New Structural Nanocomposite Based on PLGA and Al2O3 NPs as a Balance between Antibacterial Activity and Biocompatibility with Eukaryotic Cells
Abstract
:1. Introduction
2. Materials and Methods
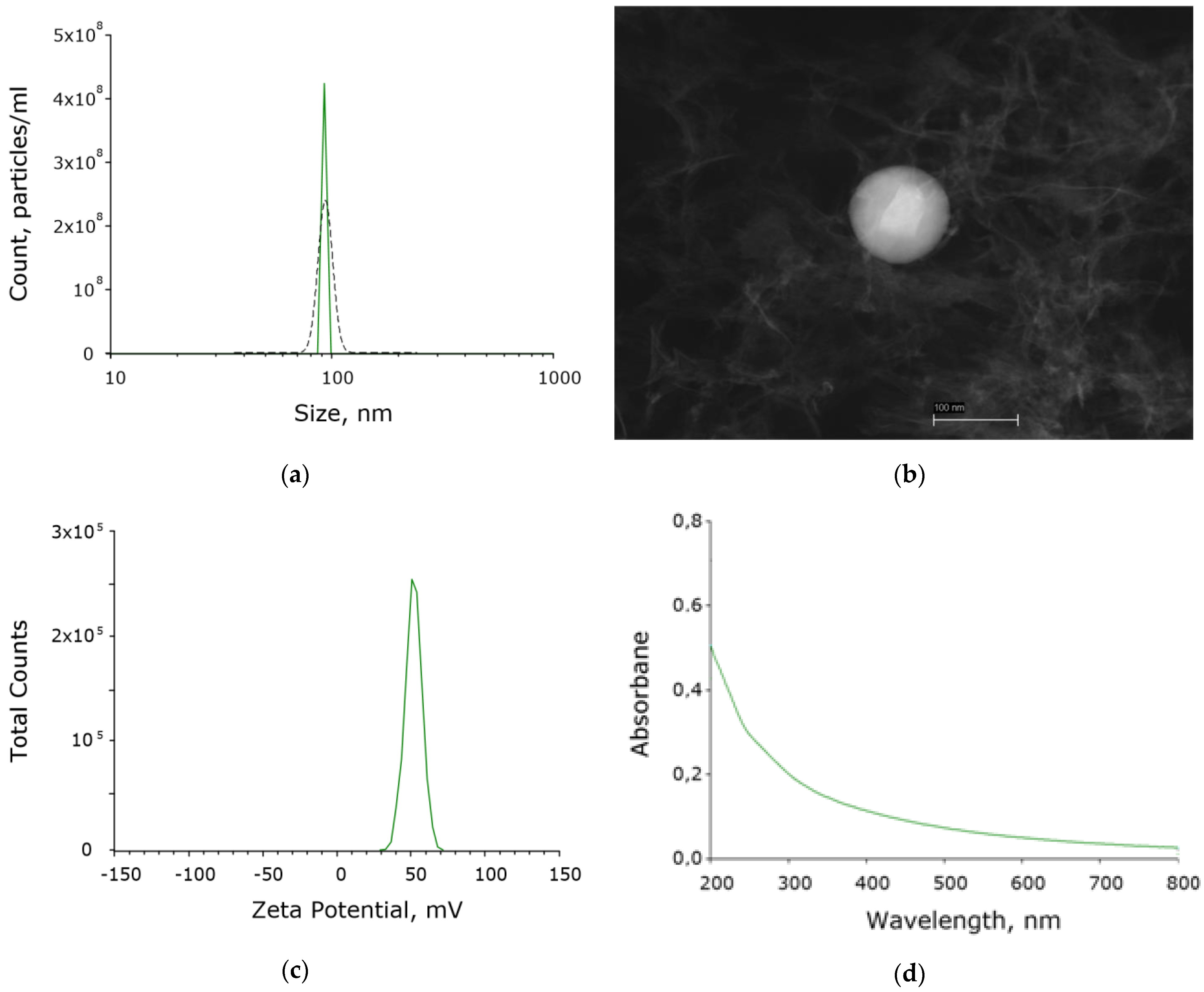
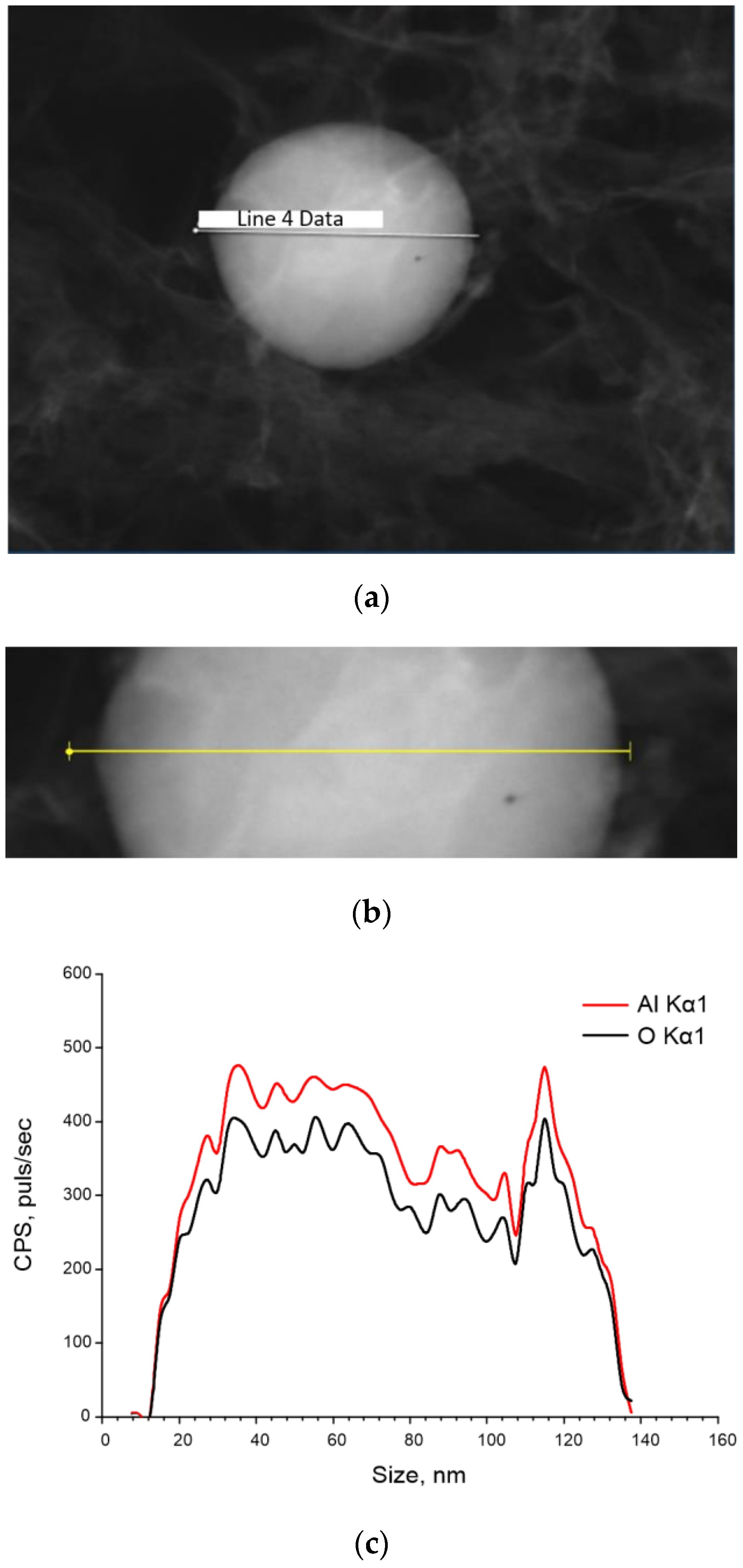
2.1. Aluminum Oxide Nanoparticles Preparation and Characterization
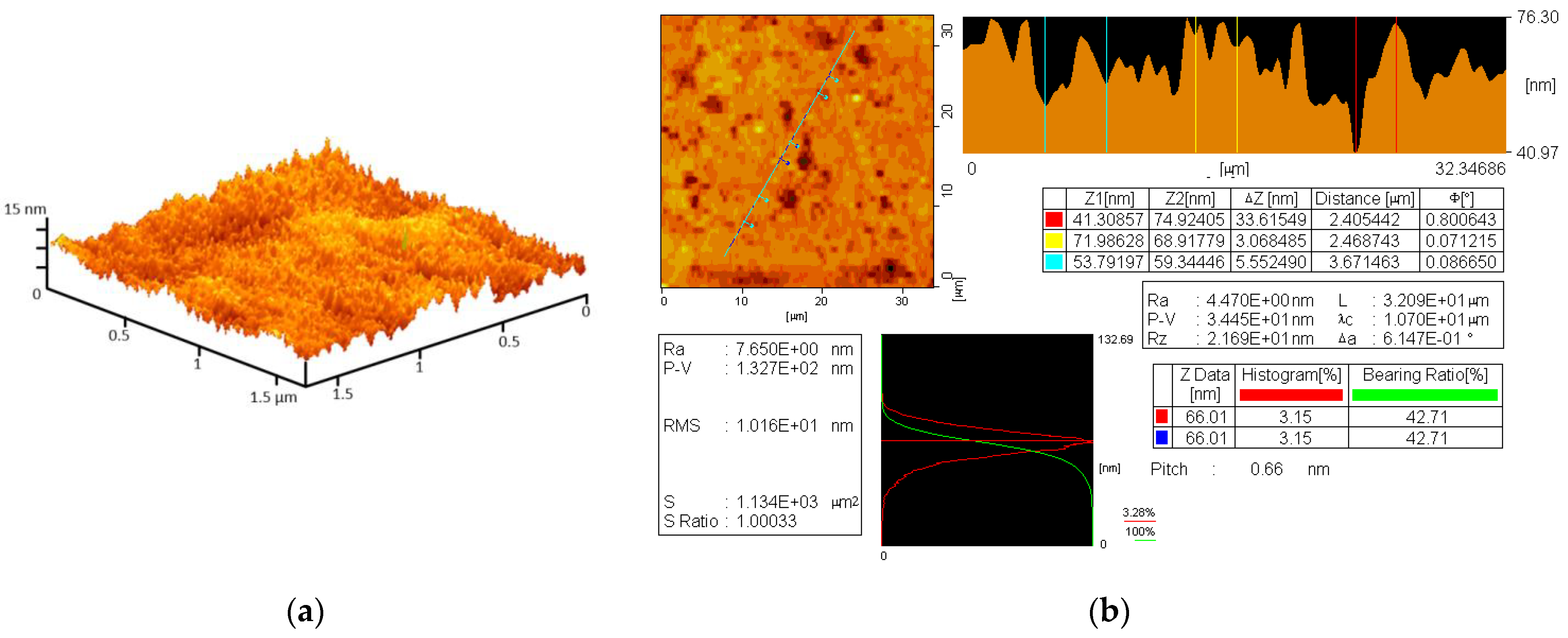
2.2. Composite Fabrication and Assay of Rheological Properties
2.3. Assay of Thermal Characteristics
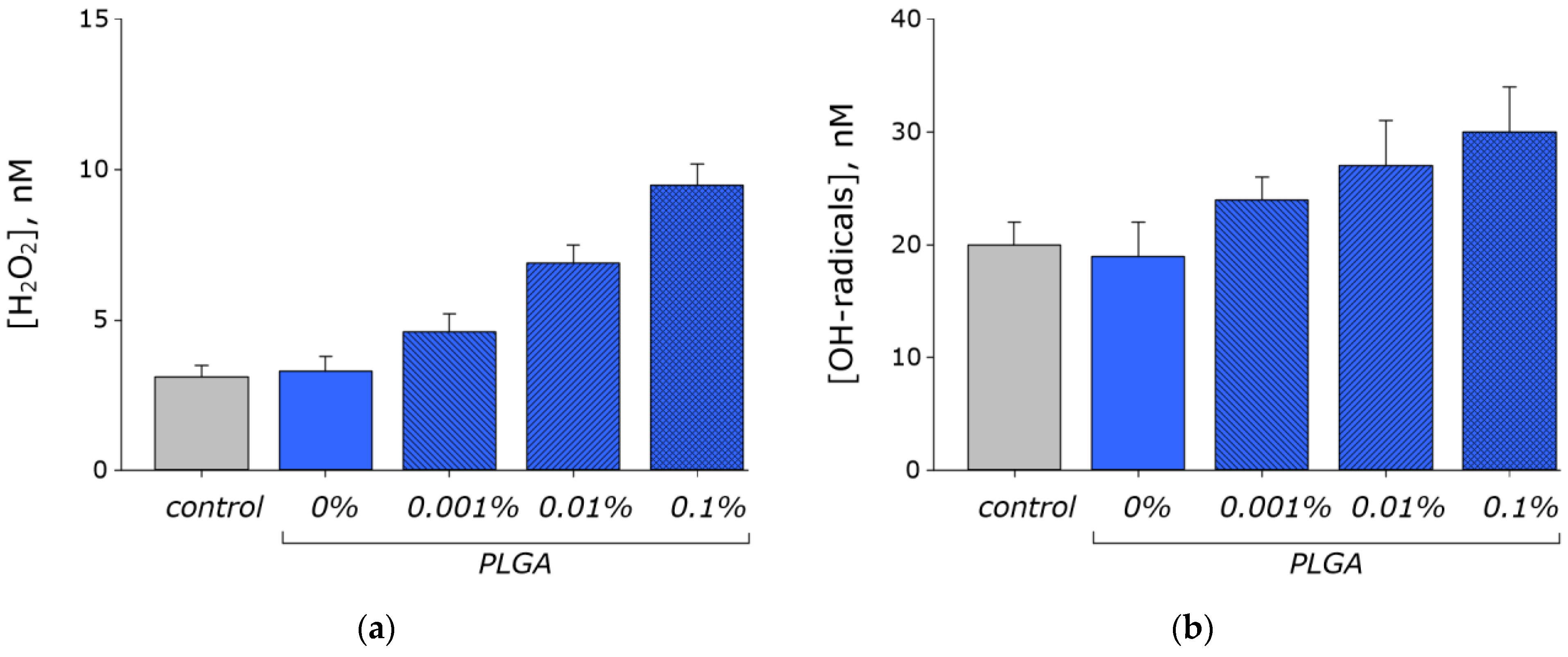
2.4. Measurement of Hydrogen Peroxide Concentration
2.5. Measurement of Hydroxyl-Radicals Concentration
2.6. Long-Lived Reactive Protein Species Detection
2.7. Mesurement of 8-Oxoguanine Concentration
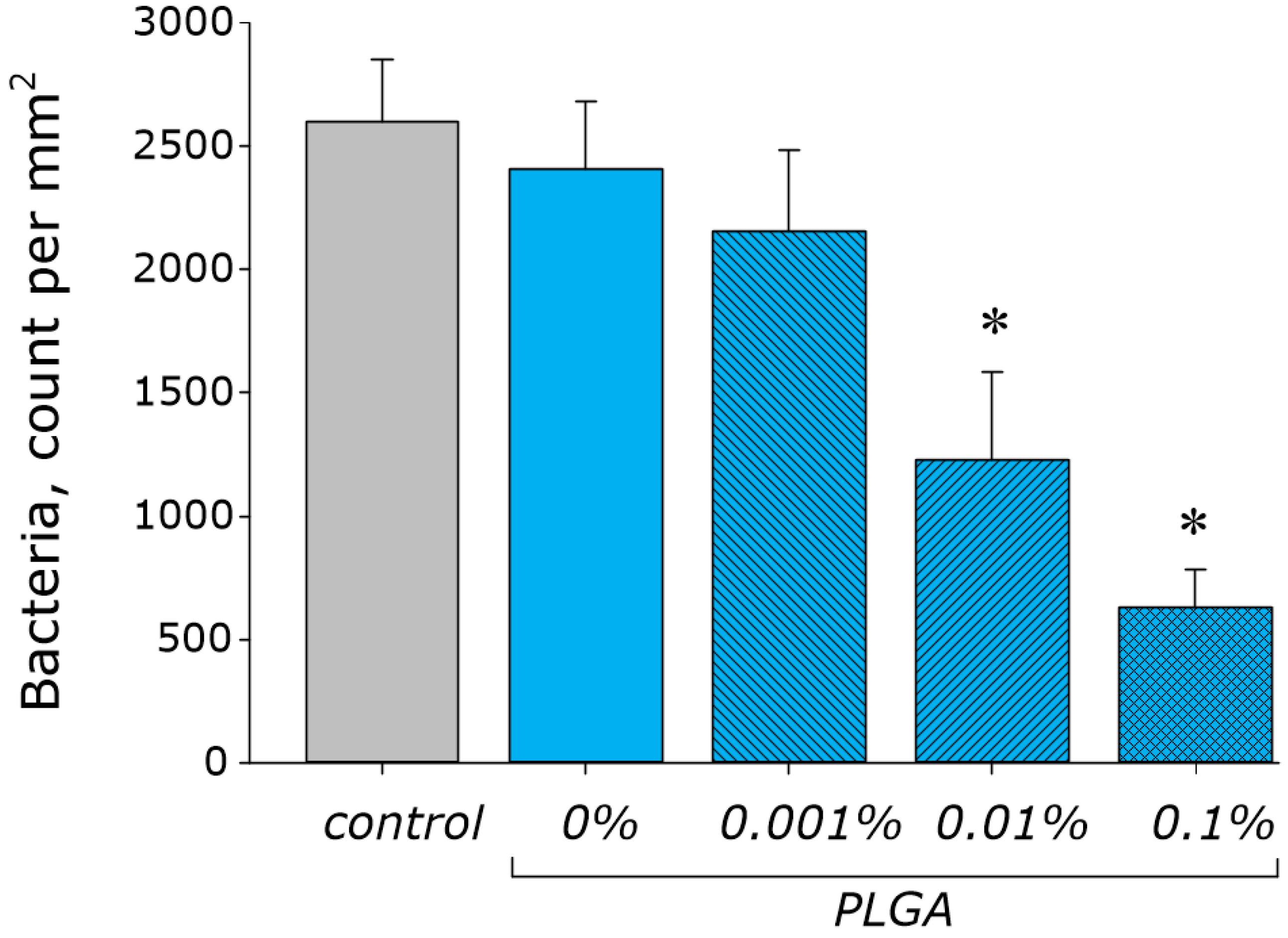
2.8. Assay of Bacteriostatic Activity
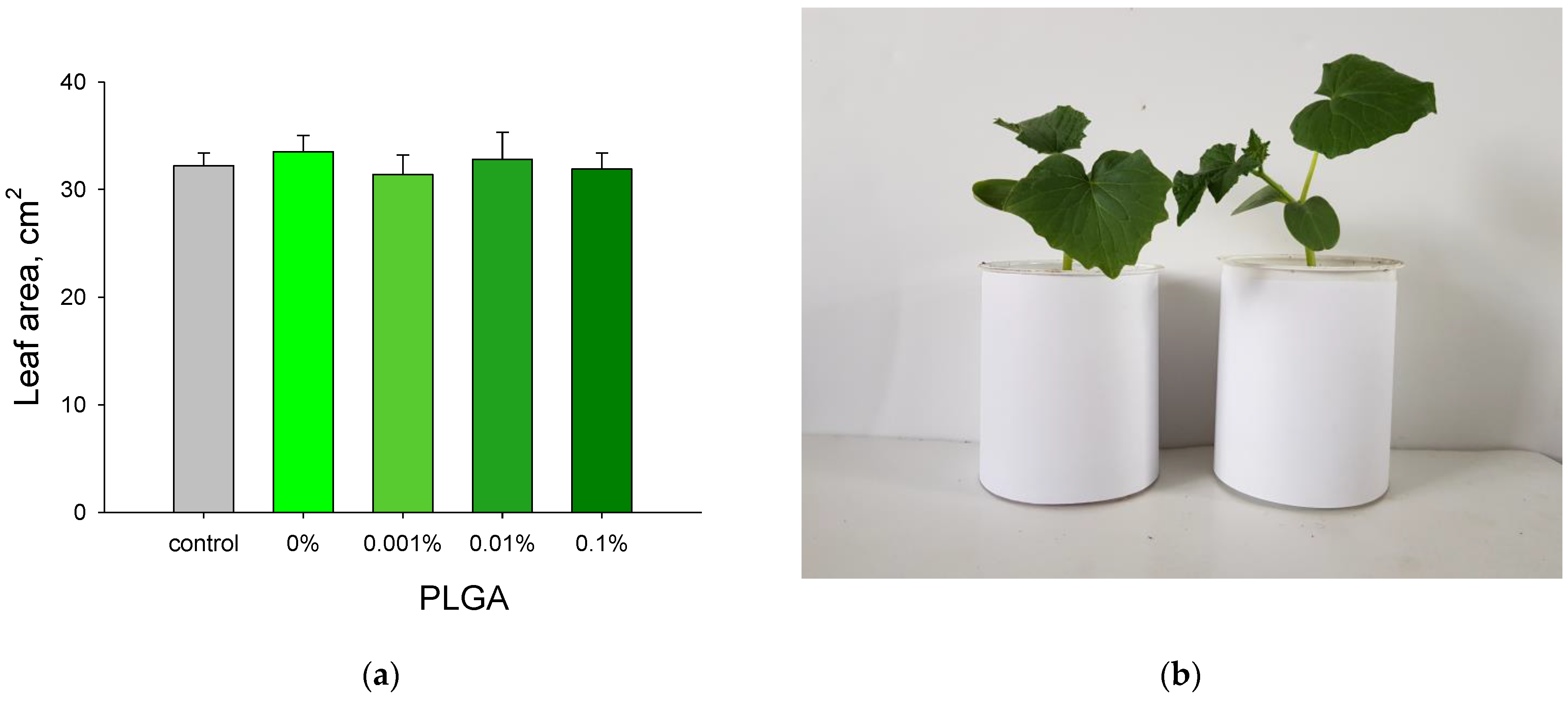
2.9. Measuring of Leaves Area
2.10. Assay of Biocompatibility with Mammalial Cells
2.11. Statistic
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdul Khalil, H.P.S.; Tye, Y.Y.; Leh, C.P.; Saurabh, C.K.; Ariffin, F.; Mohammad Fizree, H.; Mohamed, A.; Suriani, A.B. Cellulose Reinforced Biodegradable Polymer Composite Film for Packaging Applications. In Bionanocomposites for Packaging Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 49–69. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Zhang, C.-l.; Li, P. Characterisation and cooperative antimicrobial properties of chitosan/nano-ZnO composite nanofibrous membranes. Food Chem. 2012, 132, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.A.; Sergienko, K.V.; Kolmakova, A.A.; Konushkin, S.V.; Baikin, A.S.; Kolmakov, A.G.; Sevostyanov, M.A.; Kulikov, A.V.; Ivanov, V.E.; Belosludtsev, K.N.; et al. Development of a Biocompatible PLGA Polymers Capable to Release Thrombolytic Enzyme Prourokinase. J. Biomater. Sci. Polym. Ed. 2020, 31, 1405–1420. [Google Scholar] [CrossRef] [PubMed]
- Taherimehr, M.; Yousefniapasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344. [Google Scholar] [CrossRef] [PubMed]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Pinheiro, I.F.; Ferreira, F.V.; Souza, D.H.S.; Gouveia, R.F.; Lona, L.M.F.; Morales, A.R.; Mei, L.H.I. Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur. Polym. J. 2017, 97, 356–365. [Google Scholar] [CrossRef]
- Lim, X.Z. Microplastics are everywhere—But are they harmful? Nature 2022, 593, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Wirth, M.; Lorenz, C.; Gerdts, G. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal. Bioanal. Chem. 2018, 410, 5131–5141. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Han, J.S.; Park, E.J.; Seong, E.; Lee, G.H.; Kim, D.W.; Son, H.Y.; Han, H.Y.; Lee, B.S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Mohanty, S.; Nayak, S.K. Biobased polyurethane adhesive over petroleum based adhesive: Use of renewable resource. J. Macromol. Sci. Part A 2018, 55, 36–48. [Google Scholar] [CrossRef]
- Eslami, H.; Azimi Lisar, H.; Jafarzadeh Kashi, T.S.; Tahriri, M.; Ansari, M.; Rafiei, T.; Bastami, F.; Shahin-Shamsabadi, A.; Mashhadi Abbas, F.; Tayebi, L. Poly(lactic-co-glycolic acid)(PLGA)/TiO(2) nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biol. J. Int. Assoc. Biol. Stand. 2018, 53, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Biswal, A.K.; Thodikayil, A.T.; Saha, S. pH-Sensitive Acetalated Dextran/PLGA-Based Double-Layered Microparticles and Their Application in Food Preservation. ACS Appl. Bio Mater. 2021, 4, 2429–2441. [Google Scholar] [CrossRef]
- Murcia Valderrama, M.A.; Van Putten, R.J.; Gruter, G.M. PLGA Barrier Materials from CO(2). The influence of Lactide Co-monomer on Glycolic Acid Polyesters. ACS Appl. Polym. Mater. 2020, 2, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Gupta, K.C.; Kang, I.K. PLGA/nHA hybrid nanofiber scaffold as a nanocargo carrier of insulin for accelerating bone tissue regeneration. Nanoscale Res. Lett. 2014, 9, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, A.; Kwak, S.; Gupta, K.C.; Kang, I.-K. Antibacterial Activity and Cytocompatibility of PLGA/CuO Hybrid Nanofiber Scaffolds Prepared by Electrospinning. J. Nanomater. 2015, 2015, 832762. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.; Curry, A.; Gambhir, A.; Panigrahi, H.; Walker, C.; Wilkins, E.; Worsley, M.; Kay, P. Intraoperative bacterial contamination in operations for joint replacement. J. Bone Jt. Surg. Br. Vol. 1999, 81, 886–889. [Google Scholar] [CrossRef]
- Nehra, P.; Chauhan, R.; Garg, N.; Verma, K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br. J. Biomed. Sci. 2018, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Thukkaram, M.; Sitaram, S.; Subbiahdoss, G. Antibacterial efficacy of iron-oxide nanoparticles against biofilms on different biomaterial surfaces. Int. J. Biomater. 2014, 2014, 716080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, S.; Anderson, F. Infection in the Operating Room; The British Editorial Society of Bone and Joint Surgery: London, UK, 1999. [Google Scholar]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Parmar, A.; Kaur, G.; Kapil, S.; Sharma, V.; Sachar, S.; Sandhir, R.; Sharma, S. Green chemistry mediated synthesis of PLGA-Silver nanocomposites for antibacterial synergy: Introspection of formulation parameters on structural and bactericidal aspects. React. Funct. Polym. 2019, 141, 68–81. [Google Scholar] [CrossRef]
- Scavone, M.; Armentano, I.; Fortunati, E.; Cristofaro, F.; Mattioli, S.; Torre, L.; Kenny, J.M.; Imbriani, M.; Arciola, C.R.; Visai, L. Antimicrobial properties and cytocompatibility of PLGA/Ag nanocomposites. Materials 2016, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Burmistrov, D.E.; Yanykin, D.V.; Paskhin, M.O.; Nagaev, E.V.; Efimov, A.D.; Kaziev, A.V.; Ageychenkov, D.G.; Gudkov, S.V. Additive Production of a Material Based on an Acrylic Polymer with a Nanoscale Layer of Zno Nanorods Deposited Using a Direct Current Magnetron Discharge: Morphology, Photoconversion Properties, and Biosafety. Materials 2021, 14, 6586. [Google Scholar] [CrossRef]
- Almatar, M.; Makky, E.A.; Var, I.; Koksal, F. The role of nanoparticles in the inhibition of multidrug-resistant bacteria and biofilms. Curr. Drug Deliv. 2018, 15, 470–484. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Depristo, M.A.; Collins, J.J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Janani, B.; Al-Mohaimeed, A.M.; Raju, L.L.; Al Farraj, D.A.; Thomas, A.M.; Khan, S.S. Synthesis and characterizations of hybrid PEG-Fe3O4 nanoparticles for the efficient adsorptive removal of dye and antibacterial, and antibiofilm applications. J. Environ. Health Sci. Eng. 2021, 19, 389–400. [Google Scholar] [CrossRef]
- Yu, S.; Perálvarez-Marín, A.; Minelli, C.; Faraudo, J.; Roig, A.; Laromaine, A. Albumin-coated SPIONs: An experimental and theoretical evaluation of protein conformation, binding affinity and competition with serum proteins. Nanoscale 2016, 8, 14393–14405. [Google Scholar] [CrossRef] [Green Version]
- Sousa, C.U.; Sequeira, D.; Kolen’ko, Y.V.; Pinto, I.S.M.; Petrovykh, D.Y. Analytical protocols for separation and electron microscopy of nanoparticles interacting with bacterial cells. Anal. Chem. 2015, 87, 4641–4648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielyan, L.; Badalyan, H.; Gevorgyan, V.; Trchounian, A. Comparable antibacterial effects and action mechanisms of silver and iron oxide nanoparticles on Escherichia coli and Salmonella typhimurium. Sci. Rep. 2020, 10, 13145. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Serov, D.A.; Astashev, M.E.; Semenova, A.A.; Lisitsyn, A.B. Ag2O Nanoparticles as a Candidate for Antimicrobial Compounds of the New Generation. Pharmaceuticals 2022, 15, 968. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [Green Version]
- Martirosyan, A.; Schneider, Y.-J. Engineered Nanomaterials in Food: Implications for Food Safety and Consumer Health. Int. J. Environ. Res. Public Health 2014, 11, 5720–5750. [Google Scholar] [CrossRef] [Green Version]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Lin, L.-H.; Lee, H.-P.; Yeh, M.-L. Characterization of a sandwich PLGA-gallic acid-PLGA coating on Mg alloy ZK60 for bioresorbable coronary artery stents. Materials 2020, 13, 5538. [Google Scholar] [CrossRef]
- Sevostyanov, M.; Baikin, A.; Sergienko, K.; Shatova, L.; Kirsankin, A.; Baymler, I.; Shkirin, A.; Gudkov, S. Biodegradable stent coatings on the basis of PLGA polymers of different molecular mass, sustaining a steady release of the thrombolityc enzyme streptokinase. React. Funct. Polym. 2020, 150, 104550. [Google Scholar] [CrossRef]
- Scheiner, K.C.; Maas-Bakker, R.F.; Van Steenbergen, M.J.; Schwendeman, S.P.; Hennink, W.E.; Kok, R.J. Post-loading of proangiogenic growth factors in PLGA microspheres. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2021, 158, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Duan, Y.-R.; He, Q.; Lu, J.; Zhang, Z.-R. PELGE nanoparticles as new carriers for the delivery of plasmid DNA. Chem. Pharm. Bull. 2005, 53, 599–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Healthc. Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef]
- Klose, D.; Siepmann, F.; Elkharraz, K.; Siepmann, J. PLGA-based drug delivery systems: Importance of the type of drug and device geometry. Int. J. Pharm. 2008, 354, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Aazem, I.; Rathinam, P.; Pillai, S.; Honey, G.; Vengellur, A.; Bhat, S.G.; Sailaja, G. Active bayerite underpinned Ag2O/Ag: An efficient antibacterial nanohybrid combating microbial contamination. Metallomics 2021, 13, mfab049. [Google Scholar] [CrossRef] [PubMed]
- Beinert, H.; Holm, R.H.; Münck, E. Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science 1997, 277, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C 2021, 118, 111394. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Petralia, S.; Neri, G.; Corsaro, C.; Forte, L.; Squarzoni, S.; Guglielmino, S.; Traina, F.; Fazio, E.; et al. Antimicrobial Effect and Cytotoxic Evaluation of Mg-Doped Hydroxyapatite Functionalized with Au-Nano Rods. Molecules 2021, 26, 1099. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Zhang, W. Fabrication and application of nanoporous anodic aluminum oxide: A review. Nanotechnology 2021, 32, 222001. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, A.S.; Nabil, S.; Aldulaijan, S.; Ababutain, I.M.; Alghamdi, A.I.; Almubayedh, S.; Khalil, K.D. Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives. Polymers 2021, 13, 1160. [Google Scholar] [CrossRef]
- Khajeh Mehrizi, M.; Mashroteh, H.; Nabizadeh Moghadam Noghabi, N. Effect of Chitosan, Aluminum Oxide and Silver Nanoparticles on Antibacterial, Deodorizing and Moisture Absorption Properties of Nonwoven Polyester Fabrics for Use in Medical Textiles. Med. Lab. J. 2016, 10, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Vyas, S.; Shukla, A.; Shivhare, S.; Upadhyay, N. Facile synthesis and characterization of polyaniline (PANI)—Aluminium oxide (Al2O3) nanocomposites by using chemical oxidative polymerization. AIP Conf. Proc. 2021, 2369, 020192. [Google Scholar] [CrossRef]
- Manyasree, D.; Kiranmayi, P.; Ravi Kumar, R.V.S.S.N. Synthesis, characterization and antibacterial activity of aluminium oxide nanoparticles. Int. J. Pharm. Pharm. Sci. 2018, 10, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Nahrawy, A.M.E.; Abou Hammad, A.B.; Abdel-Aziz, M.S.; Wassel, A.R. Spectroscopic and Antimicrobial Activity of Hybrid Chitosan/Silica Membranes doped with Al2O3 Nanoparticles. Silicon 2019, 11, 1677–1685. [Google Scholar] [CrossRef]
- El Nahrawy, A.M.; Mansour, A.M.; Abou Hammad, A.B.; Ibrahim, R.S.; Abouelnaga, A.M.; Abdel-Aziz, M.S. Optical, Functional Impact and Antimicrobial of Chitosan/Phosphosilicate/Al2O3 Nanosheets. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3084–3094. [Google Scholar] [CrossRef]
- Astashev, M.E.; Sarimov, R.M.; Serov, D.A.; Matveeva, T.A.; Simakin, A.V.; Ignatenko, D.N.; Burmistrov, D.E.; Smirnova, V.V.; Kurilov, A.D.; Mashchenko, V.I.; et al. Antibacterial behavior of organosilicon composite with nano aluminum oxide without influencing animal cells. React. Funct. Polym. 2022, 170, 105143. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Penkov, N.V.; Fesenko, E.E.; Vedunova, M.V.; Bruskov, V.I.; Gudkov, S.V. Protective and adaptogenic role of peroxiredoxin 2 (Prx2) in neutralization of oxidative stress induced by ionizing radiation. Free Radic. Biol. Med. 2019, 134, 76–86. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Binhi, V.N.; Matveeva, T.A.; Penkov, N.V.; Gudkov, S.V. Unfolding and Aggregation of Lysozyme under the Combined Action of Dithiothreitol and Guanidine Hydrochloride: Optical Studies. Int. J. Mol. Sci. 2021, 22, 2710. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Lednev, V.N.; Simakin, A.V.; Uvarov, O.V.; Kucherov, R.N.; Ivashkin, P.I.; Dorokhov, A.S.; Izmailov, A.Y. Biosafety Construction Composite Based on Iron Oxide Nanoparticles and PLGA. Inventions 2022, 7, 61. [Google Scholar] [CrossRef]
- Chausov, D.N.; Burmistrov, D.E.; Kurilov, A.D.; Bunkin, N.F.; Astashev, M.E.; Simakin, A.V.; Vedunova, M.V.; Gudkov, S.V. New Organosilicon Composite Based on Borosiloxane and Zinc Oxide Nanoparticles Inhibits Bacterial Growth, but Does Not Have a Toxic Effect on the Development of Animal Eukaryotic Cells. Materials 2021, 14, 6281. [Google Scholar] [CrossRef]
- In Pyo Park, P.; Jonnalagadda, S. Predictors of glass transition in the biodegradable poly-lactide and poly-lactide-co-glycolide polymers. J. Appl. Polym. Sci. 2006, 100, 1983–1987. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Penkov, N.V.; Baimler, I.V.; Lyakhov, G.A.; Pustovoy, V.I.; Simakin, A.V.; Sarimov, R.M.; Scherbakov, I.A. Effect of mechanical shaking on the physicochemical properties of aqueous solutions. Int. J. Mol. Sci. 2020, 21, 8033. [Google Scholar] [CrossRef] [PubMed]
- Barmina, E.; Gudkov, S.; Simakin, A.; Shafeev, G. Stable products of laser-induced breakdown of aqueous colloidal solutions of nanoparticles. J. Laser Micro Nanoeng. 2017, 12, 254–257. [Google Scholar] [CrossRef] [Green Version]
- Baimler, I.V.; Lisitsyn, A.B.; Gudkov, S.V. Water decomposition occurring during laser breakdown of aqueous solutions containing individual gold, zirconium, molybdenum, iron or nickel nanoparticles. Front. Phys. 2020, 8, 600. [Google Scholar] [CrossRef]
- Baimler, I.; Simakin, A.; Uvarov, O.; Volkov, M.Y.; Gudkov, S. Generation of hydroxyl radicals during laser breakdown of aqueous solutions in the presence of Fe and Cu nanoparticles of different sizes. Phys. Wave Phenom. 2020, 28, 107–110. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Karp, O.E.; Bruskov, V.I. Long-lived protein radicals induced by X-ray irradiation are the source of reactive oxygen species in aqueous medium. Doklady. Biochem. Biophys. 2010, 430, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The role of peroxiredoxin 6 in neutralization of X-ray mediated oxidative stress: Effects on gene expression, preservation of radiosensitive tissues and postradiation survival of animals. Free Radic. Res. 2017, 51, 148–166. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Gudkov, S.V. Peroxiredoxin 1—Multifunctional antioxidant enzyme, protects from oxidative damages and increases the survival rate of mice exposed to total body irradiation. Arch. Biochem. Biophys. 2021, 697, 108671. [Google Scholar] [CrossRef]
- Chernikov, A.V.; Gudkov, S.V.; Shtarkman, I.N.; Bruskov, V.I. Oxygen effect in heat-mediated damage to DNA. Biofizika 2007, 52, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Shtarkman, I.; Gudkov, S.; Chernikov, A.; Bruskov, V. Effect of amino acids on X-ray-induced hydrogen peroxide and hydroxyl radical formation in water and 8-oxoguanine in DNA. Biochemistry 2008, 73, 470–478. [Google Scholar] [CrossRef]
- Barkhudarov, E.M.; Kossyi, I.A.; Anpilov, A.M.; Ivashkin, P.I.; Artem’ev, K.V.; Moryakov, I.V.; Misakyan, M.A.; Christofi, N.; Burmistrov, D.E.; Smirnova, V.V.; et al. New Nanostructured Carbon Coating Inhibits Bacterial Growth, but Does Not Influence on Animal Cells. Nanomaterials 2020, 10, 2130. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and application of photoconversion fluoropolymer films for greenhouses located at high or polar latitudes. J. Photochem. Photobiol. B Biol. 2020, 213, 112056. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Yanykin, D.V.; Simakin, A.V.; Paskhin, M.O.; Ivanyuk, V.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; Gudkov, S.V. Cultivation of Solanum lycopersicum under Glass Coated with Nanosized Upconversion Luminophore. Appl. Sci. 2021, 11, 10726. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Kolmakov, A.G.; Sergiyenko, K.V.; Kaplan, M.A.; Baikin, A.S.; Gudkov, S.V. Mechanical, physical–chemical and biological properties of the new Ti–30Nb–13Ta–5Zr alloy. J. Mater. Sci. 2020, 55, 14516–14529. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Sevostyanov, M.A.; Konushkin, S.V.; Losertová, M.; Ivannikov, A.Y.; Kolmakov, A.G.; Izmailov, A.Y. Manufacturing and study of mechanical properties, structure and compatibility with biological objects of plates and wire from new Ti-25Nb-13Ta-5Zr alloy. Metals 2020, 10, 1584. [Google Scholar] [CrossRef]
- Konushkin, S.V.; Sergiyenko, K.V.; Nasakina, E.O.; Leontyev, V.G.; Kuznetsova, O.G.; Titov, D.D.; Tsareva, A.M.; Dormidontov, N.A.; Kirsankin, A.A.; Kannykin, S.V. Study of the physicochemical and biological properties of the new promising Ti–20Nb–13Ta–5Zr alloy for biomedical applications. Mater. Chem. Phys. 2020, 255, 123557. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’yanov, M.A.; Belosludtsev, K.N.; et al. Unmodified hydrated C(60) fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 37–46. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shtarkman, I.N.; Chernikov, A.V.; Usacheva, A.M.; Bruskov, V.I. Guanosine and inosine (riboxin) eliminate the long-lived protein radicals induced X-ray radiation. Doklady. Biochem. Biophys. 2007, 413, 50–53. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Popova, N.R.; Ivanov, V.E.; Karp, O.E.; Chernikov, A.V.; Gudkov, S.V. Formation of long-lived reactive species of blood serum proteins by the action of heat. Biochem. Biophys. Res. Commun. 2014, 443, 957–961. [Google Scholar] [CrossRef]
- Kozlova, E.S.; Nikiforova, T.E. Incorporation of silver nanoparticles into a cellulose matrix for preparing package materials for foodstuffs. Russ. J. Appl. Chem. 2015, 88, 638–646. [Google Scholar] [CrossRef]
- Ivanyuk, V.V.; Shkirin, A.V.; Belosludtsev, K.N.; Dubinin, M.V.; Kozlov, V.A.; Bunkin, N.F.; Dorokhov, A.S.; Gudkov, S.V. Influence of Fluoropolymer Film Modified with Nanoscale Photoluminophor on Growth and Development of Plants. Front. Phys. 2020, 8, 616040. [Google Scholar] [CrossRef]
- Khramov, R.; Kosobryukhov, A.; Kreslavski, V.; Balakirev, D.; Khudyakova, A.; Svidchenko, E.; Surin, N.; Ponomarenko, S.; Luponosov, Y. Luminescence of Agrotextiles Based on Red-Light-Emitting Organic Luminophore and Polypropylene Spunbond Enhances the Growth and Photosynthesis of Vegetable Plants. Front. Plant Sci. 2022, 13, 827679. [Google Scholar] [CrossRef]
- Chernov, A.S.; Reshetnikov, D.A.; Kovalitskaya Yu, A.; Manokhin, A.A.; Gudkov, S.V. Influence of wideband visible light with an padding red component on the functional state of mice embryos and embryonic stem cells. J. Photochem. Photobiol. B Biol. 2018, 188, 77–86. [Google Scholar] [CrossRef]
- Mui, J.; Ngo, J.; Kim, B. Aggregation and Colloidal Stability of Commercially Available Al₂O₃ Nanoparticles in Aqueous Environments. Nanomaterials 2016, 6, 90. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, R.; Khurana, D.; Kumar, A.; Subudhi, S. Stability analysis of Al2O3/water nanofluids. J. Exp. Nanosci. 2017, 12, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Prashanth, P.A.; Raveendra, R.S.; Hari Krishna, R.; Ananda, S.; Bhagya, N.P.; Nagabhushana, B.M.; Lingaraju, K.; Raja Naika, H. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, S.; Rajakarthihan, S. Antimicrobial study on gamma-irradiated polyaniline–aluminum oxide (PANI–Al2O3) nanoparticles. Int. Nano Lett. 2020, 10, 97–110. [Google Scholar] [CrossRef]
- Yakdoumi, F.Z.; Hadj-Hamou, A.S. Effectiveness assessment of TiO2-Al2O3 nano-mixture as a filler material for improvement of packaging performance of PLA nanocomposite films. J. Polym. Eng. 2020, 40, 848–858. [Google Scholar] [CrossRef]
- Istirokhatun, T.; Yuni, U.; Andarani, P.; Susanto, H. Do ZnO and Al2O3 Nanoparticles Improve the Anti-Bacterial Properties of Cellulose Acetate-Chitosan Membrane? In MATEC Web of Conferences; EDP Sciences: Les Iles, France, 2018; Volume 156. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, V.V.; Chausov, D.N.; Serov, D.A.; Kozlov, V.A.; Ivashkin, P.I.; Pishchalnikov, R.Y.; Uvarov, O.V.; Vedunova, M.V.; Semenova, A.A.; Lisitsyn, A.B.; et al. A Novel Biodegradable Composite Polymer Material Based on PLGA and Silver Oxide Nanoparticles with Unique Physicochemical Properties and Biocompatibility with Mammalian Cells. Materials 2021, 14, 6915. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Simakin, A.V.; Smirnova, V.V.; Uvarov, O.V.; Ivashkin, P.I.; Kucherov, R.N.; Ivanov, V.E.; Bruskov, V.I.; Sevostyanov, M.A.; Baikin, A.S.; et al. Bacteriostatic and Cytotoxic Properties of Composite Material Based on ZnO Nanoparticles in PLGA Obtained by Low Temperature Method. Polymers 2021, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Maharani, D.K.; Hidayah, R.; Khasanah, L. Qualitative Study of Antibacterial Activity of Chitosan-ZnO/Al2O3 Nanocomposites. Adv. Sci. Lett. 2017, 23, 11948–11951. [Google Scholar] [CrossRef]
- Sikora, P.; Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Mijowska, E. Antimicrobial Activity of Al2O3, CuO, Fe3O4, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials. Nanomaterials 2018, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Deepika, M.S.; Thangam, R.; Sundarraj, S.; Sheena, T.S.; Sivasubramanian, S.; Kulandaivel, J.; Thirumurugan, R. Co-delivery of Diverse Therapeutic Compounds Using PEG–PLGA Nanoparticle Cargo against Drug-Resistant Bacteria: An Improved Anti-biofilm Strategy. ACS Appl. Bio Mater. 2020, 3, 385–399. [Google Scholar] [CrossRef] [Green Version]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, Y.; Fan, B.; Wang, Y.; Mao, Z.; Wang, W.; Wu, J. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release 2020, 321, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.-H.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Bacteria-Targeted Clindamycin Loaded Polymeric Nanoparticles: Effect of Surface Charge on Nanoparticle Adhesion to MRSA, Antibacterial Activity, and Wound Healing. Pharmaceutics 2019, 11, 236. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Na, Y.; Lee, J.S.; Woo, J.; Ahn, S.; Lee, E.; Choi, W.I.; Sung, D. Reactive oxygen species (ROS)-responsive ferrocene-polymer-based nanoparticles for controlled release of drugs. J. Mater. Chem. B 2020, 8, 1906–1913. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Karp, O.E.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Gudkov, S.V. Prolongation of oxidative stress by long-lived reactive protein species induced by X-ray radiation and their genotoxic action. Free Radic. Res. 2012, 46, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Levy, R.; Gao, J.; Fishbein, I.; Kousaev, V.; Sosnowski, S.; Slomkowski, S.; Golomb, G. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Ther. 2000, 7, 1896–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karp, O.E.; Gudkov, S.V.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Bruskov, V.I. Genotoxic effect of long-lived protein radicals in vivo generated by X-ray irradiation. Doklady. Biochem. Biophys. 2010, 434, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles to Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Londono, S.C.; Hartnett, H.E.; Williams, L.B. Antibacterial Activity of Aluminum in Clay from the Colombian Amazon. Environ. Sci. Technol. 2017, 51, 2401–2408. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Quinlan, G.J.; Clark, I.; Halliwell, B. Aluminium salts accelerate peroxidation of membrane lipids stimulated by iron salts. Biochim. Biophys. Acta Lipids Lipid Metab. 1985, 835, 441–447. [Google Scholar] [CrossRef]
- Morrison, K.D.; Misra, R.; Williams, L.B. Unearthing the Antibacterial Mechanism of Medicinal Clay: A Geochemical Approach to Combating Antibiotic Resistance. Sci. Rep. 2016, 6, 19043. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Paskhin, M.O.; Simakin, A.V.; Burmistrov, D.E.; Pobedonostsev, R.V.; Vyatchinov, A.A.; Vedunova, M.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; et al. Plant Photochemistry under Glass Coated with Upconversion Luminescent Film. Appl. Sci. 2022, 12, 7480. [Google Scholar] [CrossRef]
- Mirshafa, A.; Nazari, M.; Jahani, D.; Shaki, F. Size-Dependent Neurotoxicity of Aluminum Oxide Particles: A Comparison Between Nano- and Micrometer Size on the Basis of Mitochondrial Oxidative Damage. Biol. Trace Elem. Res. 2018, 183, 261–269. [Google Scholar] [CrossRef]
- Dey, M.; Singh, R.K. Neurotoxic effects of aluminium exposure as a potential risk factor for Alzheimer’s disease. Pharmacol. Rep. 2022, 74, 439–450. [Google Scholar] [CrossRef]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787. [Google Scholar]
- Thambirajoo, M.; Maarof, M.; Lokanathan, Y.; Katas, H.; Ghazalli, N.F.; Tabata, Y.; Fauzi, M.B. Potential of Nanoparticles Integrated with Antibacterial Properties in Preventing Biofilm and Antibiotic Resistance. Antibiotics 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed]





| № | Composition | Size of NPs, nm | Microorganism Strains | Effect | MIC/MBC | Results | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Commercially available Al2O3 NPs coated with chitosan | <50 | Escherichia coli ATCC2592, Pseudomonas aeruginosa ATCC 27853, Staphylococcus epidermidis ATCC 12228, Staphylococcus aureus ATCC 29213 | bacteriostatic | 5 mg/mL | Chitosan-coated Al2O3 NPs realized bacteriostatic action due to binding with the active site of the target proteins for both Gram-positive and Gram-negative bacteria. | [53] |
| 2 | Commercially available Al2O3 NPs coated with chitosan | 80 | Staphylococcus aureus ATCC 6538 | bacteriostatic | 25 μg/mL | Samples with chitosan had the highest level of antimicrobial activity (about 98%), which indicates high antibacterial activity of chitosan in the products. | [54] |
| 3 | γ-irradiated polyaniline (PANI)/Al2O3 NPs composite | - | Bacillus subtilis, Escherichia coli | bacteriostatic | - | PANI–Al2O3 NPs inhibited both Gram-positive and Gram-negative bacteria growth. | [55] |
| 4 | PANI/Al2O3 NPs composite | 14–19 | Escherichia coli, Staphylococcus aureus | bacteriostatic | 17 mg/mL | PANI–Al2O3 NPs inhibited both Gram-positive and Gram-negative bacteria growth. The bacteriostatic was two times less than effect of amikacin. | [90] |
| 5 | Chitosan/Al2O3 NPs | <10 | Aspergillus niger NRRL A-326, Candida albicans ATCC 10231, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC6538-P | bacteriostatic fungicidal | 127 mg/mL | Chitosan/silica Al2O3 NPs composite inhibited growth of S. aureus, P. aeruginosa and C. albican, but not A. niger. | [57] |
| 6 | Chitosan/P2O5:SiO2-Al2O3 NPs | 100–800 | Aspergillus niger, Candida albicans, Pseudomonas aeruginosa, Staphylococcus aureus | bacteriostatic fungicidal | 654 mg/mL | The nanocomposite inhibited growth of all studied microorganisms in equal manner. | [58] |
| 7 | Borosilaxane/Al2O3 NPs | 45 | Escherichia coli | bacteriostatic, bactericidal | 10 µg/mL | Nanocomposite had antibacterial activity via generation of reactive species but did not change SH-SY5Y cell vitality. | [59] |
| 8 | Film of polylactic acid (PLA) contained Al2O3 NPs or TiO2-Al2O3 NPs. | <10 | Escherichia coli ATCCR8739, Pseudomonas aeruginosa ATCC 10231 | bacteriostatic | ~15 mg/mL | The bacteriostatic effect of TiO2-Al2O3 nano-mixture against both bacteria was more effective than that Al2O3 NPs. | [91] |
| 9 | Commercially available Al2O3 or ZnO NPs added to cellulose acetate-chitosan membrane | <50 | Escherichia coli | no | - | Authors did not observe bacteriostatic effect of Al2O3 or ZnO NPs. Proposed case is the adsorption of NP on membrane. | [92] |
| 10 | Chitosan/ZnO-Al2O3 NPs composite synthesized with sol–gel method. | <50 | Staphylococcus aureus | bacteriostatic | 20 mg/mL | Addition of ZnO NPs enhancedantibacterial activity of Chitosan-ZnO/Al2O3 nanocomposites. | [95] |
| 11 | Commercially available Al2O3 NPs mixed with cement building material | 100 | Candida albicans, Escherichia coli ATCC 8739, Escherichia coli MG1655, Pseudomonas aeruginosa, Staphylococcus aureus | bacteriostatic, bactericidal, fungicidal | 125 μg/mL | Antimicrobial effect of Al2O3 NPs depended on species. Maximum antimicrobial activity was observed versus E. coli (both strains) and C. albicans | [96] |
| 12 | PLGA/Ag2O NPs | 35 | Escherichia coli | bacteriostatic, bactericidal | 1 µg/mL | PLGA/Ag2O NPs increased generation of ROS, damaged bacterial DNA and proteins, though didn’t show cytotoxicity for eukaryotic cells. | [93] |
| 13 | PLGA/Fe2O3 NPs | 30 | Escherichia coli | bacteriostatic, bactericidal | 1 µg/mL | Nanocomposite increased generation of ROS, 8-oxo-Gua and LRPS inhibited bacterial growth. PLGA/Fe2O3 NPs has low cytotoxicity at concentration 100 µg/mL. Nanocomposite did not interfere with plant growth. | [62] |
| 14 | PLGA/ZnO NPs | 40–70 | Escherichia coli | bacteriostatic, bactericidal | 1 µg/mL | Nanocomposite inhibited bacterial growth but had no toxic effect on the development of eukaryotic cells. | [94] |
| 15 | PEG/PLGA NPs loaded with rutin and benzamide | 260 | Pseudomonas aeruginosa, Staphylococcus aureus | bacteriostatic, bactericidal | 180 µg/mL | Drug-loaded PEG/PLGA NPs killed bacteria via cell wall disrupt. NPs demonstrated cytotoxicity for animal cell from concentration 40 µg/mL. | [97] |
| 16 | BSA/PLGA NPs loaded with sparfloxacin, tacrolimus and γ3-peptide | 166–184 | Pseudomonas aeruginosa, Staphylococcus aureus | bacteriostatic, bactericidal | 1–4 µg/mL | Strong antibacterial activity. Bactericidal activity against Gram-positive bacteria was better. | [99] |
| 17 | PLGA-polyethyleneimine (PEI) NPs loaded with clindamycin | 126–132 | Staphylococcus aureus methicillin-resistant | bacteriostatic, bactericidal | 300 µg/mL | Strong bactericidal activity. NPs did not change viability of L929 mouse fibroblast and improve wound healing in mice | [100] |
| 18 | PLGA/Al2O3 NPs | 80 | Escherichia coli | bacteriostatic, bactericidal | 10 µg/mL | PLGA/Al2O3 NPs inhibited bacterial growth via ROS generation. Nanocomposite did not influence the growth of plants. PLGA/Al2O3 NPs did not change human cell line viability and proliferation. | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simakin, A.V.; Sarimov, R.M.; Smirnova, V.V.; Astashev, M.E.; Serov, D.A.; Yanykin, D.V.; Chausov, D.N.; Shkirin, A.V.; Uvarov, O.V.; Rotanov, E.; et al. New Structural Nanocomposite Based on PLGA and Al2O3 NPs as a Balance between Antibacterial Activity and Biocompatibility with Eukaryotic Cells. J. Compos. Sci. 2022, 6, 298. https://doi.org/10.3390/jcs6100298
Simakin AV, Sarimov RM, Smirnova VV, Astashev ME, Serov DA, Yanykin DV, Chausov DN, Shkirin AV, Uvarov OV, Rotanov E, et al. New Structural Nanocomposite Based on PLGA and Al2O3 NPs as a Balance between Antibacterial Activity and Biocompatibility with Eukaryotic Cells. Journal of Composites Science. 2022; 6(10):298. https://doi.org/10.3390/jcs6100298
Chicago/Turabian StyleSimakin, Alexander V., Ruslan M. Sarimov, Veronika V. Smirnova, Maxim E. Astashev, Dmitriy A. Serov, Denis V. Yanykin, Denis N. Chausov, Alexey V. Shkirin, Oleg V. Uvarov, Evgeny Rotanov, and et al. 2022. "New Structural Nanocomposite Based on PLGA and Al2O3 NPs as a Balance between Antibacterial Activity and Biocompatibility with Eukaryotic Cells" Journal of Composites Science 6, no. 10: 298. https://doi.org/10.3390/jcs6100298






