China Rose/Hibiscus rosa-sinensis Pollen-Mediated Phytosynthesis of Silver Nanoparticles and Their Catalytic Activity
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Phytosynthesis of AgNPs Using China Rose/H. rosa-sinensis Pollen
2.3. Photocatalytic Test of AgNPs
2.4. Characterization of AgNPs
3. Results and Discussion
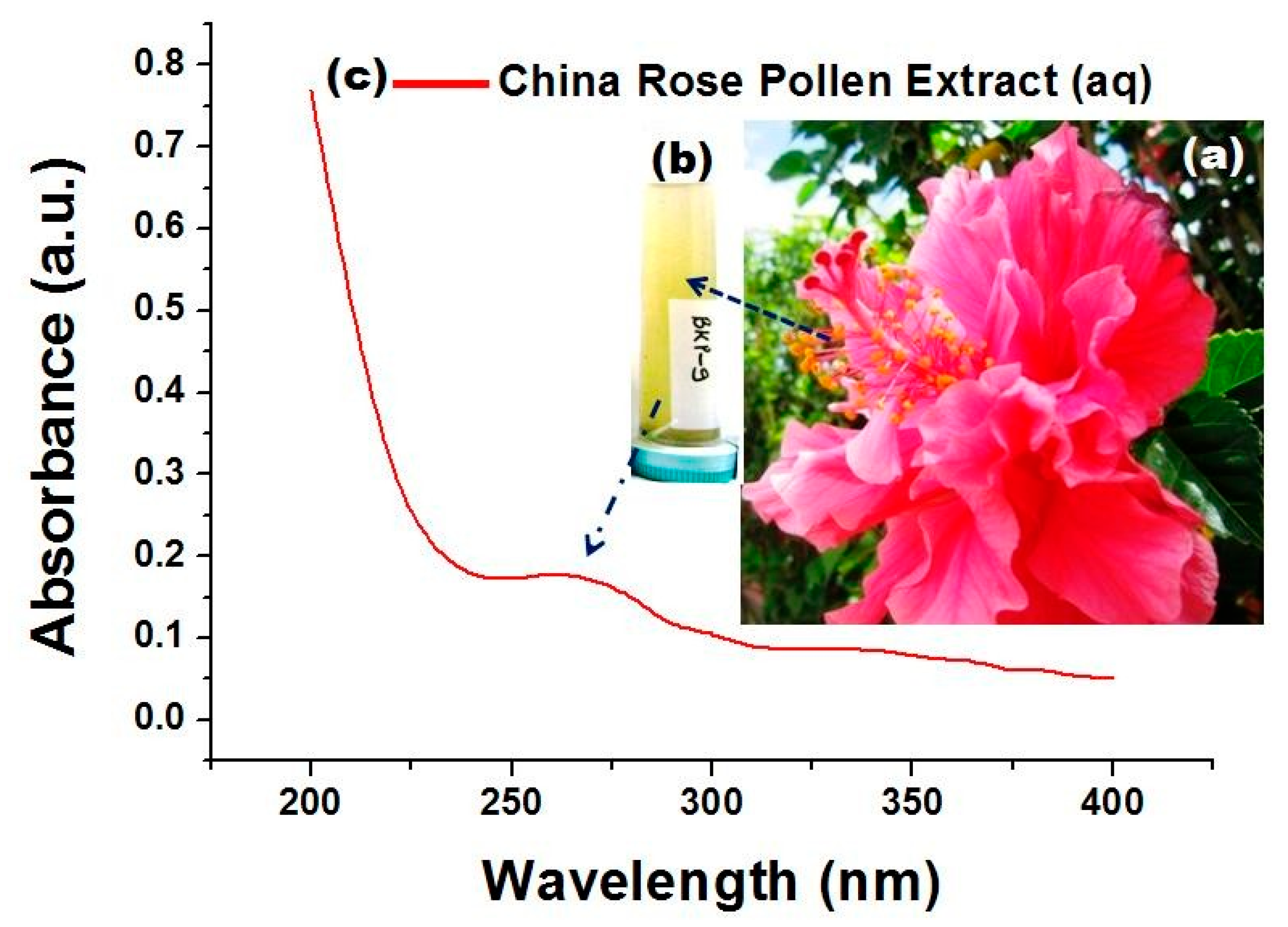
3.1. Visual and UV–Vis Studies of H. rosa-sinensis Pollen
3.2. Microscopic and XRD Studies of H. rosa-sinensis Pollen
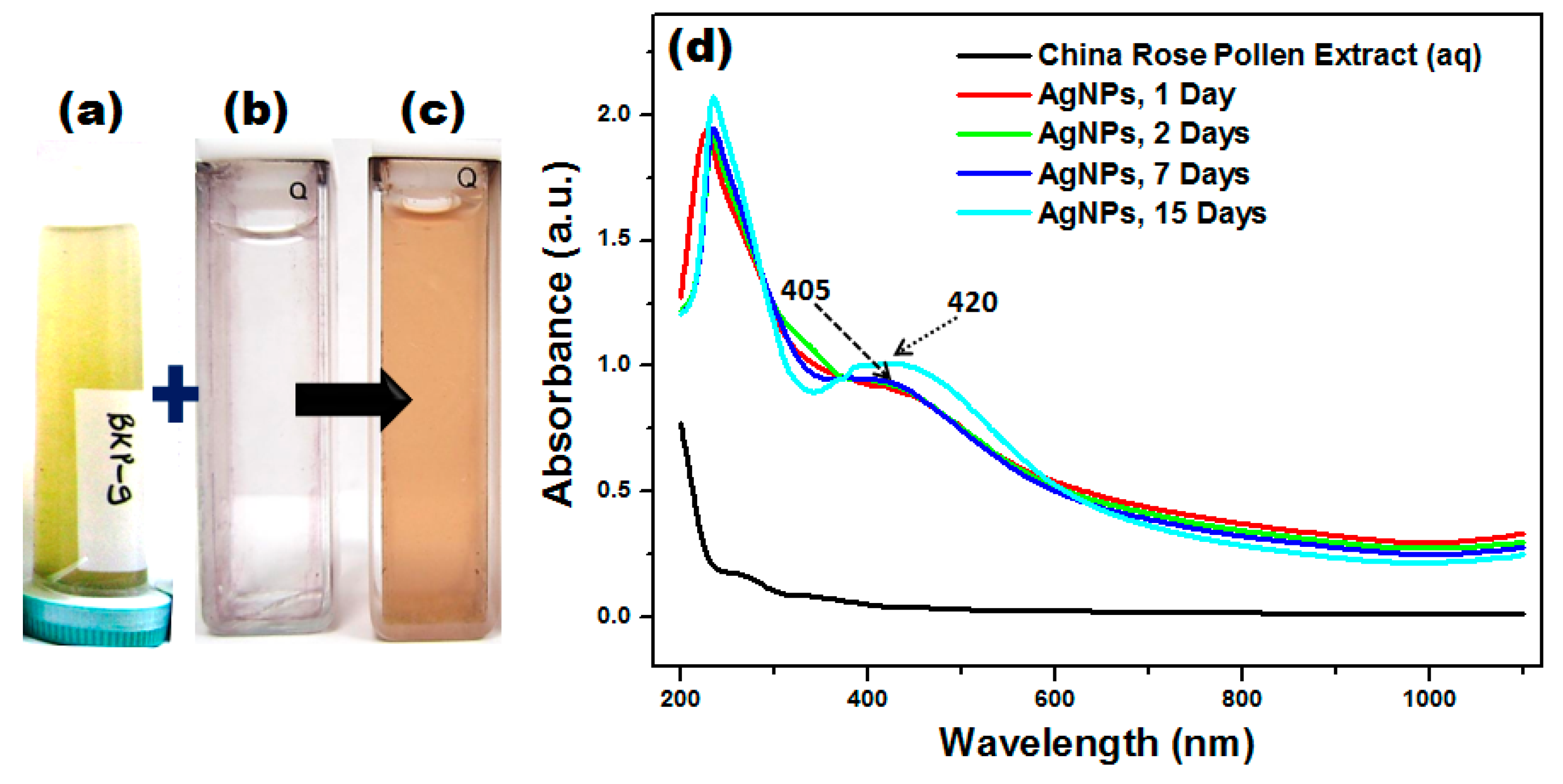
3.3. Visual and UV–Vis Studies of AgNPs Synthesized by H. rosa-sinensis Pollen
3.4. DLS Studies of AgNPs
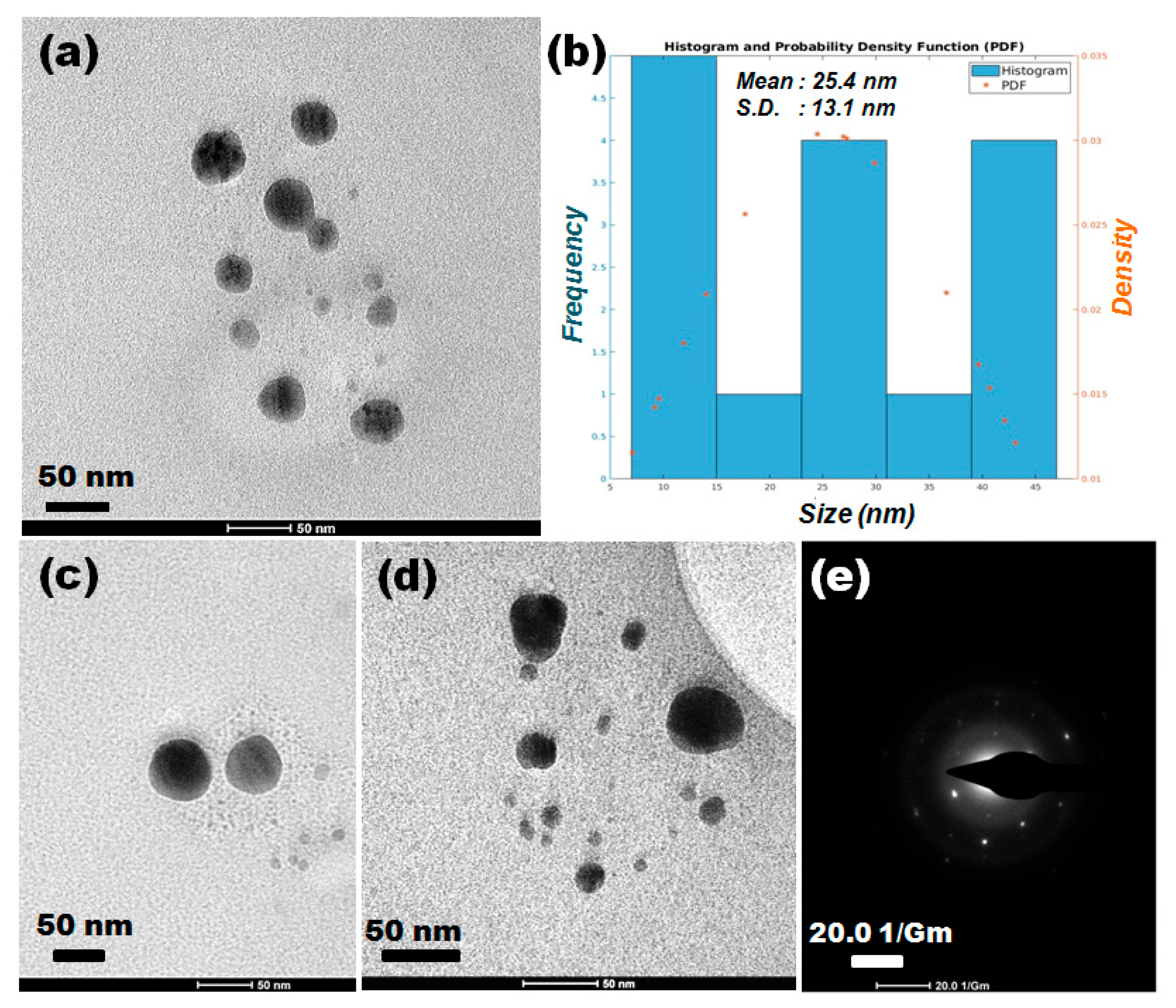
3.5. TEM–SAED Studies of AgNPs
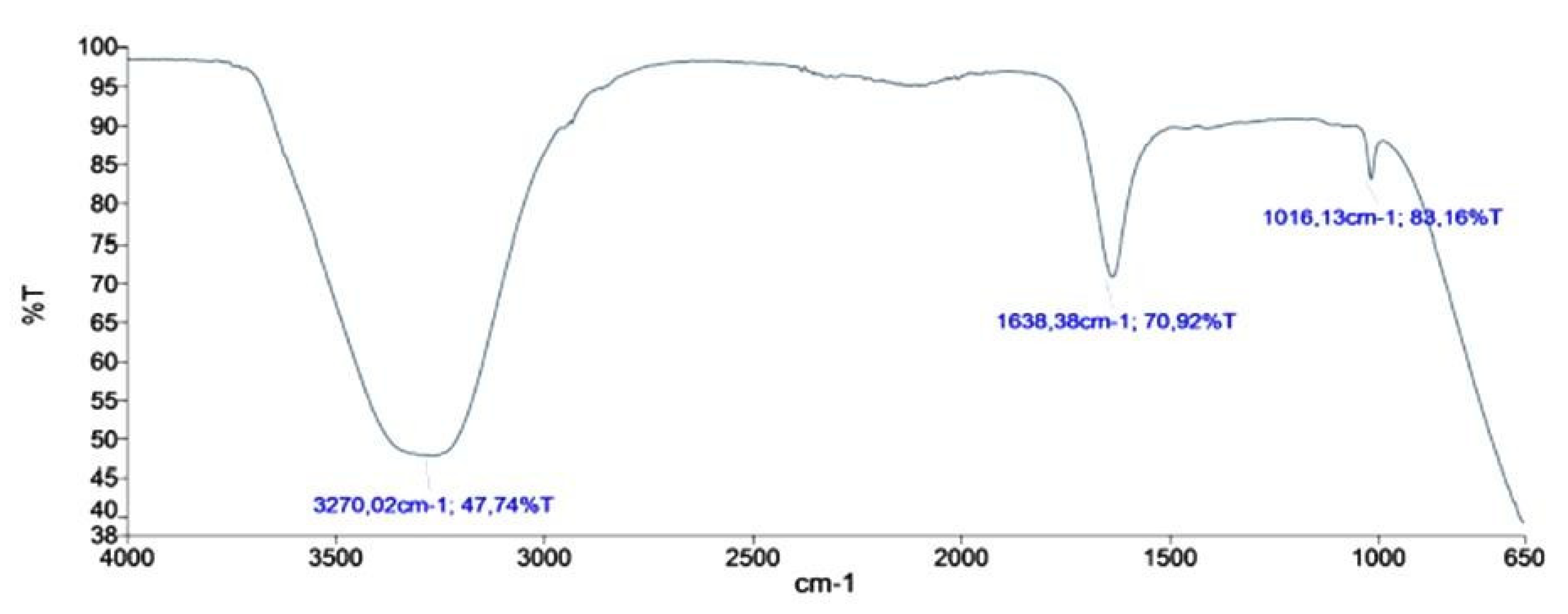
3.6. FTIR Studies of AgNPs
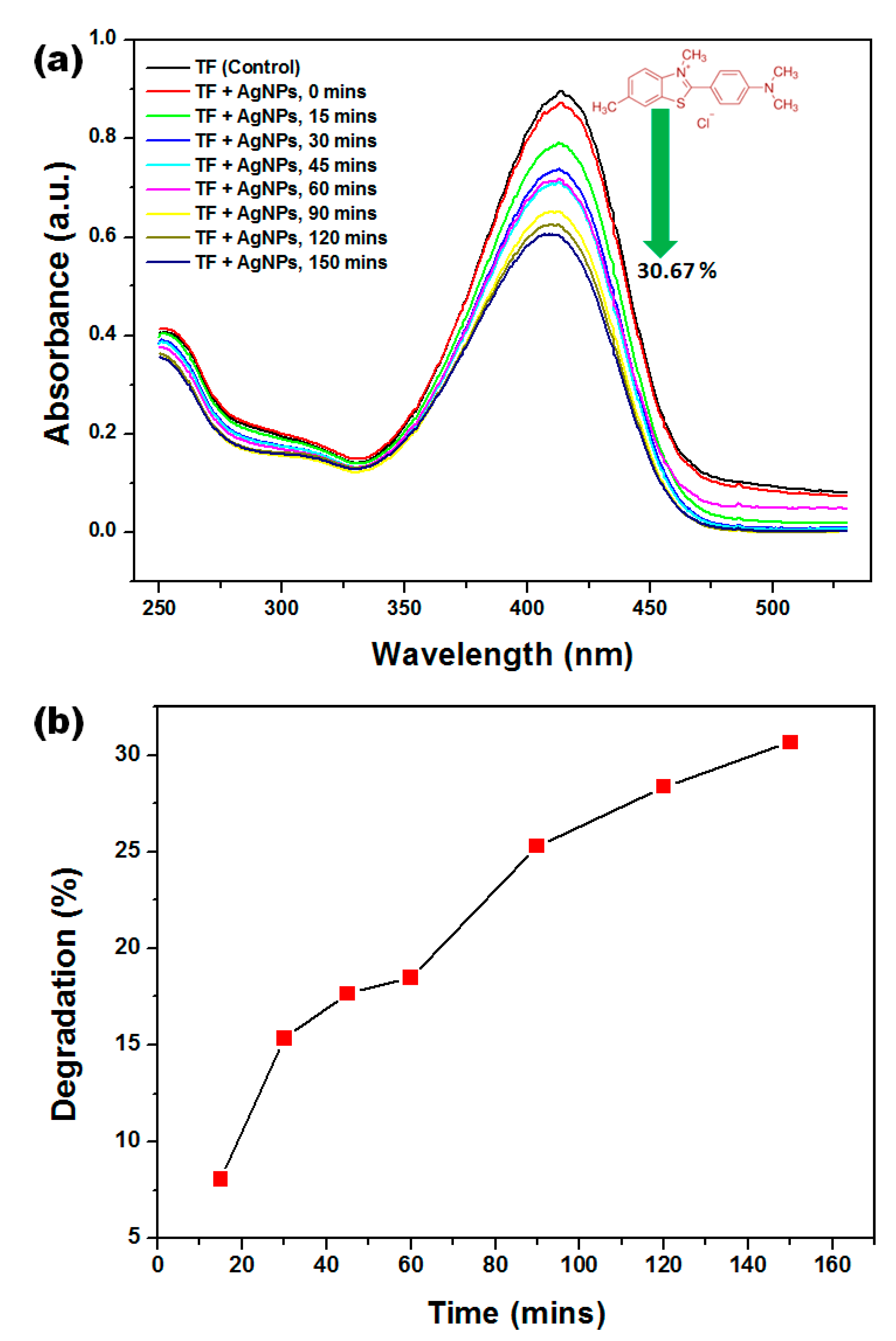
3.7. Photocatalytic Degradation Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abdullah, H.S.T.S.; Asseri, S.N.A.R.M.; Mohamad, W.N.K.W.; Kan, S.-Y.; Azmi, A.A.; Julius, F.S.Y.; Chia, P.W. Green synthesis, characterization and applications of silver nanoparticle mediated by the aqueous extract of red onion peel. Environ. Pollut. 2021, 271, 116295. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L. Biosynthesis of silver nanoparticles using Lantana camara flower extract and its application. J. Sol-Gel. Sci. Technol. 2016, 78, 285–292. [Google Scholar] [CrossRef]
- Kanniah, P.; Radhamani, J.; Chelliah, P.; Muthusamy, N.; Thangapandi, E.J.J.S.B.; Thangapandi, J.R.; Balakrishnan, S.; Shanmugam, R. Green Synthesis of multifaceted silver nanoparticles using the flower extract of Aerva lanata and evaluation of its biological and environmental applications. Chem. Sel. 2020, 5, 2322–2331. [Google Scholar] [CrossRef]
- Arroyo, G.V.; Madrid, A.T.; Gavilanes, A.F.; Naranjo, B.; Debut, A.; Arias, M.T.; Angulo, Y. Green synthesis of silver nanoparticles for application in cosmetics. J. Environ. Sci. Health Part A 2020, 55, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Awasthi, S.K.; Debut, A.; Cumbal, L. Capsicum baccatum (Andean Chilli)-assisted phytosynthesis of silver nanoparticles and their H2O2 sensing ability. Part. Sci. Technol. 2022, 40, 772–780. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mudila, H.; Gupta, G.; Sharma, A.K.; Kumar, D.; Bakshi, H.A.; Negi, P.; Kapoor, D.N.; Chellappan, D.K.; et al. Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloid Interface Sci. Commun. 2020, 35, 100244. [Google Scholar] [CrossRef]
- Istiqola, A.; Syafiuddin, A. A review of silver nanoparticles in food packaging technologies: Regulation, methods, properties, migration, and future challenges. J. Chin. Chem. Soc. 2020, 57, 1942–1956. [Google Scholar] [CrossRef]
- Yan, N.; Tang, B.Z.; Wang, W.-X. In Vivo bioimaging of silver nanoparticle dissolution in the gut environment of zooplankton. ACS Nano 2018, 12, 12212–12223. [Google Scholar] [CrossRef]
- Kalfagiannis, N.; Karagiannidis, P.G.; Pitsalidis, C.; Panagiotopoulos, N.T.; Gravalidis, C.; Kassavetis, S.; Patsalas, P.; Logothetidis, S. Plasmonic silver nanoparticles for improved organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 104, 165–174. [Google Scholar] [CrossRef]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), A review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Guzmán, K.; Kumar, B.; Vallejo, M.J.; Grijalva, M.; Debut, A.; Cumbal, L. Ultrasound-assisted synthesis and antibacterial activity of gallic acid-chitosan modified silver nanoparticles. Prog. Org. Coat. 2019, 129, 229–235. [Google Scholar] [CrossRef]
- Mahltig, B.; Gutmann, E.; Reibold, M.; Meyer, D.; Bottcher, H. Synthesis of Ag and Ag/SiO2 sols by solvothermal method and their bactericidal activity. J. Sol-Gel. Sci. Technol. 2009, 51, 204–214. [Google Scholar] [CrossRef]
- Tarimala, S.; Kothari, N.; Abidi, N.; Hequet, E.; Fralick, J.; Dai, L.L. New approach to antibacterial treatment of cotton fabric with silver nanoparticle–doped silica using sol–gel process. J. Appl. Polym. Sci. 2006, 101, 2938–2943. [Google Scholar] [CrossRef]
- Ma, H.; Yin, B.; Wang, S.; Jiao, Y.; Pan, W.; Huang, S.; Chen, S.; Meng, F. Synthesis of silver and gold nanoparticles by a novel electrochemical method. Chem. Phys. Chem. 2004, 24, 68–75. [Google Scholar] [CrossRef]
- Soukupova, J.; Kvitek, L.; Panacek, A.; Nevecna, T.; Zboril, R. Comprehensive study on surfactant role on silver nanoparticles (NPs) prepared via modified Tollens process. Mater. Chem. Phys. 2008, 111, 77–81. [Google Scholar] [CrossRef]
- Aisida, S.O.; Ugwoke, E.; Uwais, A.; Iroegbu, C.; Botha, S.; Ahmad, I.; Maaza, M.; Ezema, F.I. Incubation period induced biogenic synthesis of PEG enhanced Moringa oleifera silver nanocapsules and its antibacterial activity. J. Polym. Res. 2019, 26, 225. [Google Scholar] [CrossRef]
- Jahan, I.; Erci, F.; Isildak, I. Microwave-assisted green synthesis of non-cytotoxic silver nanoparticles using the aqueous extract of Rosa santana (rose) petals and their antimicrobial activity. Anal. Lett. 2019, 52, 1860–1873. [Google Scholar] [CrossRef]
- Babu, S.A.; Prabu, H.G. Synthesis of AgNPs using the extract of Calotropis procera flower at room temperature. Mater. Lett. 2011, 65, 1675–1677. [Google Scholar] [CrossRef]
- Ameen, F.; Srinivasan, P.; Selvankumar, T.; Kamala-Kannan, S.; Al Nadhari, S.; Almansob, A.; Dawoud, T.; Govarthanan, M. Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg. Chem. 2019, 88, 102970. [Google Scholar] [CrossRef]
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 2019, 158, 8–14. [Google Scholar] [CrossRef]
- Gogoi, N.; Babu, P.J.; Mahanta, C.; Bora, U. Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater. Sci. Eng. C 2015, 46, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, S.; Kandasamy, S.; Arumugam, S.; Seralathan, K.K.; Thangaswamy, S.; Muthusamy, G. Biomimetic synthesis of silver nanoparticles using flower extract of Bauhinia purpurea and its antibacterial activity against clinical pathogens. Environ. Sci. Pollut. Res. 2018, 25, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kaliammal, R.; Parvathy, G.; Maheshwaran, G.; Velsankar, K.; Devi, V.K.; Krishnakumar, M.; Sudhahar, S. Zephyranthes candida flower extract mediated green synthesis of silver nanoparticles for biological applications. Adv. Powder Technol. 2021, 32, 4408–4419. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Nayak, B. Biosynthesis, characterisation and antimicrobial activity of silver nanoparticles using Hibiscus rosa-sinensis petals extracts. IET Nanobiotechnol. 2015, 9, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Andrade, K.; Guerra, S.; Debut, A. Fullerene-Based Symmetry in Hibiscus rosa-sinensis Pollen. PLoS ONE 2014, 9, e102123. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Jegadeesan, U.; Sundaramoorthi, K.; Rajeswari, T.; Tokala, V.N.B.; Bhattacharya, S.; Muthusamy, S.; Sankoh, M.; Nellore, M.K. Comparative analysis of synthesis and characterization of silver nanoparticles extracted using leaf, flower, and bark of Hibiscus rosa sinensis and examine its antimicrobicidal activity. J. Nanomater. 2022, 2022, 8123854. [Google Scholar] [CrossRef]
- Kainat; Khan, M.A.; Ali, F.; Faisal, S.; Rizwan, M.; Hussain, Z.; Zaman, N.; Afsheen, Z.; Uddin, M.N.; Bibi, N. Exploring the therapeutic potential of Hibiscus rosa sinensis synthesized cobalt oxide (Co3O4-NPs) and magnesium oxide nanoparticles (MgO-NPs). Saudi J. Biol. Sci. 2021, 28, 5157–5167. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Angulo, Y.; Debut, A.; Cumbal, L. Single-step biogenic synthesis of silver nanoparticles using honeybee- collected pollen. Inorg. Nano-Met. Chem. 2022, in press. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Debut, A.; Cumbal, L. Green synthesis of cuprous oxide nanoparticles using Andean Capuli (Prunus serotina Ehrh. var. Capuli) Cherry. J. Clust. Sci. 2021, 32, 1753–1760. [Google Scholar] [CrossRef]
- Darweesh, M.A.; Elgendy, M.Y.; Ayad, M.I.; Ahmed, A.M.M.; Elsayed, N.M.K.; Hammad, W.A. A unique, inexpensive, and abundantly available adsorbent: Composite of synthesized silver nanoparticles (AgNPs) and banana leaves powder (BLP). Heliyon 2022, 8, e09279. [Google Scholar] [CrossRef]
- Atalay, F.E.; Bingol, A.; Kaya, H.; Emre, Y.; Bas, H.H.; Culum, A.A. Juglans Sporopollenin for High-Performance Supercapacitor Electrode Design. ACS Omega 2020, 5, 20417–20427. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, K.S.; Guzman, K.; Kumar, B.; Debut, A.; Cumbal, L. Shora (Capparis petiolaris) fruit mediated green synthesis and application of silver nanoparticles. Green Process Synth 2017, 6, 23–30. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Remanan, S.; Das, N.C. Temperature-dependent study of catalytic Ag nanoparticles entrapped resin nanocomposite towards reduction of 4-nitrophenol. ChemistrySelect 2019, 4, 3665–3671. [Google Scholar]
- Das, T.K.; Ganguly, S.; Remanan, S.; Ghosh, S.; Das, N.C. Mussel-inspired Ag/poly(norepinephrine)/MnO2 heterogeneous nanocatalyst for efficient reduction of 4-nitrophenol and 4-nitroaniline: An alternative approach. Res. Chem. Intermed. 2020, 46, 3629–3650. [Google Scholar] [CrossRef]
- Fodera, V.; Groenning, M.; Vetri, V.; Librizzi, F.; Spagnolo, S.; Cornett, C.; Olsen, L.; Van de Weert, M.; Leone, M. Thioflavin T hydroxylation at basic ph and its effect on amyloid fibril detection. J. Phys. Chem. B 2008, 112, 15174–15181. [Google Scholar] [CrossRef]
- Al-Maqdi, K.A.; Hisaindee, S.M.; Rauf, M.A.; Ashraf, S.S. Comparative degradation of a thiazole pollutant by an advanced oxidation process and an enzymatic approach. Biomolecules 2017, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B. Green Synthesis of Gold, Silver, and Iron nanoparticles for the degradation of organic pollutants in wastewater. J. Compos. Sci. 2021, 5, 219. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, B.; Smita, K.; Angulo, Y.; Debut, A.; Cumbal, L. China Rose/Hibiscus rosa-sinensis Pollen-Mediated Phytosynthesis of Silver Nanoparticles and Their Catalytic Activity. J. Compos. Sci. 2022, 6, 322. https://doi.org/10.3390/jcs6110322
Kumar B, Smita K, Angulo Y, Debut A, Cumbal L. China Rose/Hibiscus rosa-sinensis Pollen-Mediated Phytosynthesis of Silver Nanoparticles and Their Catalytic Activity. Journal of Composites Science. 2022; 6(11):322. https://doi.org/10.3390/jcs6110322
Chicago/Turabian StyleKumar, Brajesh, Kumari Smita, Yolanda Angulo, Alexis Debut, and Luis Cumbal. 2022. "China Rose/Hibiscus rosa-sinensis Pollen-Mediated Phytosynthesis of Silver Nanoparticles and Their Catalytic Activity" Journal of Composites Science 6, no. 11: 322. https://doi.org/10.3390/jcs6110322






