Cure Kinetics of Samarium-Doped Fe3O4/Epoxy Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Sm-Doped Fe3O4 Nanoparticles
2.2. Preparation of Epoxy/Sm-Doped Fe3O4 Nanocomposite
2.3. Characterization
3. Results and Discussion
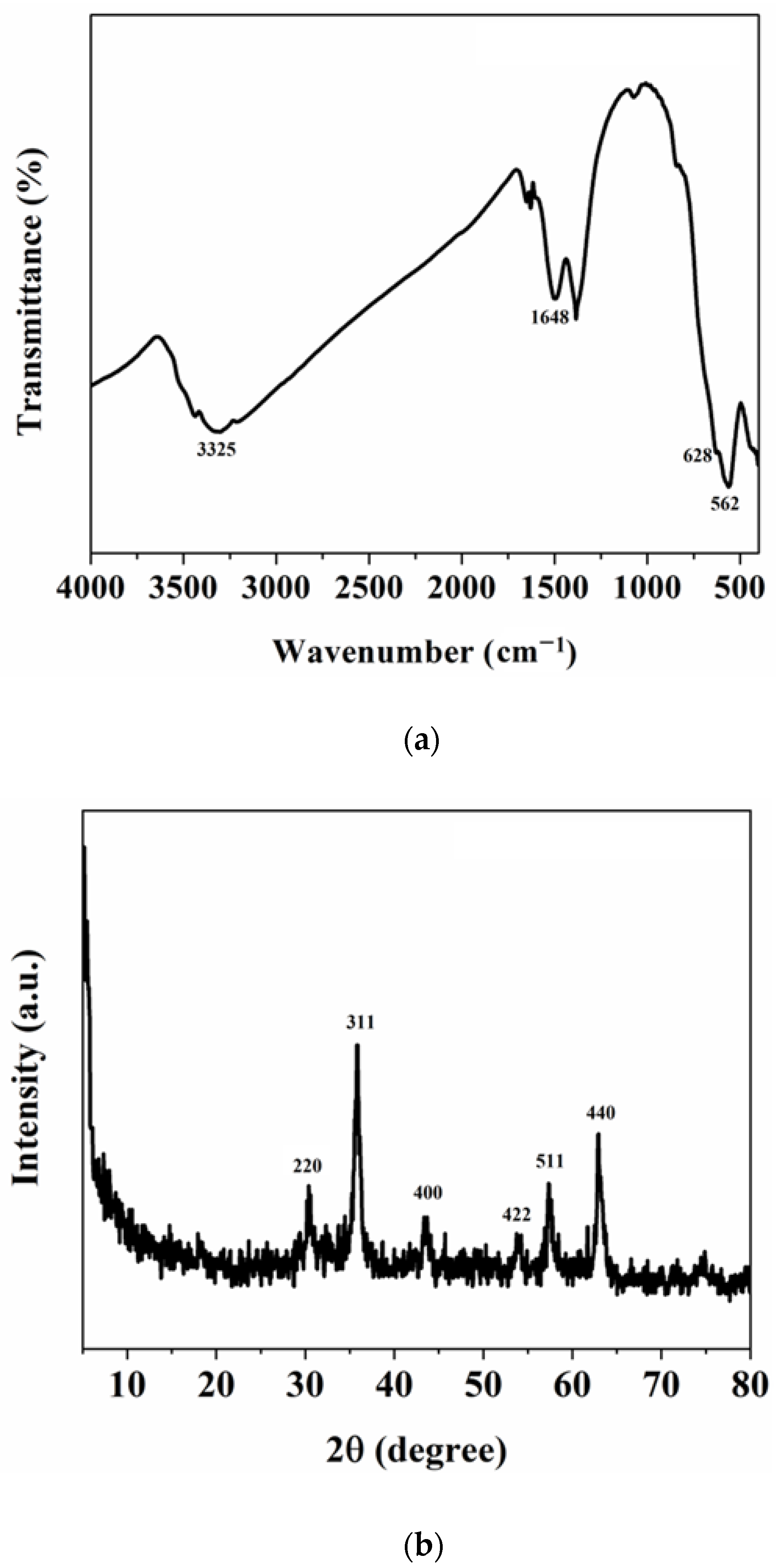
3.1. Characterization of Sm-Fe3O4 Nanoparticles
3.2. Curing Analysis
Cure State of EP/Sm-Fe3O4
3.3. Cure Kinetics of EP/Sm-Fe3O4
3.3.1. Calculation of Activation Energy
3.3.2. Determination of Reaction Model
Friedman Model
Malek Model
Validation of Isoconversional Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yavuz, C.T.; Mayo, J.; William, W.Y.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Samal, S.; Kolinova, M.; Blanco, I. The magneto-mechanical behavior of active components in iron-elastomer composite. J. Compos. Sci. 2018, 2, 54. [Google Scholar] [CrossRef] [Green Version]
- Yesmin, N.; Chalivendra, V. Electromagnetic Shielding Effectiveness of Glass Fiber/Epoxy Laminated Composites with Multi-Scale Reinforcements. J. Compos. Sci. 2021, 5, 204. [Google Scholar] [CrossRef]
- Long, Y.; Chen, Z.; Duvail, J.L.; Zhang, Z.; Wan, M. Electrical and magnetic properties of polyaniline/Fe3O4 nanostructures. Phys. B Condens. Matter 2005, 370, 121–130. [Google Scholar] [CrossRef]
- Roca, A.; Morales, M.; O’Grady, K.; Serna, C. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology 2006, 17, 2783. [Google Scholar] [CrossRef]
- Samal, S.; Škodová, M.; Blanco, I. Effects of filler distribution on magnetorheological silicon-based composites. Materials 2019, 12, 3017. [Google Scholar] [CrossRef] [Green Version]
- Bayat, M.; Yang, H.; Ko, F. Effect of iron oxide nanoparticle size on electromagnetic properties of composite nanofibers. J. Compos. Mater. 2018, 52, 1723–1736. [Google Scholar] [CrossRef]
- Yao, D.; Peng, N.; Zheng, Y. Enhanced mechanical and thermal performances of epoxy resin by oriented solvent-free graphene/carbon nanotube/Fe3O4 composite nanofluid. Compos. Sci. Technol. 2018, 167, 234–242. [Google Scholar] [CrossRef]
- Pathak, A.K.; Borah, M.; Gupta, A.; Yokozeki, T.; Dhakate, S.R. Improved mechanical properties of carbon fiber/graphene oxide-epoxy hybrid composites. Compos. Sci. Technol. 2016, 135, 28–38. [Google Scholar] [CrossRef]
- Alexopoulos, N.D.; Paragkamian, Z.; Poulin, P.; Kourkoulis, S.K. Fracture related mechanical properties of low and high graphene reinforcement of epoxy nanocomposites. Compos. Sci. Technol. 2017, 150, 194–204. [Google Scholar] [CrossRef]
- Yao, H.; Hawkins, S.A.; Sue, H.-J. Preparation of epoxy nanocomposites containing well-dispersed graphene nanosheets. Compos. Sci. Technol. 2017, 146, 161–168. [Google Scholar] [CrossRef]
- Liu, X.; Xu, F.; Zhang, K.; Wei, B.; Gao, Z.; Qiu, Y. Characterization of enhanced interfacial bonding between epoxy and plasma functionalized carbon nanotube films. Compos. Sci. Technol. 2017, 145, 114–121. [Google Scholar] [CrossRef]
- Sangermano, M.; Allia, P.; Tiberto, P.; Barrera, G.; Bondioli, F.; Florini, N.; Messori, M. Photo-Cured Epoxy Networks Functionalized With Fe3O4 Generated by Non-hydrolytic Sol–Gel Process. Macromol. Chem. Phys. 2013, 214, 508–516. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Rahmati, N.; Movahedifar, E.; Hadavand, B.S.; Karami, Z.; Ghaffari, M.; Taheri, P.; Bakhshandeh, E.; Vahabi, H.; Ganjali, M.R. Properties of nano-Fe3O4 incorporated epoxy coatings from Cure Index perspective. Prog. Org. Coat. 2019, 133, 220–228. [Google Scholar] [CrossRef]
- Lakouraj, M.M.; Rahpaima, G.; Zare, E.N. Effect of functionalized magnetite nanoparticles and diaminoxanthone on the curing, thermal degradation kinetic and corrosion property of diglycidyl ether of bisphenol A-based epoxy resin. Chin. J. Polym. Sci. 2014, 32, 1489–1499. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Do, T.V.; Ha, M.H.; Le, H.K.; Le, T.T.; Nguyen, T.N.L.; Dam, X.T.; Vu, Q.T.; Dinh, D.A.; Dang, T.C. Crosslinking process, mechanical and antibacterial properties of UV-curable acrylate/Fe3O4-Ag nanocomposite coating. Prog. Org. Coat. 2020, 139, 105325. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Khadem, S.S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/MnxFe3-xO4 nanocomposites. Prog. Org. Coat. 2020, 140, 105505. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Hamad, S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Kim, S.-J.; Zarrintaj, P.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/ZnxFe3-xO4 nanocomposites. Prog. Org. Coat. 2019, 136, 105290. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Akbari, V.; Karami, Z.; Aghazadeh, M.; Zarrintaj, P.; Saeb, M.R. Curing epoxy with polyethylene glycol (PEG) surface-functionalized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105283. [Google Scholar] [CrossRef]
- Liu, J.; Bin, Y.; Matsuo, M. Magnetic behavior of Zn-doped Fe3O4 nanoparticles estimated in terms of crystal domain size. J. Phys. Chem. C 2012, 116, 134–143. [Google Scholar] [CrossRef]
- Guo, M.; Balamurugan, J.; Li, X.; Kim, N.H.; Lee, J.H. Hierarchical 3D cobalt-doped Fe3O4 nanospheres@ NG hybrid as an advanced anode material for high-performance asymmetric supercapacitors. Small 2017, 13, 1701275. [Google Scholar] [CrossRef] [PubMed]
- Groman, E.V.; Bouchard, J.C.; Reinhardt, C.P.; Vaccaro, D.E. Ultrasmall mixed ferrite colloids as multidimensional magnetic resonance imaging, cell labeling, and cell sorting agents. Bioconjug. Chem. 2007, 18, 1763–1771. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ali, J.A.; Aghazadeh, M.; Formela, K.; Saeb, M.R.; Ranjbar, Z.; Ganjali, M.R. Curing epoxy with electrochemically synthesized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105246. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ali, J.A.; Akbari, V.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Ranjbar, Z.; Ganjali, M.R. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized MnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105247. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Karimzadeh, I.; Formela, K.; Colom, X.; Cañavate, J.; Saeb, M.R. Curing epoxy with ethylenediaminetetraacetic acid (EDTA) surface-functionalized CoxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105248. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ganjali, M.R. Samarium-doped Fe3O4 nanoparticles with improved magnetic and supercapacitive performance: A novel preparation strategy and characterization. J. Mater. Sci. 2018, 53, 295–308. [Google Scholar] [CrossRef]
- Hoz, S. Samarium Iodide Showcase: Unraveling the Mechanistic Puzzle. Acc. Chem. Res. 2020, 53, 2680–2691. [Google Scholar] [CrossRef]
- Ketteler, G.; Weiss, W.; Ranke, W.; Schlögl, R. Bulk and surface phases of iron oxides in an oxygen and water atmosphere at low pressure. Phys. Chem. Chem. Phys. 2001, 3, 1114–1122. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, M.S.; Kim, Y.I.; Lee, C.-S.; Park, J.C.; Kim, D. The enhanced anisotropic properties of the Fe3− xMxO4 (M= Fe, Co, Mn) films deposited on glass surface from aqueous solutions at low temperature. J. Phys. D Appl. Phys. 2003, 36, 1451. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kataby, G.; Ulman, A.; Cojocaru, M.; Gedanken, A. Coating a bola-amphiphile on amorphous iron nanoparticles. J. Mater. Chem. 1999, 9, 1501–1506. [Google Scholar] [CrossRef]
- Tie, S.-L.; Lee, H.-C.; Bae, Y.-S.; Kim, M.-B.; Lee, K.; Lee, C.-H. Monodisperse Fe3O4/Fe@ SiO2 core/shell nanoparticles with enhanced magnetic property. Colloids Surf. A Physicochem. Eng. Asp. 2007, 293, 278–285. [Google Scholar] [CrossRef]
- De Silva, C.R.; Smith, S.; Shim, I.; Pyun, J.; Gutu, T.; Jiao, J.; Zheng, Z. Lanthanide (III)-doped magnetite nanoparticles. J. Am. Chem. Soc. 2009, 131, 6336–6337. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Shabanian, M.; Movahedi, F.; Moghari, S.; Akbari, V.; Gabrion, X.; Laheurte, P.; Vahabi, H.; Saeb, M.R. Synthesis, characterization, and high potential of 3D metal–organic framework (MOF) nanoparticles for curing with epoxy. J. Alloys Compd. 2020, 829, 154547. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; Puglia, D.; et al. Cure Index for labeling curing potential of epoxy/LDH nanocomposites: A case study on nitrate anion intercalated Ni-Al-LDH. Prog. Org. Coat. 2019, 136, 105228. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized NixFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105198. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Zarrintaj, P.; Ganjali, M.R.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Ghaffari, M.; Saeb, M.R. Curing epoxy with electrochemically synthesized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105245. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Ganjali, M.R.; Dubois, P. Curing epoxy with polyethylene glycol (PEG) surface-functionalized NixFe3-xO4magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105250. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized MnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105199. [Google Scholar] [CrossRef]
- Akbari, V.; Jouyandeh, M.; Paran, S.M.R.; Ganjali, M.R.; Abdollahi, H.; Vahabi, H.; Ahmadi, Z.; Formela, K.; Esmaeili, A.; Mohaddespour, A.; et al. Effect of Surface Treatment of Halloysite Nanotubes (HNTs) on the Kinetics of Epoxy Resin Cure with Amines. Polymers 2020, 12, 930. [Google Scholar] [CrossRef] [Green Version]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef] [Green Version]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. ‘Cure Index’ for thermoset composites. Prog. Org. Coat. 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Thomas, S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105227. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; Puglia, D.; et al. Development of Mg-Zn-Al-CO3 ternary LDH and its curability in epoxy/amine system. Prog. Org. Coat. 2019, 136, 105264. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Hamad, S.M.; Ganjali, M.R.; Aghazadeh, M.; Torre, L.; Puglia, D.; Saeb, M.R. Curing epoxy with Mg-Al LDH nanoplatelets intercalated with carbonate ion. Prog. Org. Coat. 2019, 136, 105278. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Akbari, V.; Paran, S.M.R.; Livi, S.; Ducos, F.; Vahabi, H.; Ganjali, M.R.; Saeb, M.R. Super-crosslinked ionic liquid-intercalated montmorillonite/epoxy nanocomposites: Cure kinetics, viscoelastic behavior and thermal degradation mechanism. Polym. Eng. Sci. 2020, 60, 1940–1957. [Google Scholar] [CrossRef]
- Karami, Z.; Aghazadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Vahabi, H.; Ganjali, M.R.; Torre, L.; Puglia, D.; Saeb, M.R. Epoxy/Zn-Al-CO3 LDH nanocomposites: Curability assessment. Prog. Org. Coat. 2020, 138, 105355. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Saeb, M.R.; Ray, S.S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized NixFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105259. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Paran, S.M.R.; Esmaeili, A.; Karami, Z.; Livi, S.; Habibzadeh, S.; Vahabi, H.; Ganjali, M.R.; Saeb, M.R. Imidazole-functionalized nitrogen-rich Mg-Al-CO3 layered double hydroxide for developing highly crosslinkable epoxy with high thermal and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125826. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Hamad, S.M.; Karimzadeh, I.; Aghazadeh, M.; Karami, Z.; Akbari, V.; Shammiry, F.; Formela, K.; Saeb, M.R.; Ranjbar, Z.; et al. Unconditionally blue: Curing epoxy with polyethylene glycol (PEG) surface-functionalized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105285. [Google Scholar] [CrossRef]
- Calvino, M.M.; Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S. Non-isothermal thermogravimetry as an accelerated tool for the shelf-life prediction of paracetamol formulations. Thermochim. Acta 2021, 700, 178940. [Google Scholar] [CrossRef]
- Cavallaro, G.; Gallitto, A.A.; Lisuzzo, L.; Lazzara, G. Comparative study of historical woods from XIX century by thermogravimetry coupled with FTIR spectroscopy. Cellulose 2019, 26, 8853–8865. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vyazovkin, S. Learning about epoxy cure mechanisms from isoconversional analysis of DSC data. Thermochim. Acta 2002, 388, 289–298. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vyazovkin, S.; Mititelu, A.; Sladic, C.; Vincent, L. A study of epoxy-amine cure kinetics by combining isoconversional analysis with temperature modulated DSC and dynamic rheometry. Macromol. Chem. Phys. 2003, 204, 1815–1821. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Surface engineering of nanoparticles with macromolecules for epoxy curing: Development of super-reactive nitrogen-rich nanosilica through surface chemistry manipulation. Appl. Surf. Sci. 2018, 447, 152–164. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Bushy-surface hybrid nanoparticles for developing epoxy superadhesives. Appl. Surf. Sci. 2019, 479, 1148–1160. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Shabanian, M.; Ghiyasi, S.; Vahabi, H.; Badawi, M.; Formela, K.; Puglia, D.; Saeb, M.R. Curing behavior of epoxy/Fe3O4 nanocomposites: A comparison between the effects of bare Fe3O4, Fe3O4/SiO2/chitosan and Fe3O4/SiO2/chitosan/imide/phenylalanine-modified nanofillers. Prog. Org. Coat. 2018, 123, 10–19. [Google Scholar] [CrossRef]
- Karami, Z.; Ganjali, M.R.; Dehaghani, M.Z.; Aghazadeh, M.; Jouyandeh, M.; Esmaeili, A.; Habibzadeh, S.; Mohaddespour, A.; Inamuddin; Formela, K.; et al. Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides. Polymers 2020, 12, 1157. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Seidi, F.; Xiao, H.; Saeb, M.R. Nonisothermal Cure Kinetics of Epoxy/Polyvinylpyrrolidone Functionalized Superparamagnetic Nano-Fe3O4 Composites: Effect of Zn and Mn Doping. J. Compos. Sci. 2020, 4, 55. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Paran, S.M.R.; Mashhadzadeh, A.H.; Ganjali, M.R.; Bagheri, B.; Zarrintaj, P.; Habibzadeh, S.; Vijayan P., P.; Saeb, M.R. Effect of Nickel Doping on the Cure Kinetics of Epoxy/Fe3O4 Nanocomposites. J. Compos. Sci. 2020, 4, 102. [Google Scholar] [CrossRef]
- Moghari, S.; Jafari, S.H.; Yazdi, M.K.; Jouyandeh, M.; Hejna, A.; Zarrintaj, P.; Saeb, M.R. In-Out Surface Modification of Halloysite Nanotubes (HNTs) for Excellent Cure of Epoxy: Chemistry and Kinetics Modeling. Nanomaterials 2021, 11, 3078. [Google Scholar] [CrossRef]











| Sample | Heating Rate (°C/min) | TOnset (°C) | Tp (°C) | TEndset (°C) | ΔT (°C) | ΔH∞ (J/g) | ΔT* | ΔH* | CI |
|---|---|---|---|---|---|---|---|---|---|
| EP | 2.5 | 25.7 | 90.4 | 159.9 | 134.2 | 329.6 | N.a. | N.a. | N.a. |
| 5.0 | 37.4 | 101.7 | 171.7 | 134.4 | 336.2 | N.a. | N.a. | N.a. | |
| 7.5 | 40.7 | 110.3 | 180.7 | 140.0 | 344.1 | N.a. | N.a. | N.a. | |
| 10 | 41.4 | 119.2 | 225.3 | 183.9 | 404.3 | N.a. | N.a. | N.a. | |
| EP/Sm-Fe3O4 | 2.5 | 30.8 | 89.4 | 170.6 | 139.8 | 385.8 | 1.04 | 1.17 | 1.22 |
| 5.0 | 35.7 | 101.5 | 175.2 | 139.5 | 363.7 | 1.04 | 1.08 | 1.12 | |
| 7.5 | 39.1 | 107.7 | 207.0 | 167.9 | 296.3 | 1.2 | 0.86 | 1.03 | |
| 10 | 40.1 | 115.1 | 226.0 | 185.9 | 403.4 | 1.01 | 1.01 | 1.02 |
| Sample | Heating Rate (°C/min) | αp | αm | αp∞ |
|---|---|---|---|---|
| EP | 2.5 | 0.447 | 0.075 | 0.497 |
| 5 | 0.44 | 0.081 | 0.545 | |
| 7.5 | 0.482 | 0.087 | 0.553 | |
| 10 | 0.319 | 0.092 | 0.484 | |
| EP/Sm-Fe3O4 | 2.5 | 0.456 | 0.051 | 0.487 |
| 5 | 0.582 | 0.049 | 0.551 | |
| 7.5 | 0.429 | 0.05 | 0.51 | |
| 10 | 0.418 | 0.041 | 0.505 |
| Designation | Heating Rate (°C/min) | Friedman | KAS | ||||
|---|---|---|---|---|---|---|---|
| m | n | lnA (s−1) | m | n | lnA (s−1) | ||
| EP | 2.5 | 0.14 | 1.32 | 12.09 | 0.09 | 1.36 | 13.3 |
| 5.0 | 0.29 | 1.38 | 12.71 | 0.24 | 1.42 | 13.9 | |
| 7.5 | 0.29 | 1.36 | 12.76 | 0.24 | 1.4 | 13.92 | |
| 10 | 0.25 | 1.69 | 12.57 | 0.2 | 1.74 | 13.71 | |
| EP/Sm-Fe3O4 | 2.5 | 0.16 | 1.58 | 14.5 | 0.13 | 1.62 | 15.39 |
| 5.0 | 0.23 | 1.47 | 14.8 | 0.20 | 1.5 | 15.66 | |
| 7.5 | 0.34 | 1.72 | 15.05 | 0.30 | 1.75 | 15.9 | |
| 10 | 0.31 | 1.85 | 14.9 | 0.28 | 1.89 | 15.73 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouyandeh, M.; Ganjali, M.R.; Mehrpooya, M.; Abida, O.; Jabbour, K.; Rabiee, N.; Habibzadeh, S.; Mashahdzadeh, A.H.; García-Peñas, A.; Stadler, F.J.; et al. Cure Kinetics of Samarium-Doped Fe3O4/Epoxy Nanocomposites. J. Compos. Sci. 2022, 6, 29. https://doi.org/10.3390/jcs6010029
Jouyandeh M, Ganjali MR, Mehrpooya M, Abida O, Jabbour K, Rabiee N, Habibzadeh S, Mashahdzadeh AH, García-Peñas A, Stadler FJ, et al. Cure Kinetics of Samarium-Doped Fe3O4/Epoxy Nanocomposites. Journal of Composites Science. 2022; 6(1):29. https://doi.org/10.3390/jcs6010029
Chicago/Turabian StyleJouyandeh, Maryam, Mohammad Reza Ganjali, Mehdi Mehrpooya, Otman Abida, Karam Jabbour, Navid Rabiee, Sajjad Habibzadeh, Amin Hamed Mashahdzadeh, Alberto García-Peñas, Florian J. Stadler, and et al. 2022. "Cure Kinetics of Samarium-Doped Fe3O4/Epoxy Nanocomposites" Journal of Composites Science 6, no. 1: 29. https://doi.org/10.3390/jcs6010029
APA StyleJouyandeh, M., Ganjali, M. R., Mehrpooya, M., Abida, O., Jabbour, K., Rabiee, N., Habibzadeh, S., Mashahdzadeh, A. H., García-Peñas, A., Stadler, F. J., & Saeb, M. R. (2022). Cure Kinetics of Samarium-Doped Fe3O4/Epoxy Nanocomposites. Journal of Composites Science, 6(1), 29. https://doi.org/10.3390/jcs6010029













