.D.; Motta, M.F.; Miranda, H.C.D.; Farias, J.P. Minimization of defects in nickel-based superalloy weld overlay deposited by the GTAW cold wire feed process. Weld. Int. 2016, 30, 443–451. [Google Scholar] [CrossRef]
- Prechtl, M.; Otto, A.; Geiger, M. Rapid tooling by laminated object manufacturing of metal foil. Adv. Mater. Res. 2005, 6, 303–312. [Google Scholar] [CrossRef]
- Prechtl, M.; Otto, A.; Geiger, M. Laminated Object Manufacturing of Metal Foil—Process Chain and System Technology; Springer: Vienna, Austria, 2005; pp. 597–606. [Google Scholar]
- Bournias-Varotsis, A.; Friel, R.J.; Harris, R.A.; Engstrøm, D.S. Ultrasonic Additive Manufacturing as a form-then-bond process for embedding electronic circuitry into a metal matrix. J. Manuf. Process. 2018, 32, 664–675. [Google Scholar] [CrossRef] [Green Version]
- Mekid, S.; Daraghma, H. Experimental ultrasonic sub-surface consolidation of fiber bragg grating for sensorial materials. J. Mater. Process. Technol. 2018, 252, 673–679. [Google Scholar] [CrossRef]
- Butt, J.; Mebrahtu, H.; Shirvani, H. Microstructure and mechanical properties of dissimilar 1050 aluminium/pure copper foil composites made with a new additive manufacturing process. J. Mater. Process. Technol. 2016, 238, 96–107. [Google Scholar] [CrossRef]
- Butt, J.; Mebrahtu, H.; Shirvani, H. Rapid prototyping by heat diffusion of metal foil and related mechanical testing. Int. J. Adv. Manuf. Technol. 2016, 84, 2357–2366. [Google Scholar] [CrossRef]
- Butt, J.; Mebrahtu, H.; Shirvani, H. A novel rapid prototyping process for the production of metal parts. In Proceedings of the Second International Conference on Advances in Civil, Structural and Mechanical Engineering-CSM, Birmingham, UK, 16–17 November 2014; pp. 26–29. [Google Scholar]
- Butt, J. A Novel Additive Manufacturing Process for the Production of Metal Parts. Doctoral Dissertation, Anglia Ruskin University, Cambridge, UK, 2016. [Google Scholar]
- Butt, J.; Mebrahtu, H.; Shirvani, H. Tensile lap-shear and flexural behaviour of aluminium metal foil parts made by composite metal foil manufacturing. Prog. Addit. Manuf. 2018, 1–9. [Google Scholar] [CrossRef]
- Butt, J.; Mebrahtu, H.; Shirvani, H. Peel and tensile test investigation of aluminium 1050 foil parts made with a new additive manufacturing process. Int. J. Rapid Manuf. 2015, 5, 95–115. [Google Scholar] [CrossRef]
- Butt, J.; Mebrahtu, H.; Shirvani, H. Numerical and experimental analysis of product development by composite metal foil manufacturing. Int. J. Rapid Manuf. 2018, 7, 59–82. [Google Scholar] [CrossRef]
- Butt, J.; Mebrahtu, H.; Shirvani, H. Thermo-Mechanical Analysis of Dissimilar Al/Cu Foil Single Lap Joints Made by Composite Metal Foil Manufacturing. World Acad. Sci. Eng. Technol. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2015, 10, 41–46. [Google Scholar]
- Nammi, S.K.; Butt, J.; Mauricette, J.L.; Shirvani, H. December. Numerical Analysis of Thermal Stresses around Fasteners in Composite Metal Foils. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 280, p. 012016. [Google Scholar]
- BS EN ISO 6892-1:2009. Metallic Materials. Tensile Testing. Method of Test at Ambient Temperature; British, European and International Standard: London, UK, 2009. [Google Scholar]
- BS EN ISO 11130:2010. Corrosion of Metals and Alloys—Alternate Immersion Test in Salt Solution; British, European and International Standard: London, UK, 2010. [Google Scholar]
- Murakami, T.; Nakata, K.; Tong, H.; Ushio, M. Dissimilar metal joining of aluminum to steel by MIG arc brazing using flux cored wire. ISIJ Int. 2003, 43, 1596–1602. [Google Scholar] [CrossRef]
- Fratini, L.; Zuccarello, B. An analysis of through-thickness residual stresses in aluminium FSW butt joints. Int. J. Mach. Tools Manuf. 2006, 46, 611–619. [Google Scholar] [CrossRef]
- Qiao, X.G.; Starink, M.J.; Gao, N. Hardness inhomogeneity and local strengthening mechanisms of an Al1050 aluminium alloy after one pass of equal channel angular pressing. Mater. Sci. Eng. A 2009, 513, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Butt, J.; Mebrahtu, H.; Shirvani, H. Strength analysis of aluminium foil parts made by composite metal foil manufacturing. Prog. Addit. Manuf. 2016, 1, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Butt, J.; Mebrahtu, H.; Shirvani, H. Metal Rapid Prototyping Technologies. In Advances in Engineering Research; Petrova, V.M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; Volume 14, Chapter 2; pp. 13–52. [Google Scholar]
- Butt, J.; Shirvani, H. Additive, Subtractive, and Hybrid Manufacturing Processes. In Advances in Manufacturing and Processing of Materials and Structures; CRC Press: Boca Raton, FL, USA, 2018; pp. 187–218. [Google Scholar]














| Properties | Materials | |
|---|---|---|
| Al 1050 | Brazing Paste | |
| Yield Strength (MPa) | 105–145 | 45 |
| Tensile Strength (MPa) | 120 | 60 |
| Young’s Modulus (GPa) | 69 | 60 |
| Thermal Conductivity (W/(m·°C)) | 205 | 900 |
| Specific Heat (J/(kg·°C)) | 130 | 480 |
| Test | Specimens | Fracture Load (kN) | Total Elongation (%) | Ultimate Tensile Strength (UTS) (MPa) | Difference in UTS (%) |
|---|---|---|---|---|---|
| Before corrosion testing | Al 1050 | 4.48 | 21.5 | 132.8 | Compared to Al 1050 |
| 0.05 mm | 4.92 | 2.6 | 145.8 | 9.8 | |
| 0.1 mm | 4.85 | 2.8 | 143.8 | 8.3 | |
| 0.2 mm | 4.75 | 3.1 | 140.8 | 6.0 | |
| Compared to corresponding foil parts | |||||
| After corrosion testing | 0.05 mm | 4.9 | 2.5 | 145.2 | −0.4 |
| 0.1 mm | 4.88 | 2.6 | 144.6 | +0.5 | |
| 0.2 mm | 4.71 | 2.8 | 139.5 | −0.9 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butt, J.; Ghorabian, M.; Ahmed, A.; Shirvani, H. Finite Element Modelling and Validation of Thermomechanical Behaviour for Layered Aluminium Parts Made by Composite Metal Foil Manufacturing. J. Compos. Sci. 2018, 2, 68. https://doi.org/10.3390/jcs2040068
Butt J, Ghorabian M, Ahmed A, Shirvani H. Finite Element Modelling and Validation of Thermomechanical Behaviour for Layered Aluminium Parts Made by Composite Metal Foil Manufacturing. Journal of Composites Science. 2018; 2(4):68. https://doi.org/10.3390/jcs2040068
Chicago/Turabian StyleButt, Javaid, Mohammad Ghorabian, Abed Ahmed, and Hassan Shirvani. 2018. "Finite Element Modelling and Validation of Thermomechanical Behaviour for Layered Aluminium Parts Made by Composite Metal Foil Manufacturing" Journal of Composites Science 2, no. 4: 68. https://doi.org/10.3390/jcs2040068
APA StyleButt, J., Ghorabian, M., Ahmed, A., & Shirvani, H. (2018). Finite Element Modelling and Validation of Thermomechanical Behaviour for Layered Aluminium Parts Made by Composite Metal Foil Manufacturing. Journal of Composites Science, 2(4), 68. https://doi.org/10.3390/jcs2040068






