Optimal Modified Starch Content in UF Resin for Glulam Based on Bonding Strength Using Artificial Neural Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Production of Layer
2.1.2. Preparation of Starch Adhesive and UF Resin
2.1.3. Nano-Zinc Oxide
2.2. Methods
2.2.1. Preparation of the Starch Adhesive
First Stage: Chemical Modification of Starch
Second Stage: Preparation of the Starch Adhesive
2.2.2. Fabrication of Glulam
2.3. Experimental Design
2.4. Characterization of Modified Starch and UF Resin
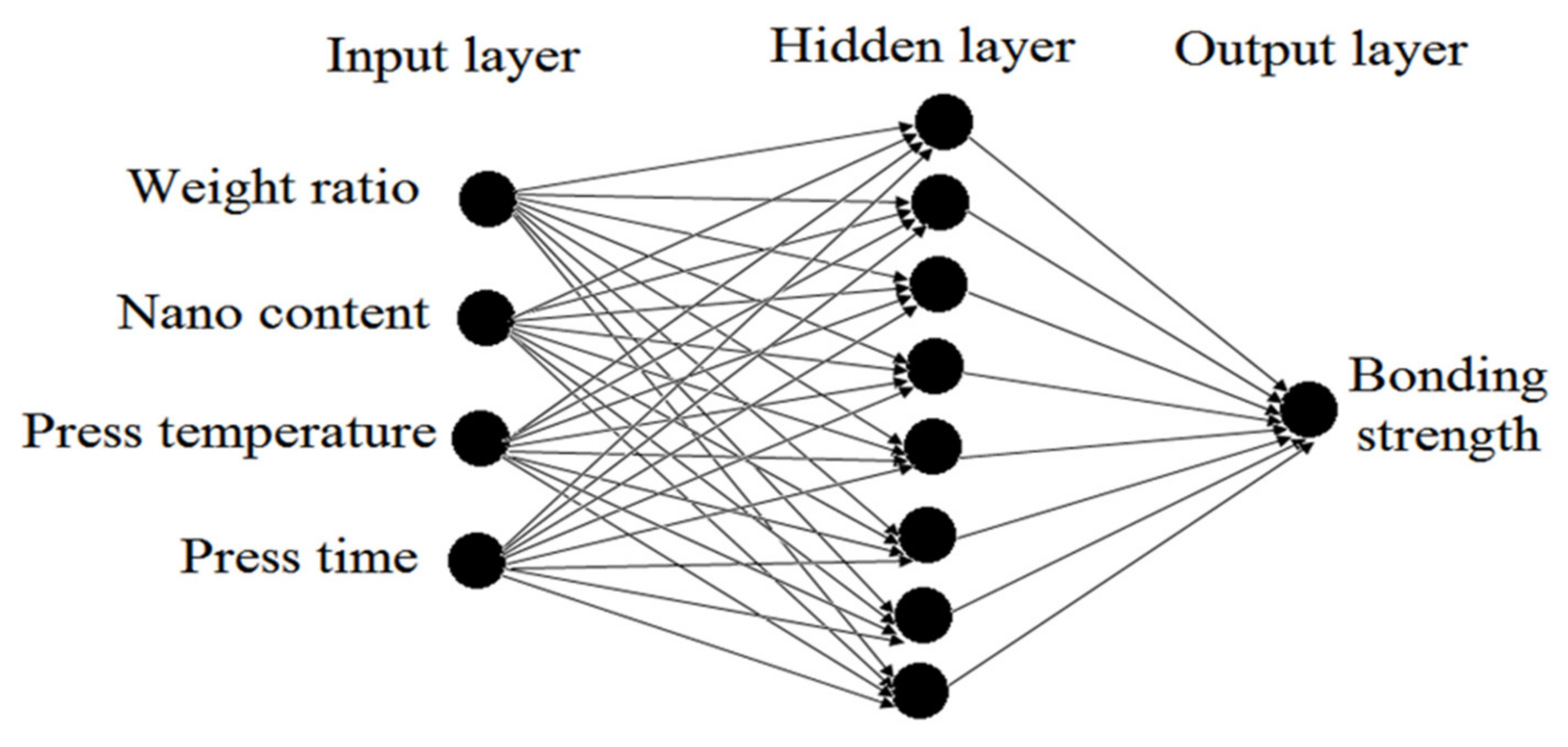
2.5. Prediction and Optimization of the Bonding Strength Using ANN
3. Results and Discussion
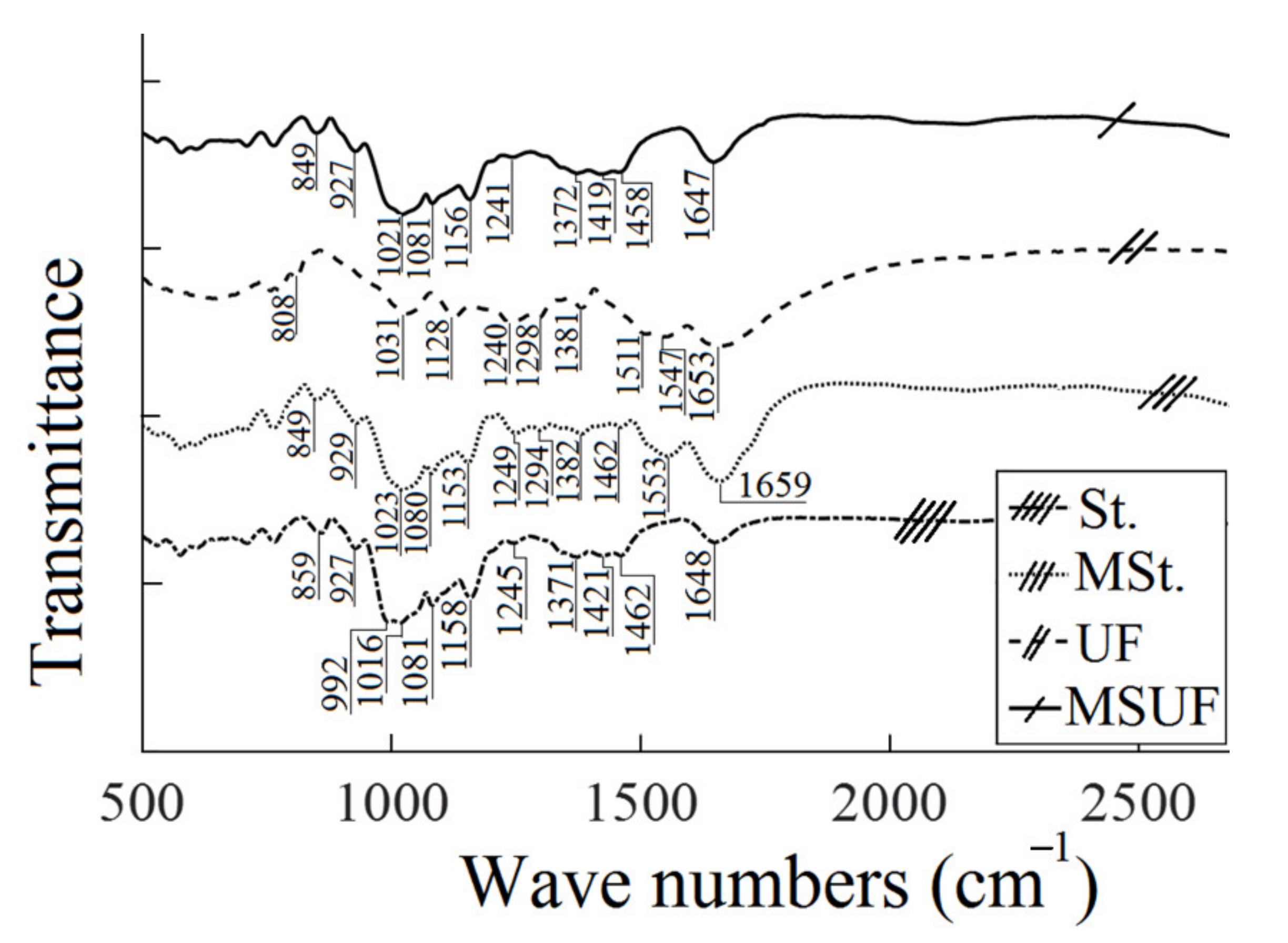
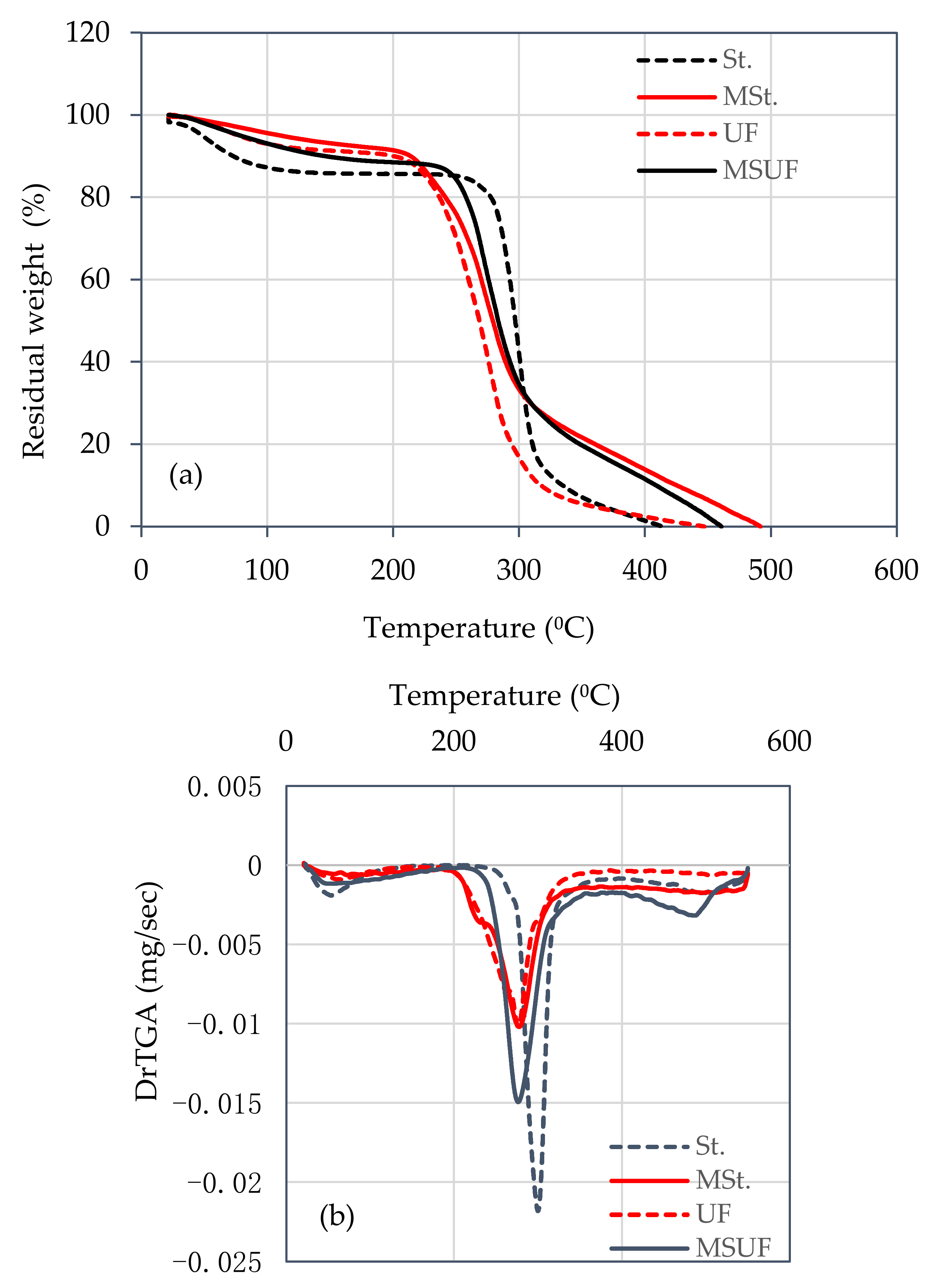
3.1. Characterization: FTIR and TGA Analysis
3.2. Statistical Analysis
3.3. ANN Results
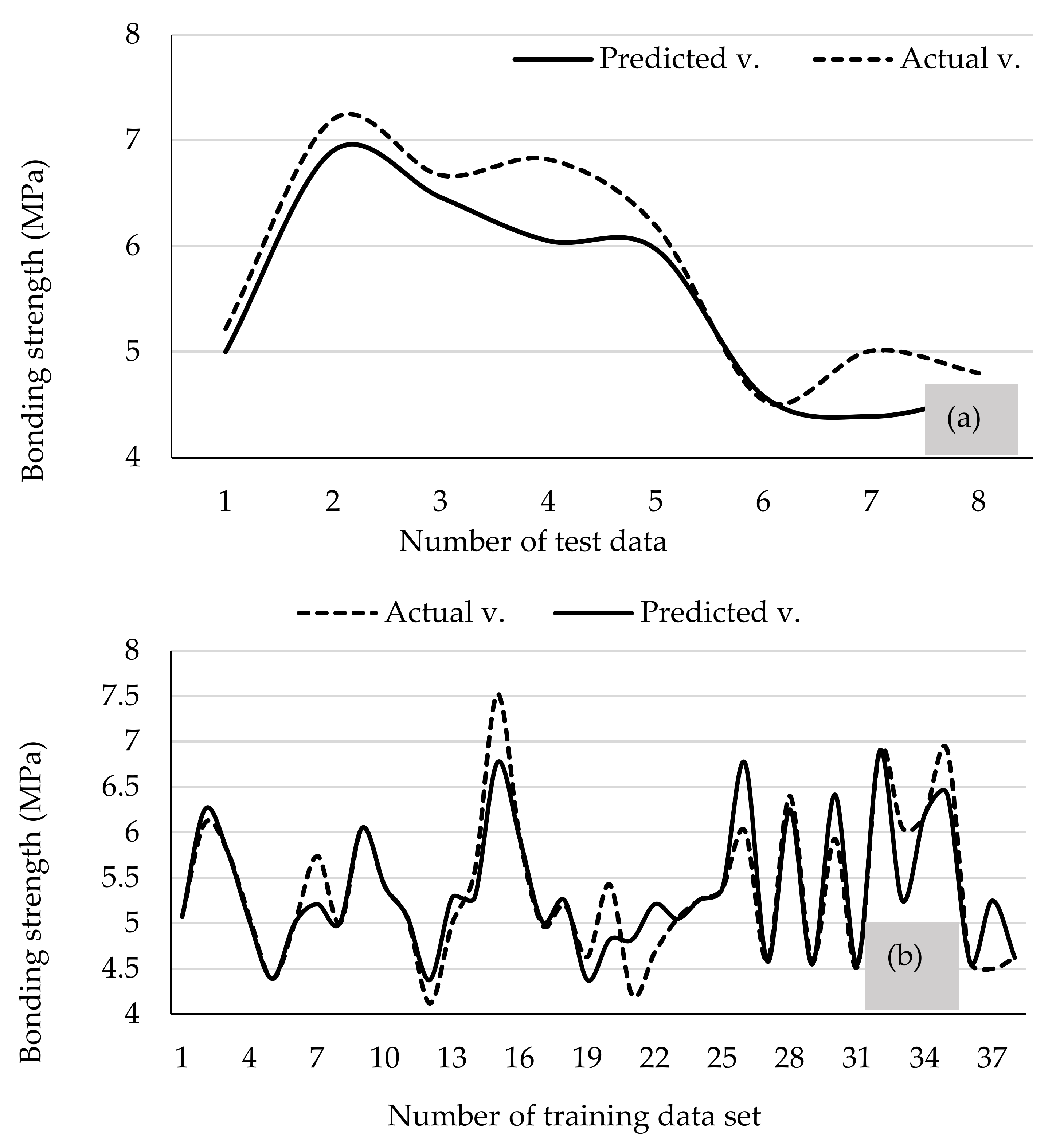
3.4. Prediction of Bonding Strength Using ANN
3.5. Discussion
3.6. Optimization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.X.; Shen, J.; Lei, C.S.; Feng, Q. Volatile organic compound and formaldehyde emissions from Populus davidiana wood treated with low molecular weight urea-formaldehyde resin. J. Environ. Biol. 2014, 35, 989–994. [Google Scholar] [PubMed]
- Zhu, X.; Xu, E.; Lin, R.; Wang, X. Decreasing the formaldehyde emission in urea-formaldehyde using modified starch by strongly acid process. J. Appl. Polym. Sci. 2014, 131, 2–7. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Starch-based adhesives for wood/wood composite bonding: Review. Open J. Polym. Chem. 2017, 7, 19–32. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; Garcia, M.A. Sustainable panels design based on modified cassava starch bioadhesives and wood processing byproducts. Ind. Crops Prod. 2019, 137, 171–179. [Google Scholar] [CrossRef]
- Amini, M.H.M.; Hashim, R.; Sulaiman, N.S.; Mohamed, M.; Sulaiman, O. Citric acid-modified starch as an environmentally friendly binder for wood composite making. Bioresources 2020, 15, 4234–4238. [Google Scholar] [CrossRef]
- Hogger, E.M.; van Herwijnen, H.W.G.; Moser, J.; Kantner, W.; Konnerth, J. Systematic assessment of wheat extenders in condensation resins for plywood production: Part I—Physico-chemical adhesive properties. J. Adhes. 2021, 97, 1404–1422. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Evaluation of starch as an environmental-friendly bioresource for the development of wood bioadhesives. Molecules 2021, 26, 4526. [Google Scholar] [CrossRef]
- Islam, M.N.; Faruk, M.O.; Rana, M.N.; Das, A.K.; Habib, A. Preparation and evaluation of rice bran-modified urea formaldehyde as environmental friendly wood adhesive. Glob. Chall. 2021, 27, 2000044. [Google Scholar] [CrossRef]
- Amini, M.H.M.; Hashim, R.; Sulaiman, N.S.; Sulaiman, O.; Selamat, M.E. Glutardialdehyde modified starch from waste oil palm trunks as a binder for wood composite making. Int. J. Adhes. Adhes. 2021, 104, 102757. [Google Scholar] [CrossRef]
- Zakaria, R.; Bawon, P.; Lee, S.H.; Salim, S.; Lum, W.C.; Al-Edrus, S.S.O.; Ibrahim, Z. Properties of particleboard from oil palm biomasses bonded with citric acid and tapioca starch. Polymers 2021, 13, 3494. [Google Scholar] [CrossRef]
- Luo, F.-X.; Fu, X.; Huang, Q.; Li, L. Properties and cross linking mechanism of starches oxidized by sodium hypochlorite at low level. Nat. Sci. 2006, 34, 79–83. [Google Scholar]
- Sukhija, S.; Singh, S.; Riar, C.S. Effect of oxidation, cross-linking and dual modification on physicochemical, crystallinity, morphological, pasting and thermal characteristics of elephant foot yam (Amorphophallus paeoniifolius) starch. Food Hydrocoll. 2015, 55, 56–64. [Google Scholar] [CrossRef]
- Patel, K.F.; Mehta, H.U.; Srivastava, H.C. Kinetics and mechanism of oxidation of starch with sodium hypochlorite. J. Appl. Polym. Sci. 1974, 18, 389–399. [Google Scholar] [CrossRef]
- Forssell, P.; Hamunen, A.; Autio, M.K.; Suortti, P.; Poutanen, K. Hypochlorite oxidation of barley and potato starch. Starch 1995, 47, 371–377. [Google Scholar] [CrossRef]
- Karazi, S.M.; Issa, A.; Brabazon, D. Comparison of ANN and DoE for the prediction of laser-machined micro-channel dimensions. Opt. Lasers Eng. 2009, 47, 956–964. [Google Scholar] [CrossRef]
- Kazi, M.K.; Eljack, F.; Mahdi, E. Optimal filler content for cotton fiber/PP composite based on mechanical properties using artificial neural network. Compos. Struct. 2020, 251, 112654. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.A.; Kushvaha, V. Effect of aspect ratio on dynamic fracture toughness of particulate polymer composite using artificial neural network. Eng. Fract. Mech. 2020, 228, 106907. [Google Scholar] [CrossRef]
- Demir, A.; Aydin, I. Modeling the thermal conductivity of veneer sheets the different moisture content using artificial neural network. Proligno 2021, 17, 3–12. [Google Scholar]
- De Palacios, P.; Fernández, F.G.; García-Iruela, A.; González-Rodrigo, B.; Esteban, L.G. Study of the influence of the physical properties of particleboard type P2 on the internal bond of panels using artificial neural networks. Comput. Electron. Agric. 2018, 155, 142–149. [Google Scholar] [CrossRef]
- Aysenur, G.; Derya, U.; Sibel, Y. Application of artificial neural network to predict the effect of paraffin addition on water absorption and thickness swelling of MDF. Drv. Ind. 2019, 70, 247–255. [Google Scholar] [CrossRef]
- Mei, H.; Lang, L.; Li, X.; Mirza, H.A.; Yang, X. Prediction of tensile strength and deformation of diffusion bonding joint for inconel 718 using deep neural network. Metals 2020, 10, 1266. [Google Scholar] [CrossRef]
- Silva, F.A.N.; Delgado, J.M.P.Q.; Cavalcanti, R.S.; Azevedo, A.C.; Guimarães, A.S.; Lima, A.G.B. Use of nondestructive testing of ultrasound and artificial neural networks to estimate compressive strength of concrete. Buildings 2021, 11, 44. [Google Scholar] [CrossRef]
- Kuakpetoon, D.; Wang, Y.J. Characterization of different starches oxidized by hypochlorite. Starch 2001, 53, 211–218. [Google Scholar] [CrossRef]
- Aini, N.; Purwiyatno, H. Gelatinization properties of white maize starch from three varieties of corn subject to oxidized and acetylated-oxidized modification. Int. Food Res. J. 2010, 17, 961–968. [Google Scholar]
- Nazerian, M.; Razavi, S.A.; Partoviniya, A.; Vatankhah, E.; Razmpour, Z. Comparison of different modeling methods toward predictive capability evaluation of the bonding strength of wood laminated products. Proc. Inst. Mech. Eng. Part E J. Process. Mech. Eng. 2021, 236, 991–1003. [Google Scholar] [CrossRef]
- EN 302-1:2013; Adhesives for Load-Bearing Timber Structures—Test Methods—Part 1: Determination of Longitudinal Tensile Shear Strength. European Committee for Standardization: Brussels, Belgium, 2013.
- Zorba, T.; Papadopoulou, E.; Hatjiissaak, A.; Paraskevopoulos, K.M.; Chrissafis, K. Urea-formaldehyde resins characterized by thermal analysis and FTIR method. J. Therm. Anal. Calorim. 2008, 92, 29–33. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, Q.H.; Xu, X.C.; Jiang, W.Y.; Gan, S.C.; Zuo, H.F. Oxidation of cornstarch using oxygen as oxidant without catalyst. LWT-Food Sci. Technol. 2011, 44, 139–144. [Google Scholar] [CrossRef]
- Lin, Q.; Pan, J.; Lin, Q.; Liu, Q. Microwave synthesis and adsorption performance of a novel crosslinked starch microsphere. J. Hazard. Mater. 2013, 263, 517–524. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, L.; Wang, H.; Wang, Y.; Zhang, H. Environment-friendly urea-oxidized starch adhesive with zero formaldehyde-emission. Carbohydr. Polym. 2018, 181, 1112–1118. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Chi, Y.; Yan, J. TG-FTIR study on urea-formaldehyde resin residue during pyrolysis and combustion. J. Hazard. Mater. 2010, 173, 205–210. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starch. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Roumeli, E.; Papadopoulou, E.; Pavlidou, E.; Bikiaris, D.; Paraskevopoulos, K.M.; Chrissafis, K. Synthesis, characterization and thermal analysis of urea–formaldehyde/nanoSiO2 resins. Thermochim. Acta 2012, 527, 33–39. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.D. Influence of initial molar ratios on the performance of low molar ratio urea-formaldehyde resin adhesives. J. Korean Wood Sci. Technol. 2020, 48, 136–153. [Google Scholar] [CrossRef]
- Sun, Q.N.; Hse, C.Y.; Shupe, T.F. Characterization and performance of melamine enhanced urea formaldehyde resin for bonding southern pine particleboard. J. Appl. Polym. Sci. 2011, 119, 3538–3543. [Google Scholar] [CrossRef]
- Tang, M.; Wen, S.; Liu, D. Effects of heating- or caustic-digested starch on its flocculation on hematite. Miner. Process. Extr. Metall. Rev. 2016, 37, 49–57. [Google Scholar] [CrossRef]
- Golova, O.P.; Nosova, N.I. Degradation of cellulose by alkaline oxidation. Russ. Chem. Rev. 1973, 42, 327–338. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Hong, Y.; Cheng, L.; Li, Z. Bonding strength and water resistance of starch-based wood adhesive improved by silica nanoparticles. Carbohydr. Polym. 2011, 86, 72–76. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Lawal, O.S. Functional properties and retrogradation behaviour of native and chemically modified starch of mucuna bean (Mucuna pruriens). J. Sci. Food Agric. 2003, 83, 1541–1546. [Google Scholar] [CrossRef]
- Garza-Ulloa, J. Application of Mathematical Models in Biomechatronics: Artificial Intelligence and Time-Frequency Analysis; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 373–524. [Google Scholar]
- Canakci, A.; Ozsahin, S.; Varol, T. Modeling the influence of a process control agent on the properties of metal matrix composite powders using artificial neural networks. Powder Technol. 2012, 228, 26–35. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yang, J.; Yu, J. Preparation and properties of glycerol plasticized-pea starch/zinc oxide-starch bionanocomposites. Carbohydr. Polym. 2009, 75, 472–478. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Liu, B.; Ma, X. Preparation and characterization of glycerol plasticized-pea starch/ZnO carboxy methyl cellulose sodium nanocomposites. Bioresour. Technol. 2009, 100, 2832–2841. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Putri, O.D.; Fikriyyah, A.K.; Nissa, R.C.; Hidayat, S.; Septiyanto, R.F.; Karina, M.; Satoto, R. Harnessing the excellent mechanical, barrier and antimicrobial properties of zinc oxide (ZnO) to improve the performance of starch-based bioplastic. Polym.-Plast. Technol. Mater. 2020, 59, 1259–1267. [Google Scholar] [CrossRef]
- Basta, A.H.; El-Saied, H.; Winandy, J.E.; Sabo, R. Preformed amide-containing biopolymer for improving the environmental performance of synthesized urea–formaldehyde in agro-fiber composites. J. Polym. Environ. 2011, 19, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Li, Y.; Xu, B.; Ren, G.; Li, P.; Han, S.; Liu, J. Effects of inulin with different degree of polymerization on gelatinization and retrogradation of wheat starch. Food. Chem. 2017, 229, 35–43. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, Y.; Wu, Y.; Xiao, J.; Zhu, Y.; Zhao, X. Effect of acid hydrolysis and oxidation on the properties of dialdehyde starch. Mater. Rev. 2017, 31, 41–45. [Google Scholar]
- Frihart, C.R.; Pizzi, A.; Xi, X.; Lorenz, L.F. Reactions of soy flour and soy protein by non-volatile aldehydes generation by specific oxidation. Polymers 2019, 11, 1478. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Gonçalves, J.R.; El-Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Jacques, A.C.; Zavareze, E.R. Oxidation of potato starch with different sodium hypochlorite concentrations and its effect on biodegradable films. LWT-Food. Sci. Technol. 2015, 60, 714–720. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, J.; Gao, Q.; Mao, A.; Li, J. Toughening and enhancing melamine–urea–formaldehyde resin properties via in situ polymerization of dialdehyde starch and microphase separation. Polymers 2019, 11, 1167. [Google Scholar] [CrossRef]
- Zhang, D.S.; Zhang, Y.R.; Wang, X.L.; Wang, Y.Z. High carbonyl content oxidized starch prepared by hydrogen peroxide and its thermoplastic application. Starch 2009, 61, 646–655. [Google Scholar] [CrossRef]
- Dias, A.R.G.; da Rosa-Zavareze, E.; Helbig, E.; de Moura, F.A.; Vargas, C.G.; Ciacco, C.F. Oxidation of fermented cassava starch using hydrogen peroxide. Carbohydr. Polym. 2011, 86, 185–191. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Simasatitkul, P.; Chaiwan, W.; Watthanaworasakun, Y. Effect of sodium hydroxide concentration on properties of carboxymethyl rice starch. Int. Food Res. J. 2012, 19, 923–931. [Google Scholar]
- Sulaiman, N.S.; Hashim, R.; Sulaiman, O.; Nasir, M.; Amini, M.H.M.; Hiziroglu, S. Partial replacement of urea-formaldehyde with modified oil palm starch based adhesive to fabricate particleboard. Int. J. Adhes. Adhes. 2018, 84, 1–8. [Google Scholar] [CrossRef]
- Pizzi, A. Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. II. Tannin adhesive preparation, characteristics, and application. J. Appl. Polym. Sci. 1979, 24, 1257–1268. [Google Scholar] [CrossRef]
- Fan, D.B.; Li, J.Z.H.; Chang, J.M.; Gou, J.S.H.; Jiang, J.X. Chemical structure and curing behavior of phenol-urea-formaldehyde condensed resins of high urea content. J. Adhes. Sci. Technol. 2009, 23, 1787–1797. [Google Scholar] [CrossRef]
- Fan, D.B.; Chang, J.M.; Li, J.Z.; Xia, B.H.; Sang, Z.T. Cure properties and adhesive performances of cure-accelerated phenol-urea-formaldehyde resins. Eur. J. Wood Prod. 2011, 69, 213–220. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H. Improving physical and mechanical properties of new lignin-urea-glyoxal resin by nanoclay. Eur. J. Wood Wood Prod. 2017, 75, 885–891. [Google Scholar] [CrossRef]
- Bardak, T.; Sozen, E.; Kayahan, K.; Bardak, S. The impact of nanoparticles and moisture content on bonding strength of urea formaldehyde resin adhesive. Drv. Ind. 2018, 69, 247–252. [Google Scholar] [CrossRef]
- Veigel, S.; Muller, U.; Keckes, J.; Obersriebnig, M.; Gindl-Altmutter, W. Cellulose nanofibrils as filler for adhesives: Effect on specific fracture energy of solid wood-adhesive bonds. Cellulose 2011, 18, 1227–1237. [Google Scholar] [CrossRef]
- Moubarik, A.; Charrier, B.; Allal, A.; Charrier, F.; Pizzi, A. Development and optimization of a new formaldehyde-free cornstarch and tannin wood adhesive. Eur. J. Wood Wood Prod. 2009, 68, 167–177. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Thanomsilp, C.; Suwantong, O.A. A review: Starch-based composite foams. Compos. Part A Appl. Sci. Manuf. 2015, 78, 246–263. [Google Scholar] [CrossRef]
- Yongfeng, A.; Jane, J. Gelatinization and rheological properties of starch. Starch 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Stoeckel, F.; Konnerth, J.; Gindl-Altmutter, W. Mechanical properties of adhesives for bonding wood—A review. Int. J. Adhes. Adhes. 2013, 45, 32–41. [Google Scholar] [CrossRef]





| Variables | Unit | Coded Values of Variables | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Modified starch (x1) | % | 10 | 30 | 50 | 70 | 90 |
| Nano content (x2) | % | 0 | 1 | 2 | 3 | 4 |
| Press temperature (x3) | °C | 120 | 140 | 160 | 180 | 200 |
| Press time (x4) | min | 14 | 16 | 18 | 20 | 22 |
| Run | Coded Values | Actual Values | ||||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | Modified Starch (St., %) | Nano Content (NC, %) | Press Temperature (PTem, °C) | Press Time (PTim, min) | |
| 1 | −1 | 1 | 1 | 1 | 30 | 3 | 180 | 20 |
| 2 | 0 | 0 | 0 | 2 | 50 | 2 | 160 | 22 |
| 3 | 1 | 1 | 1 | 1 | 70 | 3 | 180 | 20 |
| 4 | 2 | 0 | 0 | 0 | 90 | 2 | 160 | 18 |
| 5 | −1 | −1 | −1 | 1 | 30 | 1 | 140 | 20 |
| 6 | 1 | 1 | −1 | −1 | 70 | 3 | 140 | 16 |
| 7 | 0 | 0 | 0 | 0 | 50 | 2 | 160 | 18 |
| 8 | −1 | 1 | −1 | −1 | 30 | 3 | 140 | 16 |
| 9 | −1 | −1 | 1 | 1 | 30 | 1 | 180 | 20 |
| 10 | −2 | 0 | 0 | 0 | 10 | 2 | 160 | 18 |
| 11 | 1 | −1 | −1 | 1 | 70 | 1 | 140 | 20 |
| 12 | −1 | 1 | −1 | 1 | 30 | 3 | 140 | 20 |
| 13 | 1 | −1 | −1 | −1 | 70 | 1 | 140 | 16 |
| 14 | 1 | 1 | 1 | −1 | 70 | 3 | 180 | 16 |
| 15 | −1 | −1 | 1 | −1 | 30 | 1 | 180 | 16 |
| 16 | 1 | 1 | −1 | 1 | 70 | 3 | 140 | 20 |
| 17 | 1 | −1 | 1 | 1 | 70 | 1 | 180 | 20 |
| 18 | 0 | 2 | 0 | 0 | 50 | 4 | 160 | 18 |
| 19 | 1 | −1 | 1 | −1 | 70 | 1 | 180 | 16 |
| 20 | 0 | 0 | −2 | 0 | 50 | 2 | 120 | 18 |
| 21 | 0 | −2 | 0 | 0 | 50 | 0 | 160 | 18 |
| 22 | 0 | 0 | 2 | 0 | 50 | 2 | 200 | 18 |
| 23 | 0 | 0 | 0 | −2 | 50 | 2 | 160 | 14 |
| 24 | −1 | 1 | 1 | −1 | 30 | 3 | 180 | 16 |
| 25 | −1 | −1 | −1 | −1 | 30 | 1 | 140 | 16 |
| Run | Experimental Value (MPa) | Predicted Value (MPa) |
|---|---|---|
| 1 | 5.08 (0.7) | 5.07 |
| 2 | 6.10 (0.1) | 6.25 |
| 3 | 5.22 (0.8) | 5.00 |
| 4 | 5.80 (0.1) | 5.80 |
| 5 | 6.62 (0.1) | 6.20 |
| 6 | 5.06 (0.4) | 5.02 |
| 7 | 5.55 (0.3) | 5.25 |
| 8 | 5.50 (0.7) | 5.25 |
| 9 | 4.39 (0.8) | 4.39 |
| 10 | 5.00 (1.0) | 5.00 |
| 11 | 5.74 (0.6) | 5.21 |
| 12 | 5.00 (0.4) | 5.00 |
| 13 | 7.20 (1.1) | 6.90 |
| 14 | 5.53 (0.1) | 5.41 |
| 15 | 6.05 (0.1) | 6.10 |
| 16 | 5.06 (0.7) | 5.10 |
| 17 | 6.67 (0.3) | 6.50 |
| 18 | 4.12 (0.2) | 4.37 |
| 19 | 4.50 (0.6) | 5.25 |
| 20 | 5.00 (0.4) | 5.28 |
| 21 | 6.82 (1.0) | 6.10 |
| 22 | 5.57 (0.4) | 5.28 |
| 23 | 6.19 (0.9) | 5.97 |
| 24 | 4.54 (0.6) | 4.58 |
| 25 | 5.94 (0.4) | 6.00 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 25.7 | 8 | 3.21 | 9.32 | <0.0001 |
| x1 | 4.22 | 1 | 4.22 | 12.2 | 0.00107 |
| x1x2 | 2.69 | 1 | 2.69 | 7.81 | 0.00761 |
| x1x4 | 2.57 | 1 | 2.57 | 7.45 | 0.009 |
| x2x3 | 3.46 | 1 | 3.46 | 10 | 0.00276 |
| x2x4 | 1.25 | 1 | 1.25 | 3.64 | 0.0429 |
| x3x4 | 6.42 | 1 | 6.42 | 18.6 | <0.0001 |
| x12 | 2.69 | 1 | 2.69 | 7.79 | 0.00768 |
| x42 | 2.96 | 1 | 2.96 | 8.58 | 0.00532 |
| Residual | 15.5 | 45 | 0.345 | ||
| Lack of Fit | 8.7 | 19 | 0.458 | 1.75 | 0.0924 |
| Pure Error | 6.82 | 26 | 0.262 | ||
| R2 | 0.624 | Adj. R2 | 0.557 | ||
| Pred. R2 | 0.481 | Adeq. precision | 10.5 | ||
| Performance Criteria | Data Set (Bonding Strength, MPa) | |||
|---|---|---|---|---|
| Training | Validation | Testing | All | |
| R2 | 0.8128 | 0.9023 | 0.9388 | 0.7921 |
| MAPE | 3.8263 | 10.2074 | 5.5234 | 5.0231 |
| RMSE | 0.3422 | 0.6023 | 0.3965 | 0.3993 |
| MAE | 0.2076 | 0.4300 | 0.3248 | 0.2579 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazerian, M.; Akbarzade, M.; Ghorbanezdad, P.; Papadopoulos, A.N.; Vatankhah, E.; Foti, D.; Koosha, M. Optimal Modified Starch Content in UF Resin for Glulam Based on Bonding Strength Using Artificial Neural Network. J. Compos. Sci. 2022, 6, 279. https://doi.org/10.3390/jcs6100279
Nazerian M, Akbarzade M, Ghorbanezdad P, Papadopoulos AN, Vatankhah E, Foti D, Koosha M. Optimal Modified Starch Content in UF Resin for Glulam Based on Bonding Strength Using Artificial Neural Network. Journal of Composites Science. 2022; 6(10):279. https://doi.org/10.3390/jcs6100279
Chicago/Turabian StyleNazerian, Morteza, Masood Akbarzade, Payam Ghorbanezdad, Antonios N. Papadopoulos, Elham Vatankhah, Dafni Foti, and Mojtaba Koosha. 2022. "Optimal Modified Starch Content in UF Resin for Glulam Based on Bonding Strength Using Artificial Neural Network" Journal of Composites Science 6, no. 10: 279. https://doi.org/10.3390/jcs6100279
APA StyleNazerian, M., Akbarzade, M., Ghorbanezdad, P., Papadopoulos, A. N., Vatankhah, E., Foti, D., & Koosha, M. (2022). Optimal Modified Starch Content in UF Resin for Glulam Based on Bonding Strength Using Artificial Neural Network. Journal of Composites Science, 6(10), 279. https://doi.org/10.3390/jcs6100279







