Vanadium Dioxide–Iridium Composite Development: Specific Near Infrared Surface Plasmon Resonance
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Colloidal Lithography: 2D Arrangement of the Polystyrene Microspheres (P-Spheres) and Optimization Processes
3.2. VO2–Ir Microtriangles Composite Development
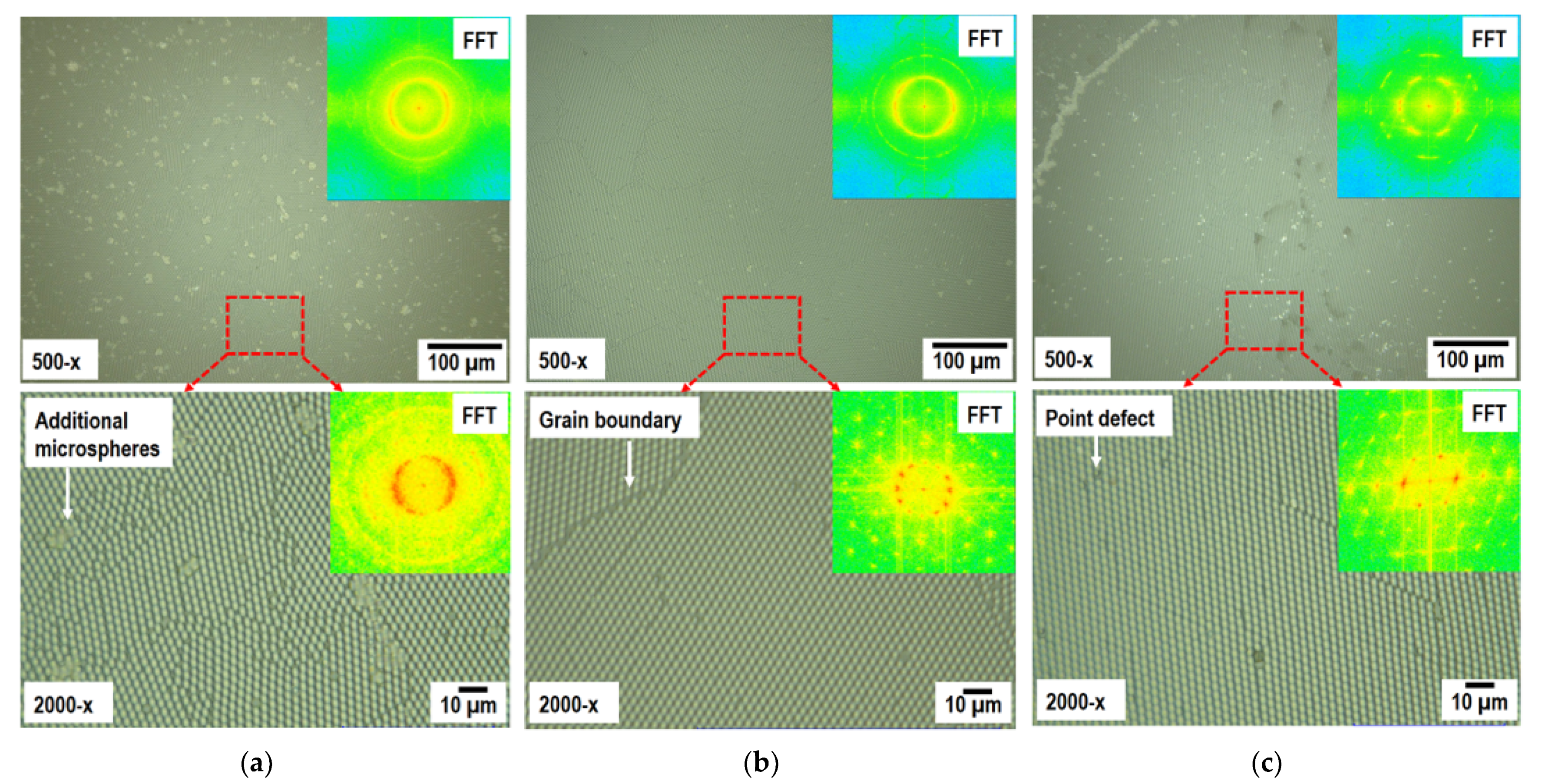
3.2.1. VO2–Ir Microtriangles Composite Development and Structural Characteristics
3.2.2. Compositional Characteristics of the VO2–Ir Microtriangles Composite
3.3. Specific Optical Properties of the VO2–Ir Composite
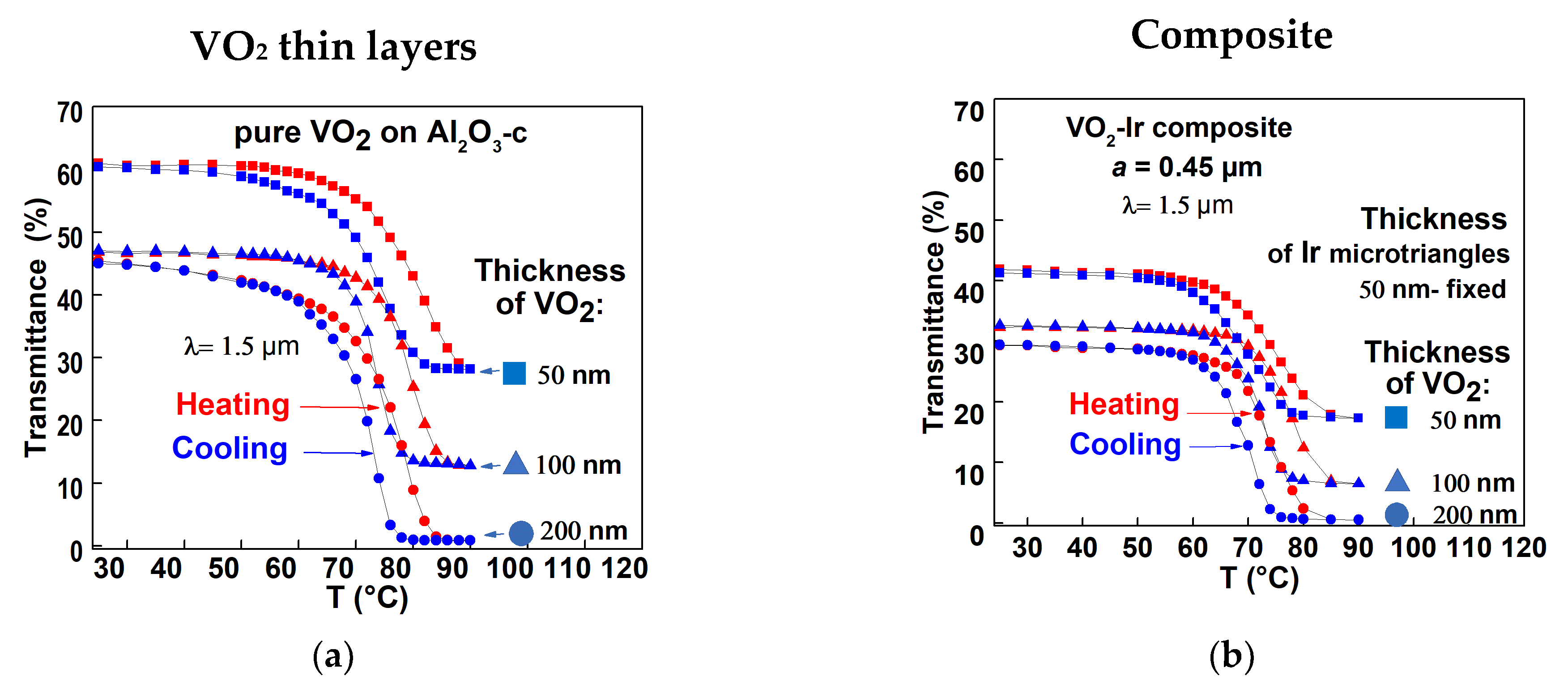
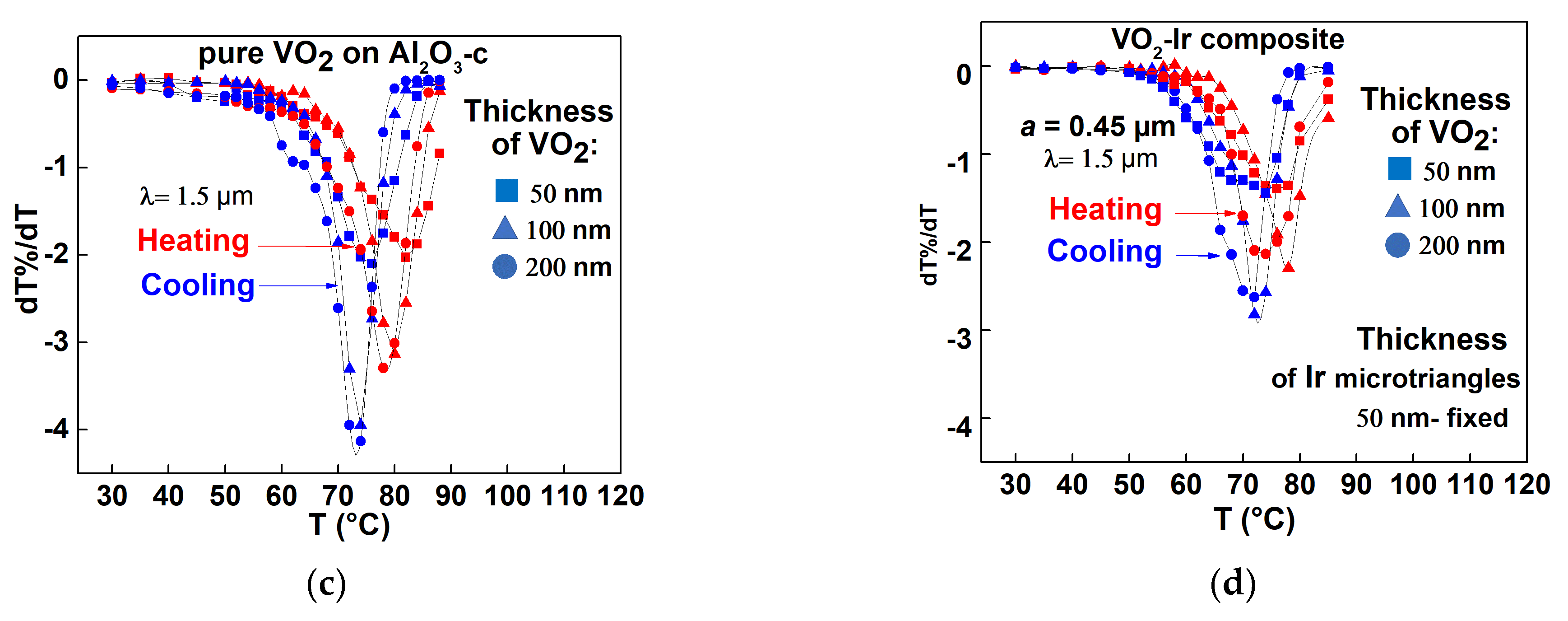
3.3.1. VO2–Ir Microtriangles Composite’s Optical Transmittance Characteristics
3.3.2. Plasmonic Properties of the VO2–Ir Microtriangles Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghidini, M.; Asti, G.; Pellicelli, R.; Pernechele, C.; Solzi, M. Hard–Soft composite magnets. J. Magn. Magn. Mater. 2007, 316, 159–165. [Google Scholar] [CrossRef]
- Wei, H.; Xu, H. Plasmonics in composite nanostructures. Mater. Today 2014, 17, 372–380. [Google Scholar] [CrossRef]
- Gaudin, M.; Carles, P.; Laborde, E.; Champeaux, C.; Dumas-Bouchiat, F. A dual nanosecond-pulsed laser setup for nanocomposite synthesis—Ag nanoparticles in Al2O3/VO2 matrix. J. Appl. Phys. 2019, 125, 054301. [Google Scholar] [CrossRef]
- Chen, Y.M.; Cai, J.H.; Huang, Y.S.; Lee, K.Y.; Tsai, D.S. Preparation and characterization of iridium dioxide–Carbon nanotube nanocomposites for supercapacitors. Nanotechnology 2011, 22, 115706. [Google Scholar] [CrossRef]
- Morin, F.J. Oxides Which Show a Metal-to-Insulator Transition at the Neel Temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Dumas-Bouchiat, F.; Champeaux, C.; Catherinot, A.; Crunteanu, A.; Blondy, P. rf-microwave switches based on reversible semiconductor-metal transition of VO2 thin films synthesized by pulsed-laser deposition. Appl. Phys. Lett. 2007, 91, 223505. [Google Scholar] [CrossRef]
- Forero-Sandoval, I.Y.; Chan-Espinoza, J.A.; Ordonez-Miranda, J.; Alvarado-Gil, J.J.; Dumas-Bouchiat, F.; Champeaux, C.; Joulain, K.; Ezzahri, Y.; Drevillon, J.; Gomez-Heredia, C.L.; et al. VO2 Substrate Effect on the Thermal Rectification of a Far-Field Radiative Diode. Phys. Rev. Appl. 2020, 14, 034023. [Google Scholar] [CrossRef]
- Sadiq, M.N.; Roy, M.L.; Perennec, A.; Laurent, P.; Martin, N.; Passerieux, D.; Crunteanu, A.; Boyer, R.; Dumas-Bouchiat, F.; Martin, M.-B.; et al. Design and Characterisation of VO2 Based Switches for Ultra-Fast Reconfigurable Devices. In Proceedings of the 2019 IEEE MTT-S International Microwave Workshop Series on Advanced Materials and Processes for RF and THz Applications (IMWS-AMP), Bochum, Germany, 16–18 July 2019; pp. 172–174. [Google Scholar] [CrossRef]
- Stinson, H.T.; Sternbach, A.; Najera, O.; Jing, R.; Mcleod, A.S.; Slusar, T.V.; Mueller, A.; Anderegg, L.; Kim, H.T.; Rozenberg, M.; et al. Imaging the nanoscale phase separation in vanadium dioxide thin films at terahertz frequencies. Nat. Commun. 2018, 9, 3604. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Cao, X.; Luo, H.; Jin, P. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Mater. 2018, 10, 581–605. [Google Scholar] [CrossRef] [Green Version]
- Kumi-Barimah, E.; Anagnostou, D.E.; Jose, G. Phase changeable vanadium dioxide (VO2) thin films grown from vanadium pentoxide (V2O5) using femtosecond pulsed laser deposition. AIP Adv. 2020, 10, 065225. [Google Scholar] [CrossRef]
- Liu, K.; Lee, S.; Yang, S.; Delaire, O.; Wu, J. Recent progresses on physics and applications of vanadium dioxide. Mater. Today 2018, 21, 875–896. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Biegler, Z.; Back, T.; Sarangan, A. Vanadium dioxide phase change thin films produced by thermal oxidation of metallic vanadium. Thin Solid Films 2020, 707, 138117. [Google Scholar] [CrossRef]
- Nag, J.; Haglund, R.F., Jr. Synthesis of vanadium dioxide thin films and nanoparticles. J. Phys. Condens. Matter 2008, 20, 264016. [Google Scholar] [CrossRef]
- Garry, G.; Durand, O.; Lordereau, A. Structural, electrical and optical properties of pulsed laser deposited VO2 thin films on R- and C-sapphire planes. Thin Solid Films 2004, 453–454, 427–430. [Google Scholar] [CrossRef]
- Soltani, M.; Chaker, M.; Haddad, E.; Kruzelecky, R.V.; Nikanpour, D. Optical switching of vanadium dioxide thin films deposited by reactive pulsed laser deposition. J. Vac. Sci. Technol. A 2004, 22, 859–864. [Google Scholar] [CrossRef]
- Mattinen, M.; Hämäläinen, J.; Vehkamäki, M.; Heikkilä, M.J.; Mizohata, K.; Jalkanen, P.; Räisänen, J.; Ritala, M.; Leskelä, M. Atomic Layer Deposition of Iridium Thin Films Using Sequential Oxygen and Hydrogen Pulses. J. Phys. Chem. C 2016, 120, 15235–15243. [Google Scholar] [CrossRef]
- Galeazzi, M.; Chen, C.; Cohn, J.L.; Gundersen, J.O. Iridium thin films deposited via pulsed laser deposition for future applications as transition-edge sensors. Nucl. Instrum. Methods Phys. Res. 2004, 520, 293–295. [Google Scholar] [CrossRef]
- Ferrara, D.W.; Nag, J.; MacQuarrie, E.R.; Kaye, A.B.; Haglund, R.F. Plasmonic Probe of the Semiconductor to Metal Phase Transition in Vanadium Dioxide. Nano Lett. 2013, 13, 4169–4175. [Google Scholar] [CrossRef]
- Xu, G.; Chen, Y.; Tazawa, M.; Jin, P. Surface Plasmon Resonance of Silver Nanoparticles on Vanadium Dioxide. J. Phys. Chem. B 2006, 110, 2051–2056. [Google Scholar] [CrossRef]
- Takeya, H.; Frame, J.; Tanaka, T.; Urade, Y.; Fang, X.; Kubo, W. Bolometric photodetection using plasmon-assisted resistivity change in vanadium dioxide. Sci. Rep. 2018, 8, 12764. [Google Scholar] [CrossRef]
- Kallepalli, L.N.D.; Constantinescu, C.; Delaporte, P.; Utéza, O.; Grojo, D. Ultra-high ordered, centimeter scale preparation of microsphere Langmuir films. J. Colloid Interface Sci. 2015, 446, 237–243. [Google Scholar] [CrossRef]
- Lotito, V.; Zambelli, T. Approaches to self-assembly of colloidal monolayers: A guide for nanotechnologists. Adv. Colloid Interface Sci. 2017, 246, 217–274. [Google Scholar] [CrossRef]
- Van Dommelen, R.; Fanzio, P.; Sasso, L. Surface self-assembly of colloidal crystals for micro- and nano-patterning. Adv. Colloid Interface Sci. 2018, 251, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Stavroulakis, P.; Boden, S.; Johnson, T.; Bagnall, D.M. Suppression of backscattered diffraction from sub-wavelength “moth-eye” arrays. Opt. Express 2013, 21, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee, R.; Goswami, A.; Pal, S.; Schofield, K.; Bukhari, S.A.M.; Thundat, T. Sharpness and intensity modulation of the metal-insulator transition in ultrathin VO2 films by interfacial structure manipulation. Phys. Rev. Mater. 2018, 2, 034605. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Hong, B.; Wang, L.; Luo, Z.; Li, X.; Kang, C.; Li, M.; Zong, H.; Gao, C. Surface-growth-mode-induced strain effects on the metal–insulator transition in epitaxial vanadium dioxide thin films. RSC Adv. 2015, 5, 80122–80128. [Google Scholar] [CrossRef]
- Fan, L.L.; Chen, S.; Luo, Z.L.; Liu, Q.H.; Wu, Y.F.; Song, L.; Ji, D.X.; Wang, P.; Chu, W.S.; Gao, C.; et al. Strain Dynamics of Ultrathin VO2 Film Grown on TiO2 (001) and the Associated Phase Transition Modulation. Nano Lett. 2014, 14, 4036–4043. [Google Scholar] [CrossRef]
- Liu, J.; He, H.; Xiao, D.; Yin, S.; Ji, W.; Jiang, S.; Luo, D.; Wang, B.; Liu, Y. Recent Advances of Plasmonic Nanoparticles and their Applications. Materials 2018, 11, 1833. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.R.; Malinsky, M.D.; Haynes, C.L.; Van Duyne, R.P. Nanosphere Lithography: Tunable Localized Surface Plasmon Resonance Spectra of Silver Nanoparticles. J. Phys. Chem. B 2000, 104, 10549–10556. [Google Scholar] [CrossRef]



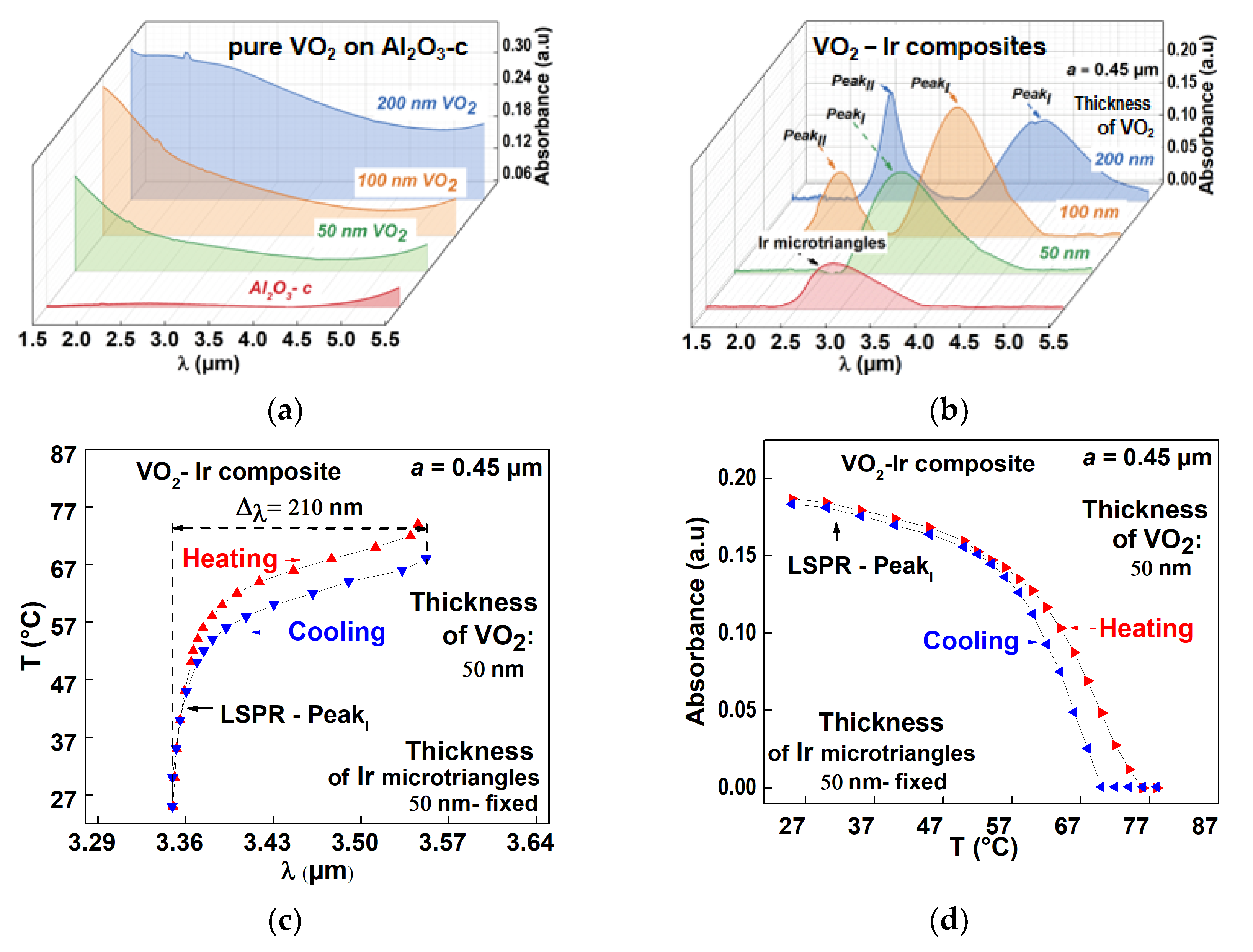
| Ir Microtriangles a”—Characteristic Size | VO2–Ir Composite Thickness of VO2 Matrix (nm) | LSPR PeakI Position/Tunability | LSPR PeakII Position/Tunability |
|---|---|---|---|
| 0.45 µm | 50 | 3.34 µm/210 nm | - |
| 100 | 3.68 µm/396 nm | 2.35 µm/150 nm | |
| 200 | 4.26 µm/196 nm | 2.60 µm/100 nm | |
| 0.70 µm | 50 | 4.71 µm/123 nm | - |
| 100 | - | 3.43 µm/120 nm | |
| 200 | - | 3.91 µm/134 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bercea, A.I.; Champeaux, C.; Constantinescu, C.D.; Dumas-Bouchiat, F. Vanadium Dioxide–Iridium Composite Development: Specific Near Infrared Surface Plasmon Resonance. J. Compos. Sci. 2021, 5, 193. https://doi.org/10.3390/jcs5070193
Bercea AI, Champeaux C, Constantinescu CD, Dumas-Bouchiat F. Vanadium Dioxide–Iridium Composite Development: Specific Near Infrared Surface Plasmon Resonance. Journal of Composites Science. 2021; 5(7):193. https://doi.org/10.3390/jcs5070193
Chicago/Turabian StyleBercea, Adrian Ionut, Corinne Champeaux, Catalin Daniel Constantinescu, and Frédéric Dumas-Bouchiat. 2021. "Vanadium Dioxide–Iridium Composite Development: Specific Near Infrared Surface Plasmon Resonance" Journal of Composites Science 5, no. 7: 193. https://doi.org/10.3390/jcs5070193
APA StyleBercea, A. I., Champeaux, C., Constantinescu, C. D., & Dumas-Bouchiat, F. (2021). Vanadium Dioxide–Iridium Composite Development: Specific Near Infrared Surface Plasmon Resonance. Journal of Composites Science, 5(7), 193. https://doi.org/10.3390/jcs5070193







