Removal of Pb(II) Ions from Aqueous Solution Using Modified Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Cassava Starch
2.3. Preparation of Adsorbents
- NCS—Native commercially available starch;
- NWS—Native starch extracted by the wet method;
- MCS—Modified native commercially available starch;
- MWS—Modified native starch extracted by the wet method.
2.4. Characterization of Samples
2.4.1. Nitrogen (N2) Adsorption Studies
2.4.2. Fourier Transforms Infrared Spectroscopy (FTIR)
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. X-Ray Diffraction (XRD)
2.4.5. Atomic Absorption Spectrophotometer (AAS)
2.5. Adsorption Studies
2.5.1. Adsorption Kinetic Studies
2.5.2. Equilibrium Adsorption Kinetics
2.5.3. Desorption Experiments
3. Results and Discussion
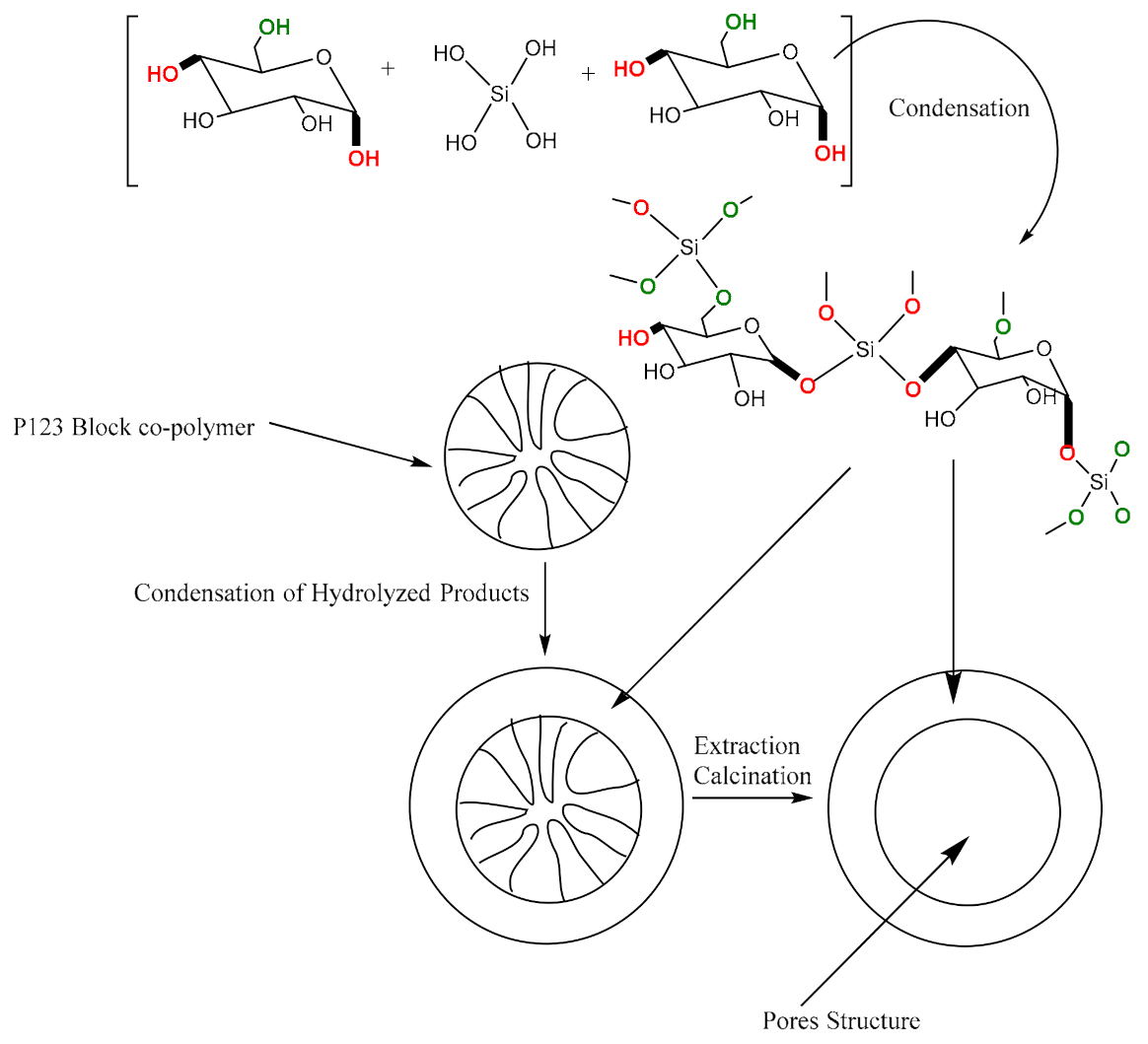
3.1. Synthesis of Modified Starch
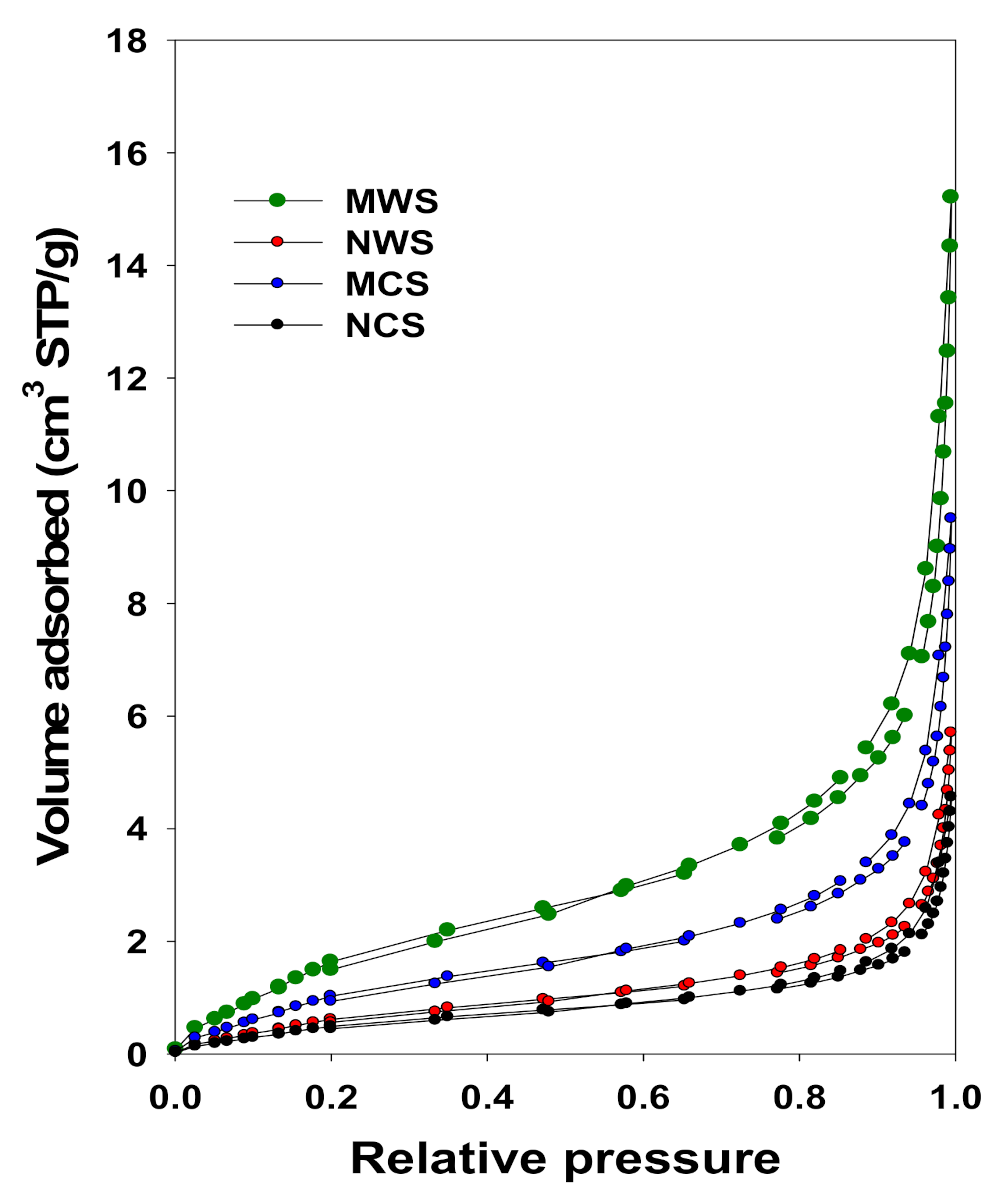
3.2. N2 Adsorption Studies
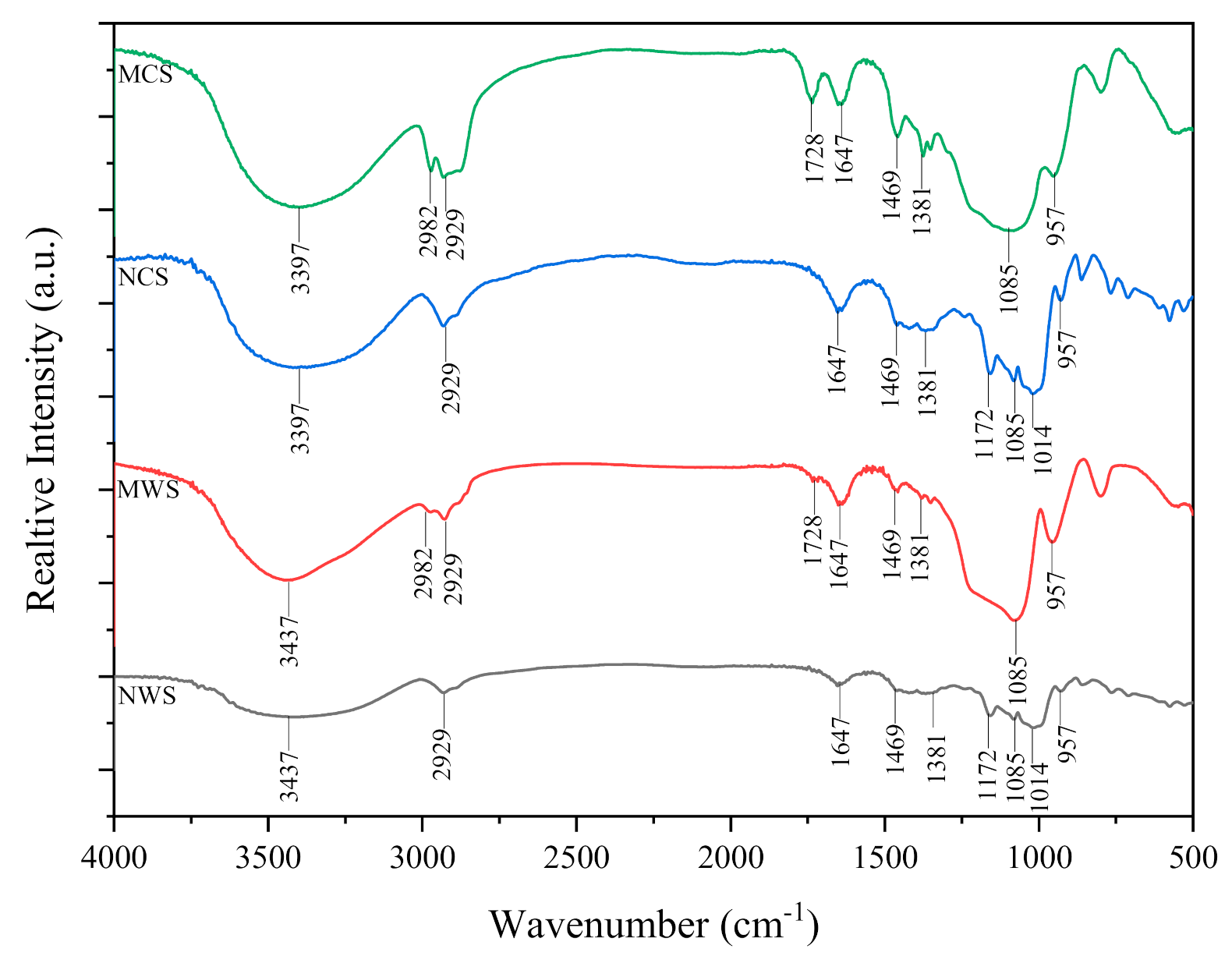
3.3. Fourier Transforms Infrared Spectroscopy (FTIR)
3.4. Thermogravimetric Analysis (TGA)
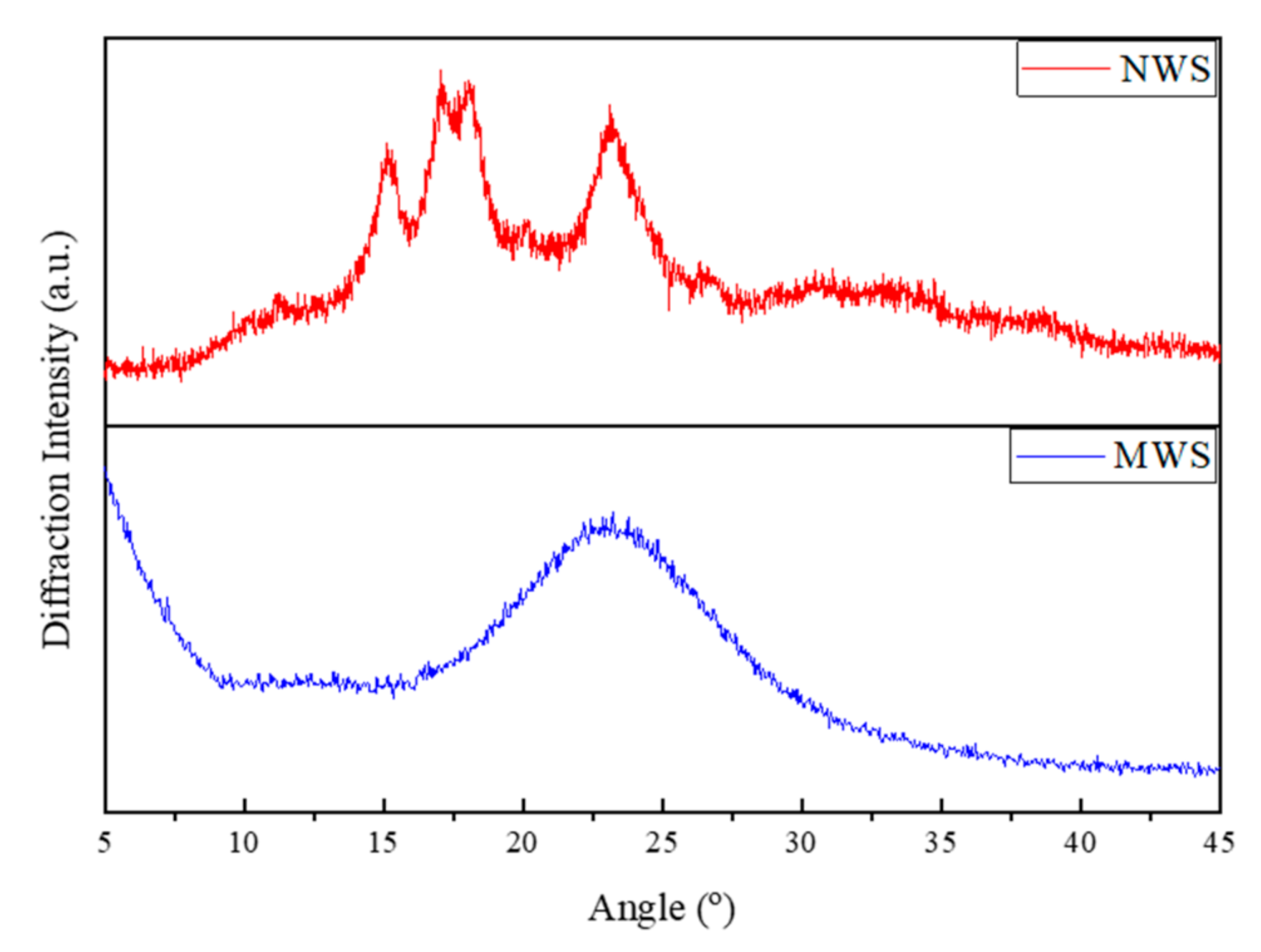
3.5. X-Ray Diffraction (XRD) Analysis
3.6. Adsorption and Kinetic Studies
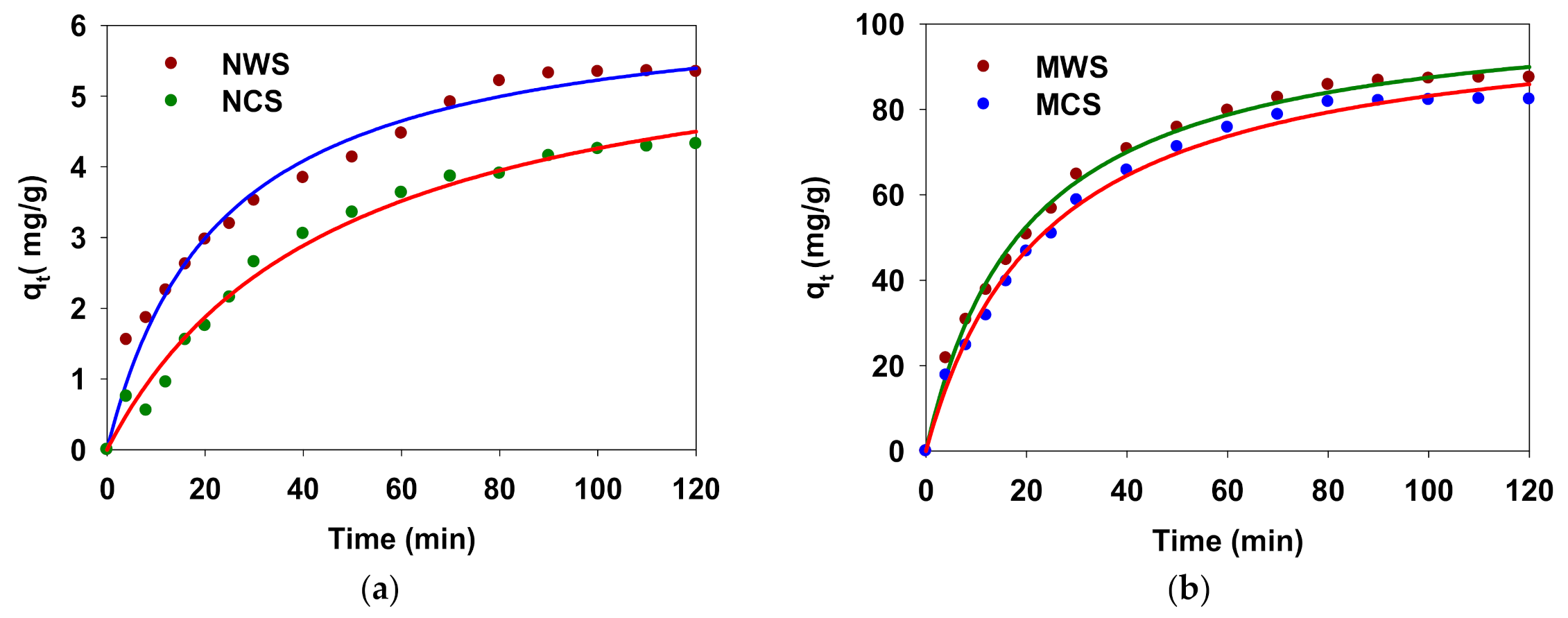
3.6.1. The Effect of Contact Time on Adsorption Capacity
3.6.2. Pseudo-First Order Kinetic Model
3.6.3. Pseudo-Second-Order Model
3.7. Effect of Initial Concentration on Adsorption Capacity
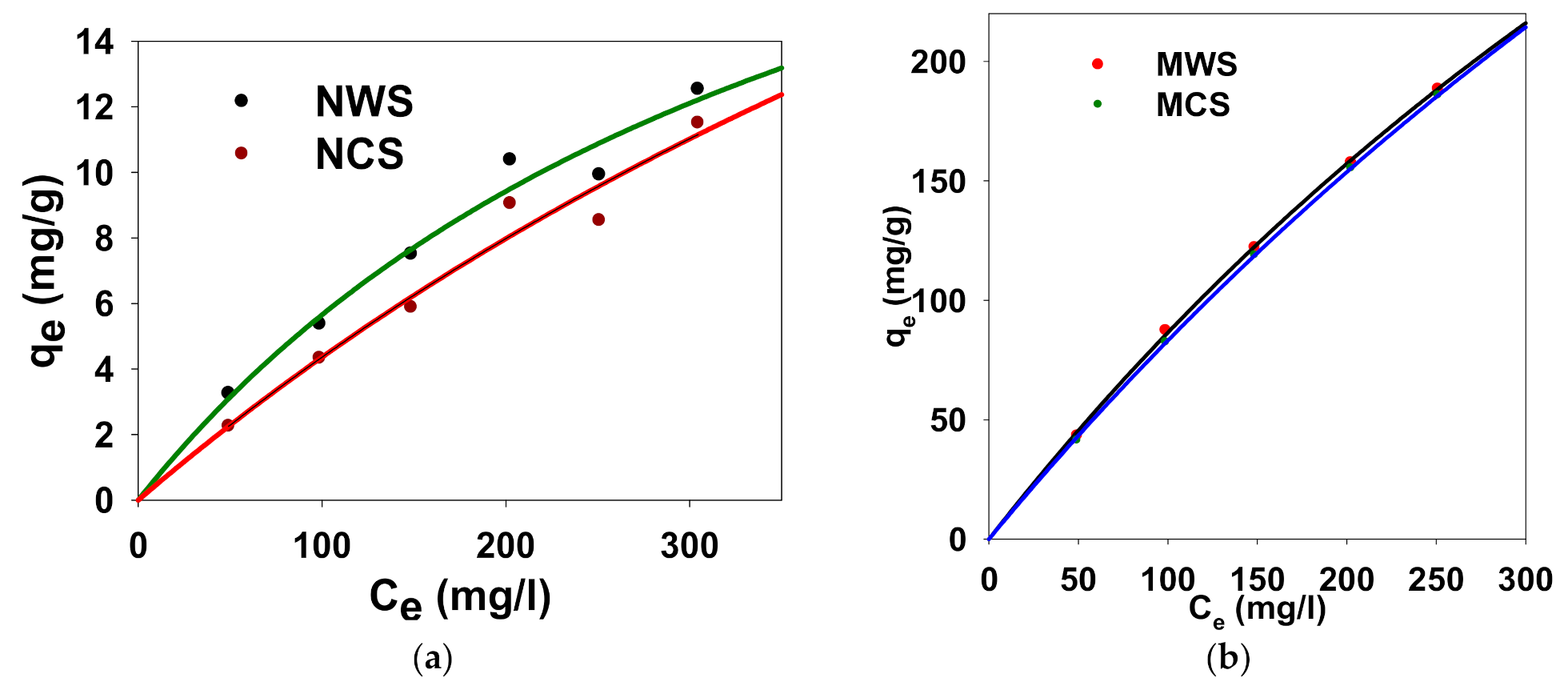
3.8. Adsorption Isotherms
3.8.1. Langmuir Model
3.8.2. Freundlich Model
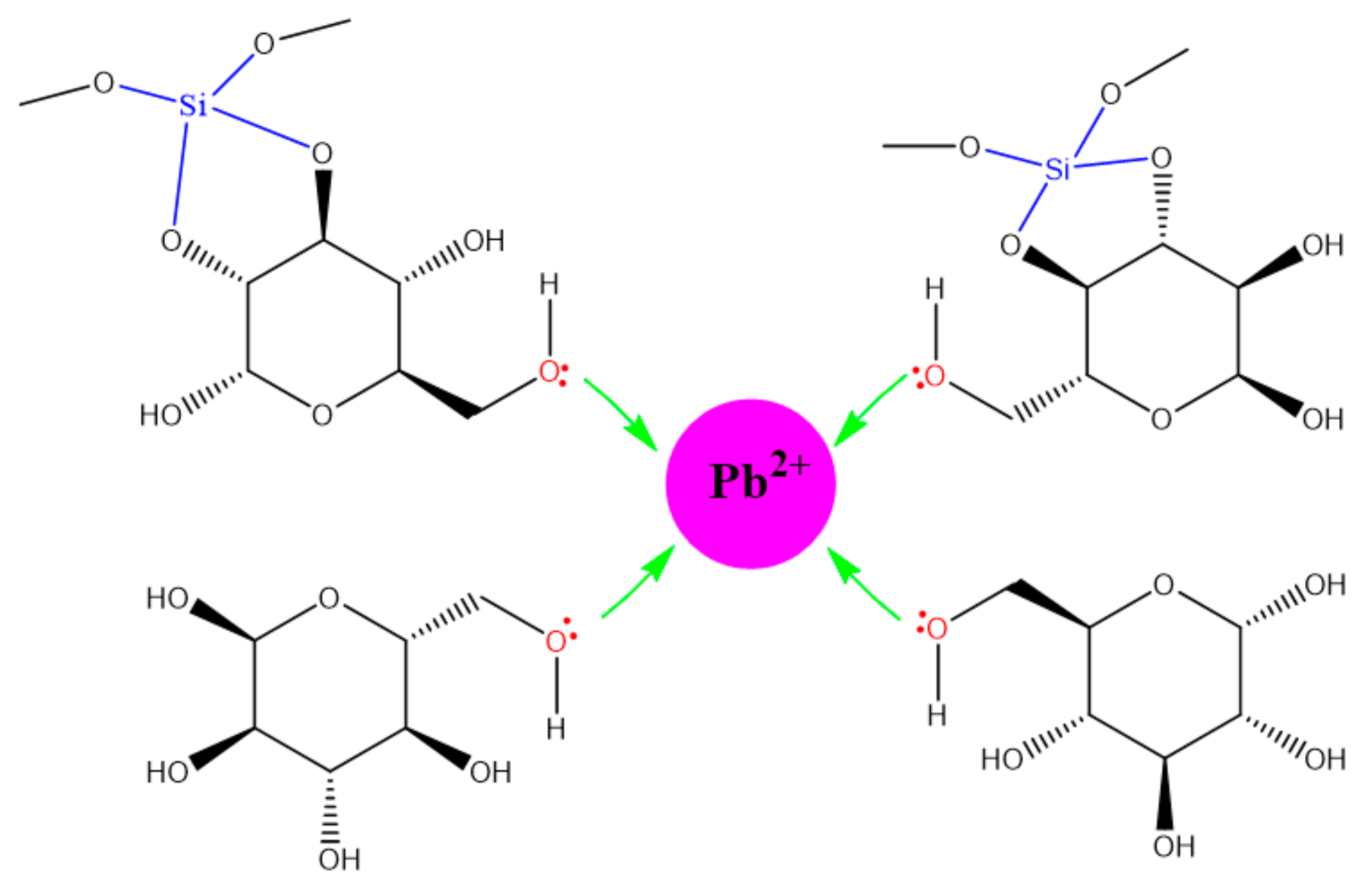
3.9. Lead Adsorption Mechanism
3.10. Desorption Studies
3.11. Comparison with Other Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, B.; Fan, W.; Yi, X.; Wang, Z.; Gao, F.; Li, Y.; Gu, H. Dithiocarbamate-modified starch derivatives with high heavy metal adsorption performance. Carbohydr. Polym. 2016, 136, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Apopei, D.F.; Dinu, M.V.; Trochimczuk, A.W.; Dragan, E.S. Sorption isotherms of heavy metal ions onto semi-interpenetrating polymer network cryogels based on polycrylamide and anionically modified potato starch. Ind. Eng. Chem. Res. 2012, 51, 10462–10471. [Google Scholar] [CrossRef]
- Bradl, H. Heavy Metals in the Environment: Origin, Interaction and Remediation; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Nriagu, J.O. A global assessment of natural sources of atmospheric trace metals. Nature 1989, 338, 47–49. [Google Scholar] [CrossRef]
- Bhat, M.A.; Chisti, H.; Shah, S.A. Removal of heavy metal ions from water by cross-linked potato di-starch phosphate polymer. Sep. Sci. Technol. 2015, 50, 1741–1747. [Google Scholar] [CrossRef]
- Ibrahim, B.; Fakhre, N. Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int. J. Biol. Macromol. 2019, 123, 70–80. [Google Scholar] [CrossRef]
- Ferguson, J.E. The Heavy Elements. In Chemistry, Environmental Impact and Health Effects; Pergamon Press: Oxford, UK, 1990; pp. 211–212. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Overall evaluations of carciogeniety: An updating of IARC Monographs volumes 1 to 42. IARC Monogr. Eval. Carcinog. Risks Hum. Suppl. 1987, 7, 1–440. [Google Scholar]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Goyer, R.A.; Clarkson, T.W. Toxic effects of metals. Casarett Doull’s Toxicol. Basic Sci. Poisons 1996, 5, 696–698. [Google Scholar]
- Herawati, N.; Suzuki, S.; Hayashi, K.; Rivai, I.; Koyama, H. Cadmium, copper, and zinc levels in rice and soil of Japan, Indonesia, and China by soil type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef]
- Shallari, S.; Schwartz, C.; Hasko, A.; Morel, J.-L. Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci. Total Environ. 1998, 209, 133–142. [Google Scholar] [CrossRef]
- Ashizawa, A.; Faroon, O.; Ingerman, L.; Jenkins, K.; Tucker, P.; Wright, S. Draft Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2008. [Google Scholar]
- Bardos, P. Composting of Mechanically Segregated Fractions of Municipal Solid Waste—A review. SITA Environmental Trust. 2004. Available online: http://www.compostinfo.info/content/set%20critical%20review%20msw%20composting.pdf (accessed on 1 January 2021).
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Abdel-Aal, S.; Gad, Y.; Dessouki, A. The use of wood pulp and radiation-modified starch in wastewater treatment. J. Appl. Polym. Sci. 2006, 99, 2460–2469. [Google Scholar] [CrossRef]
- Tchounwou, P.; Wilson, B.; Abdelghani, A.; Ishaque, A.; Patlolla, A. Differential cytotoxicity and gene expression in human liver carcinoma (HepG2) cells exposed to arsenic trioxide, and monosodium acid methanearsonate (MSMA). Int. J. Mol. Sci. 2002, 3, 1117–1132. [Google Scholar] [CrossRef] [Green Version]
- Yedjou, C.G.; Moore, P.; Tchounwou, P.B. Dose-and time-dependent response of human leukemia (HL-60) cells to arsenic trioxide treatment. Int. J. Environ. Res. Public Health 2006, 3, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Centeno, J.A.; Patlolla, A.K. Arsenic toxicity, mutagenesis, and carcinogenesis—A health risk assessment and management approach. Mol. Cell. Biochem. 2004, 255, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R. A commentary on the impacts of metals and metalloids in the environment upon the metabolism of drugs and chemicals. Toxicol. Lett. 2004, 148, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Centeno, J.A.; Tchounwou, P.B.; Patlolla, A.K.; Mullick, F.G.; Murakata, L.; Meza, E.; TodorTodorov, D.L.; Yedjou, C.G. Environmental pathology and health effects of arsenic poisoning. In Managing Arsenic in the Environment: From Soil to Human Health; CSIRO Publishing: Clayton, Victoria, Australia, 2006; pp. 311–327. [Google Scholar]
- Okuda, J.; Iida, K. Preparation of a “chronological table of main diseases in Japanese history” for pharmacy students of the 6-year program. Yakushigaku Zasshi 2005, 40, 137–146. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar]
- Meena, A.K.; Mishra, G.; Rai, P.; Rajagopal, C.; Nagar, P. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef]
- Jain, S.; Vasudevan, P.; Jha, N. Removal of some heavy metals from polluted water by aquatic plants: Studies on duckweed and water velvet. Biol. Wastes 1989, 28, 115–126. [Google Scholar] [CrossRef]
- Flora, S.J.; Flora, G.; Saxena, G. Environmental occurrence, health effects and management of lead poisoning. In Lead; Elsevier: Amsterdam, The Netherlands, 2006; pp. 158–228. [Google Scholar]
- Harvey, B. Managing Elevated Blood Lead Levels among Young Children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention. Available online: https://www.cdc.gov/nceh/lead/casemanagement/casemanage_main.htm (accessed on 4 January 2021).
- Abdul-Raheim, A.-R.M.; El-Saeed Shimaa, M.; Farag Reem, K.; Abdel-Raouf Manar, E. Low cost biosorbents based on modified starch iron oxide nanocomposites for selective removal of some heavy metals from aqueous solutions. Adv. Mater. Lett. 2016, 7, 402–409. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Nabil, G.M.; Zaki, M.M.; Saleh, M.M. Starch functionalization of iron oxide by-product from steel industry as a sustainable low cost nanocomposite for removal of divalent toxic metal ions from water. Int. J. Biol. Macromol. 2019, 137, 455–468. [Google Scholar] [CrossRef]
- Feng, K.; Wen, G. Absorbed Pb2+ and Cd2+ ions in water by cross-linked starch xanthate. Int. J. Polym. Sci. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Barczak, M.; Michalak-Zwierz, K.; Gdula, K.; Tyszczuk-Rotko, K.; Dobrowolski, R.; Dąbrowski, A. Ordered mesoporous carbons as effective sorbents for removal of heavy metal ions. Microporous Mesoporous Mater. 2015, 211, 162–173. [Google Scholar] [CrossRef]
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical modification of starch and its application as an adsorbent material. RSC Adv. 2016, 6, 78264–78285. [Google Scholar] [CrossRef]
- Xie, G.; Shang, X.; Liu, R.; Hu, J.; Liao, S. Synthesis and characterization of a novel amino modified starch and its adsorption properties for Cd (II) ions from aqueous solution. Carbohydr. Polym. 2011, 84, 430–438. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Lu, H.; Li, M.; Liu, J.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced dispersion stability and heavy metal ion adsorption capability of oxidized starch nanoparticles. Food Chem. 2018, 242, 256–263. [Google Scholar] [CrossRef]
- Awokoya, K.; Moronkola, B. Preparation and characterization of succinylated starch as adsorbent for the removal of Pb (II) ions from aqueous media. Int. J. Eng. Sci 2012, 1, 18–24. [Google Scholar]
- Kweon, D.-K.; Choi, J.-K.; Kim, E.-K.; Lim, S.-T. Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohydr. Polym. 2001, 46, 171–177. [Google Scholar] [CrossRef]
- Güçlü, G.; Al, E.; Emik, S.; İyim, T.B.; Özgümüş, S.; Özyürek, M. Removal of Cu 2+ and Pb 2+ ions from aqueous solutions by starch-graft-acrylic acid/montmorillonite superabsorbent nanocomposite hydrogels. Polym. Bull. 2010, 65, 333–346. [Google Scholar] [CrossRef]
- Xu, S.-M.; Feng, S.; Peng, G.; Wang, J.-D.; Yushan, A. Removal of Pb (II) by crosslinked amphoteric starch containing the carboxymethyl group. Carbohydr. Polym. 2005, 60, 301–305. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, S.-F.; Ju, B.-Z.; Yang, J.-Z.; Quan, X. Removal of Pb (II) from aqueous solution by cross-linked starch phosphate carbamate. J. Polym. Res. 2006, 13, 213–217. [Google Scholar] [CrossRef]
- Soto, D.; Urdaneta, J.; Pernía, K.; León, O.; Muñoz-Bonilla, A.; Fernandez-García, M. Removal of heavy metal ions in water by starch esters. Starch-Stärke 2016, 68, 37–46. [Google Scholar] [CrossRef]
- Kolya, H.; Das, S.; Tripathy, T. Synthesis of Starch-g-Poly- (N-methylacrylamide-co-acrylic acid) and its application for the removal of mercury (II) from aqueous solution by adsorption. Eur. Polym. J. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Irani, M.; Ismail, H.; Ahmad, Z.; Fan, M. Synthesis of linear low-density polyethylene-g-poly (acrylic acid)-co-starch/organo-montmorillonite hydrogel composite as an adsorbent for removal of Pb (ΙΙ) from aqueous solutions. J. Environ. Sci. 2015, 27, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Benesi, I.R.; Labuschagne, M.T.; Dixon, A.G.; Mahungu, N.M. Stability of native starch quality parameters, starch extraction and root dry matter of cassava genotypes in different environments. J. Sci. Food Agric. 2004, 84, 1381–1388. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Methods 920.36 (Moisture), 984.13 (Protein), 948.22(Lipid and Oil) and 923.03 (Ash of Flour), 17th ed.; AOAC (2000) Official Methods of Analysis; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Method 962.09 (Crude Fibber), 18th ed.; AOAC (2005) Official Method of Analysis; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Stawski, D. New determination method of amylose content in potato starch. Food Chem. 2008, 110, 777–781. [Google Scholar] [CrossRef]
- Gunathilake, C.; Kadanapitiye, M.S.; Dudarko, O.; Huang, S.D.; Jaroniec, M. Adsorption of lead ions from aqueous phase on mesoporous silica with P-containing pendant groups. ACS Appl. Mater. Interfaces 2015, 7, 23144–23152. [Google Scholar] [CrossRef]
- Gunathilake, C.; Gangoda, M.; Jaroniec, M. Mesoporous isocyanurate-containing organosilica–alumina composites and their thermal treatment in nitrogen for carbon dioxide sorption at elevated temperatures. J. Mater. Chem. 2013, 1, 8244–8252. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. The important role of crystallinity and amylose ratio in thermal stability of starches. J. Therm. Anal. Calorim. 2018, 131, 2555–2567. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.B.; Stephani, R.; Poppi, R.J.; de Oliveira, L.F.C. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Utrilla-Coello, R.G.; Hernández-Jaimes, C.; Carrillo-Navas, H.; González, F.; Rodríguez, E.; Bello-Perez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Acid hydrolysis of native corn starch: Morphology, crystallinity, rheological and thermal properties. Carbohydr. Polym. 2014, 103, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Badmus, M.; Audu, T.; Anyata, B. Removal of lead ion from industrial wastewaters by activated carbon prepared from periwinkle shells (Typanotonus fuscatus). Turk. J. Eng. Environ. Sci. 2007, 31, 251–263. [Google Scholar]
- Vojoudi, H.; Badiei, A.; Bahar, S.; Ziarani, G.M.; Faridbod, F.; Ganjali, M.R. A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J. Magn. Magn. Mater. 2017, 441, 193–203. [Google Scholar] [CrossRef]
- Pavlovic, I.; Pérez, M.; Barriga, C.; Ulibarri, M. Adsorption of Cu2+, Cd2+ and Pb2+ ions by layered double hydroxides intercalated with the chelating agents diethylenetriaminepentaacetate and meso-2, 3-dimercaptosuccinate. Appl. Clay Sci. 2009, 43, 125–129. [Google Scholar] [CrossRef]


| NWS | NCS | |
|---|---|---|
| Components | Percentage (%) | Percentage (%) |
| Moisture Content | 12 ± (0.01) | 14 |
| Amylose | 21.04 | - |
| Crude fibre | 0.2 ± (0.01) | 0.2 |
| Gelatinization Temperature | 43.27 °C | - |
| Particle Size | <0.149 mm | <0.037 mm |
| Sample | Vsp (cm3/g) | Vmi (cm3/g) | SBET (m2/g) | Vtot (cm3/g) |
|---|---|---|---|---|
| NCS | 0.006 | <0.01 | 1.7 ± 0.1 | 0.007 |
| MCS | 0.013 | <0.01 | 3.6 ± 0.1 | 0.014 |
| NWS | 0.007 | <0.01 | 2.1 ± 0.1 | 0.009 |
| MWS | 0.021 | <0.01 | 5.8 ± 0.1 | 0.022 |
| Sample | First-Order Rate Constant (k1), min−1 | R2 |
|---|---|---|
| NCS | 4.35 × 10−2 | 0.94 |
| MCS | 5.46 × 10−2 | 0.98 |
| NWS | 4.77 × 10−2 | 0.94 |
| MWS | 6.67 × 10−2 | 0.92 |
| Adsorbent | qe (mg·g−1) | h (mg·g−1 min−1) | k2 (g mg−1 min−1) | R2 |
|---|---|---|---|---|
| NCS | 21.77 | 0.15 | 6.65 × 10−4 | 0.98 |
| MCS | 261.01 | 4.51 | 4.42 × 10−4 | 0.99 |
| NWS | 26.39 | 0.29 | 7.15 × 10−4 | 0.99 |
| MWS | 303.09 | 5.52 | 5.2 × 10−4 | 0.99 |
| Sample | qm (mg·g−1) | KL (L mg−1) | R2 |
|---|---|---|---|
| NCS | 13.36 | 3.64 × 10−3 | 0.99 |
| MCS | 294.12 | 3.06 × 10−2 | 0.98 |
| NWS | 18.91 | 1.18 × 10−3 | 0.99 |
| MWS | 330.37 | 1.64 × 10−2 | 0.99 |
| Sample | N | KF | R2 |
|---|---|---|---|
| NCS | 0.87 | 0.08 | 0.98 |
| MCS | 0.67 | 11.65 | 0.98 |
| NWS | 0.73 | 0.20 | 0.98 |
| MWS | 0.56 | 18.59 | 0.97 |
| Adsorbent | Desorption Efficiency (%) | ||
|---|---|---|---|
| 10 min | 20 min | 30 min | |
| MCS | 72.4 | 85.6 | 98.3 |
| MWS | 76.4 | 89.8 | 97.5 |
| Adsorbent | qm (mg/g) | Reference |
|---|---|---|
| Starch esters | 25.16 | [40] |
| Oxidized starch | 48.27 | [36] |
| Hydrothermally modified chitosan | 97.97 | [34] |
| Hydroxyethyl starch-graft polyacrylamide | 103.6 | [41] |
| Cross-linked amphoteric starch | 152.74 | [38] |
| Activated carbon prepared by periwinkle shells | 234.4 | [54] |
| Silica modified through co-precipitation | 270 | [55] |
| MCS | 294.12 | Present study |
| Cross-linked phosphate carbonate | 316.47 | [39] |
| MWS | 330.37 | Present study |
| ZnAl-dmsa (meso-2,3-dimercaptosuccinic acid) | 404 | [56] |
| Polyethylene-graftpoly (acrylic acid)-co-starch/organo- montmorillonite hydrogel composite | 430 | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunawardene, O.H.P.; Gunathilake, C.A.; Amaraweera, A.P.S.M.; Fernando, N.M.L.; Manipura, A.; Manamperi, W.A.; Kulatunga, K.M.A.K.; Rajapaksha, S.M.; Gamage, A.; Dassanayake, R.S.; et al. Removal of Pb(II) Ions from Aqueous Solution Using Modified Starch. J. Compos. Sci. 2021, 5, 46. https://doi.org/10.3390/jcs5020046
Gunawardene OHP, Gunathilake CA, Amaraweera APSM, Fernando NML, Manipura A, Manamperi WA, Kulatunga KMAK, Rajapaksha SM, Gamage A, Dassanayake RS, et al. Removal of Pb(II) Ions from Aqueous Solution Using Modified Starch. Journal of Composites Science. 2021; 5(2):46. https://doi.org/10.3390/jcs5020046
Chicago/Turabian StyleGunawardene, O. H. P., C. A. Gunathilake, A. P. S. M. Amaraweera, N. M. L. Fernando, A. Manipura, W. A. Manamperi, K. M. A. K. Kulatunga, S. M. Rajapaksha, A. Gamage, R. S. Dassanayake, and et al. 2021. "Removal of Pb(II) Ions from Aqueous Solution Using Modified Starch" Journal of Composites Science 5, no. 2: 46. https://doi.org/10.3390/jcs5020046








