A Better Understanding of the SBA-15 Pores Filling through Textural Changes in CMK-3 Carbon Synthesis and Its CO2:CH4 Adsorption Selectivity
Abstract
:1. Introduction
2. Materials and Methods
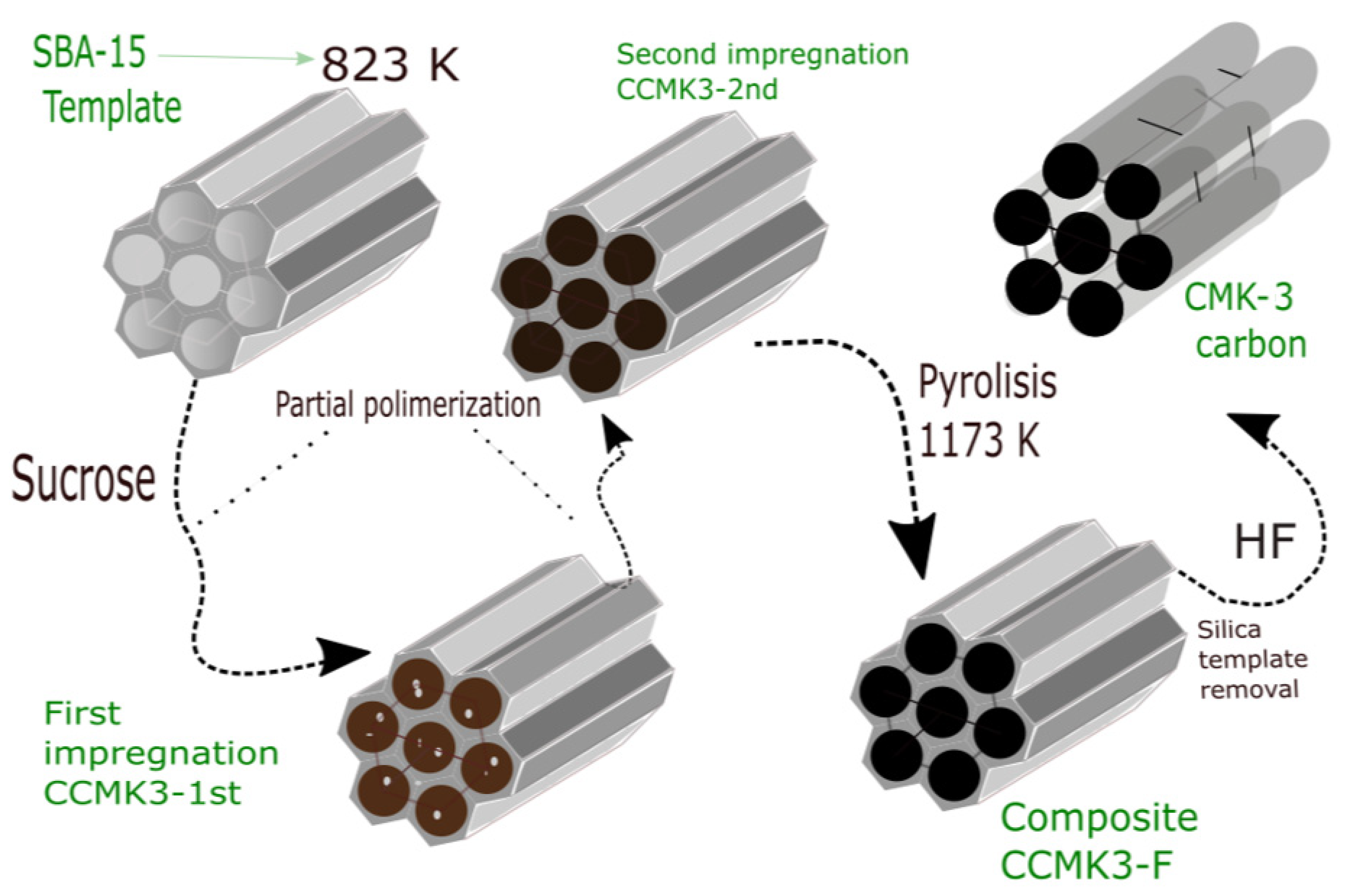
2.1. SBA-15 and CMK-3 Carbon Synthesis
2.2. Morphological, Textural, and Structural Characterizations
2.3. Evaluation of CO2 and CH4 Adsorption
3. Results and Discussions
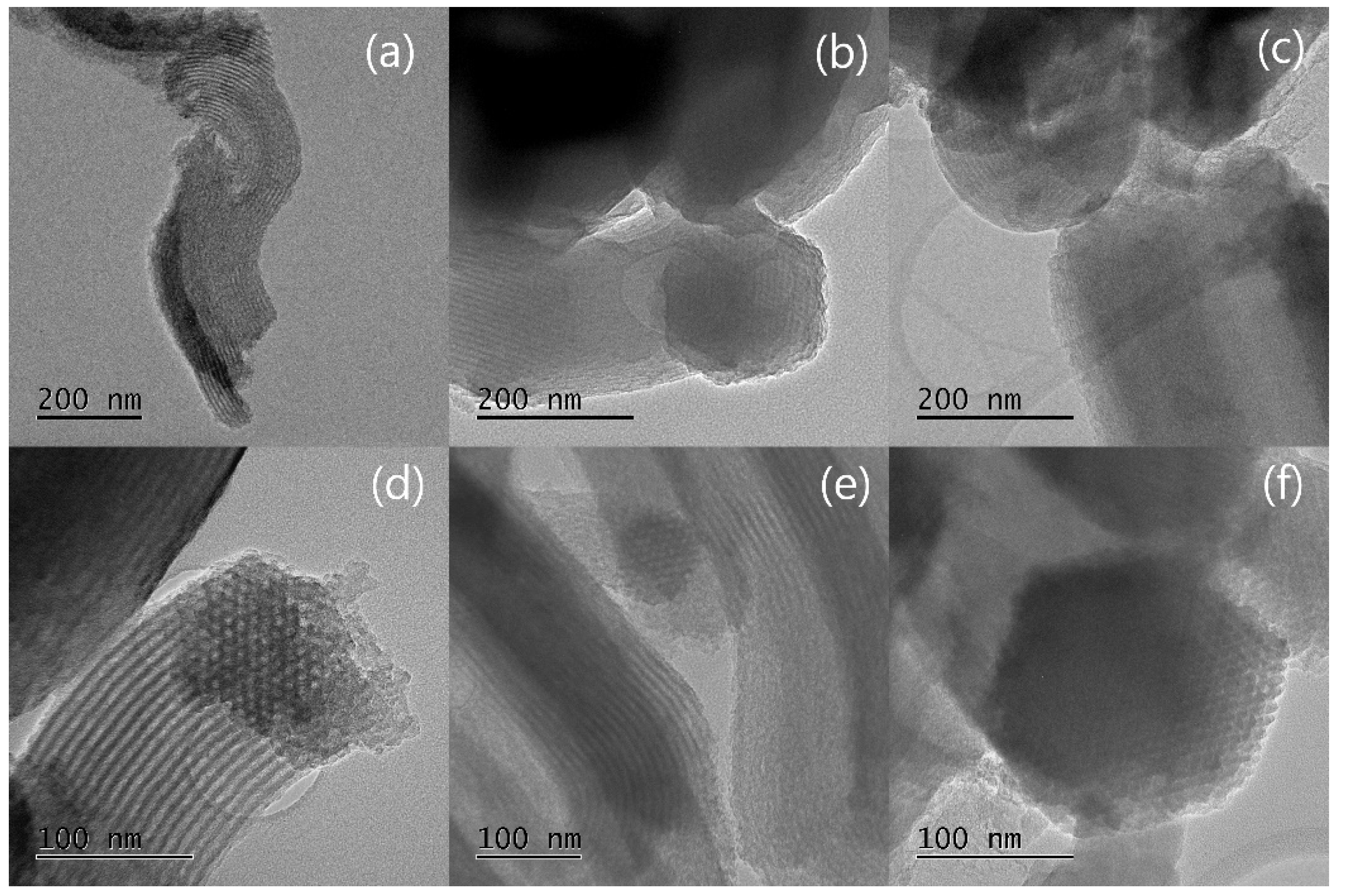
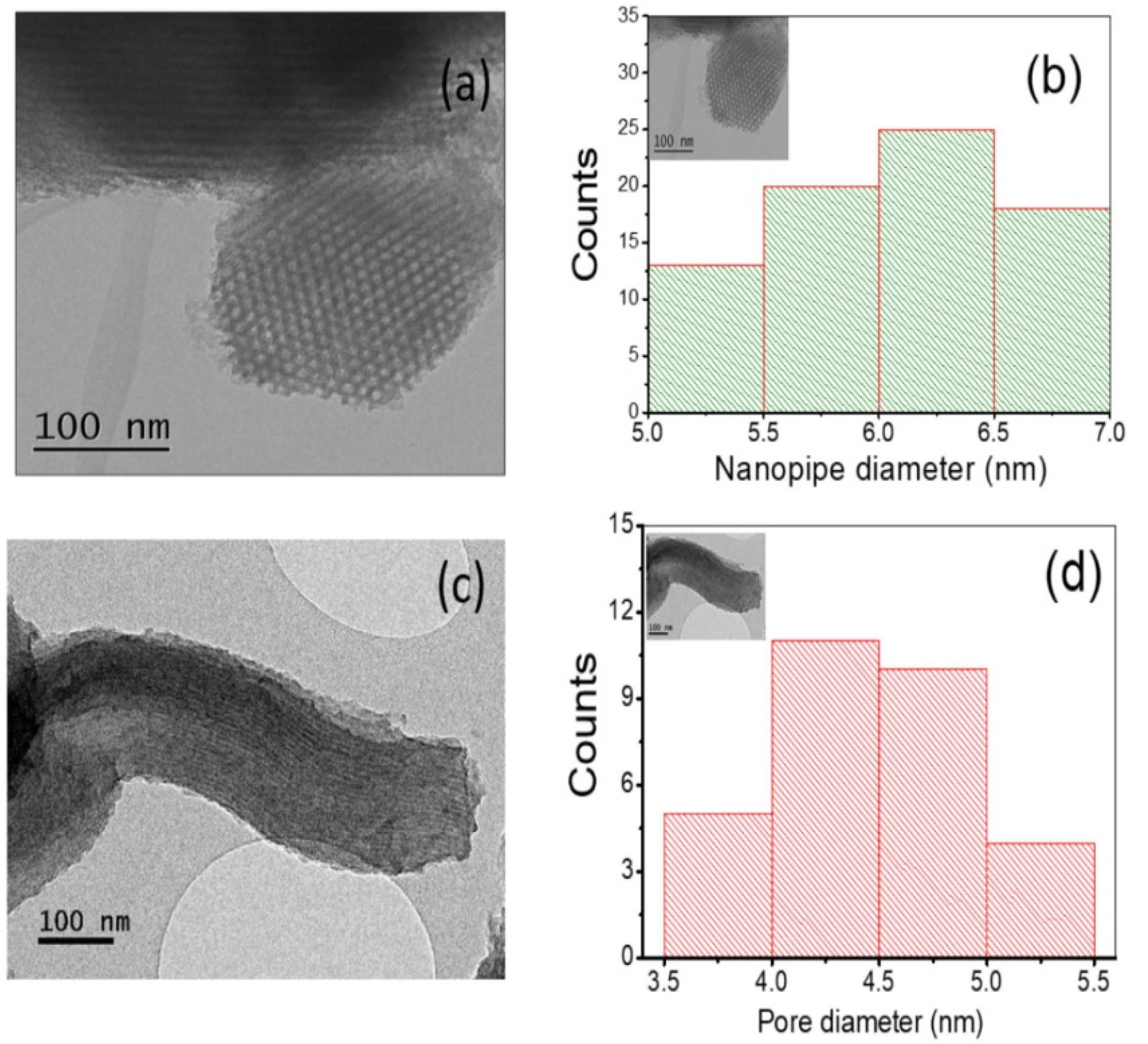
3.1. Morphological Sequence in the CMK-3 Synthesis Stages
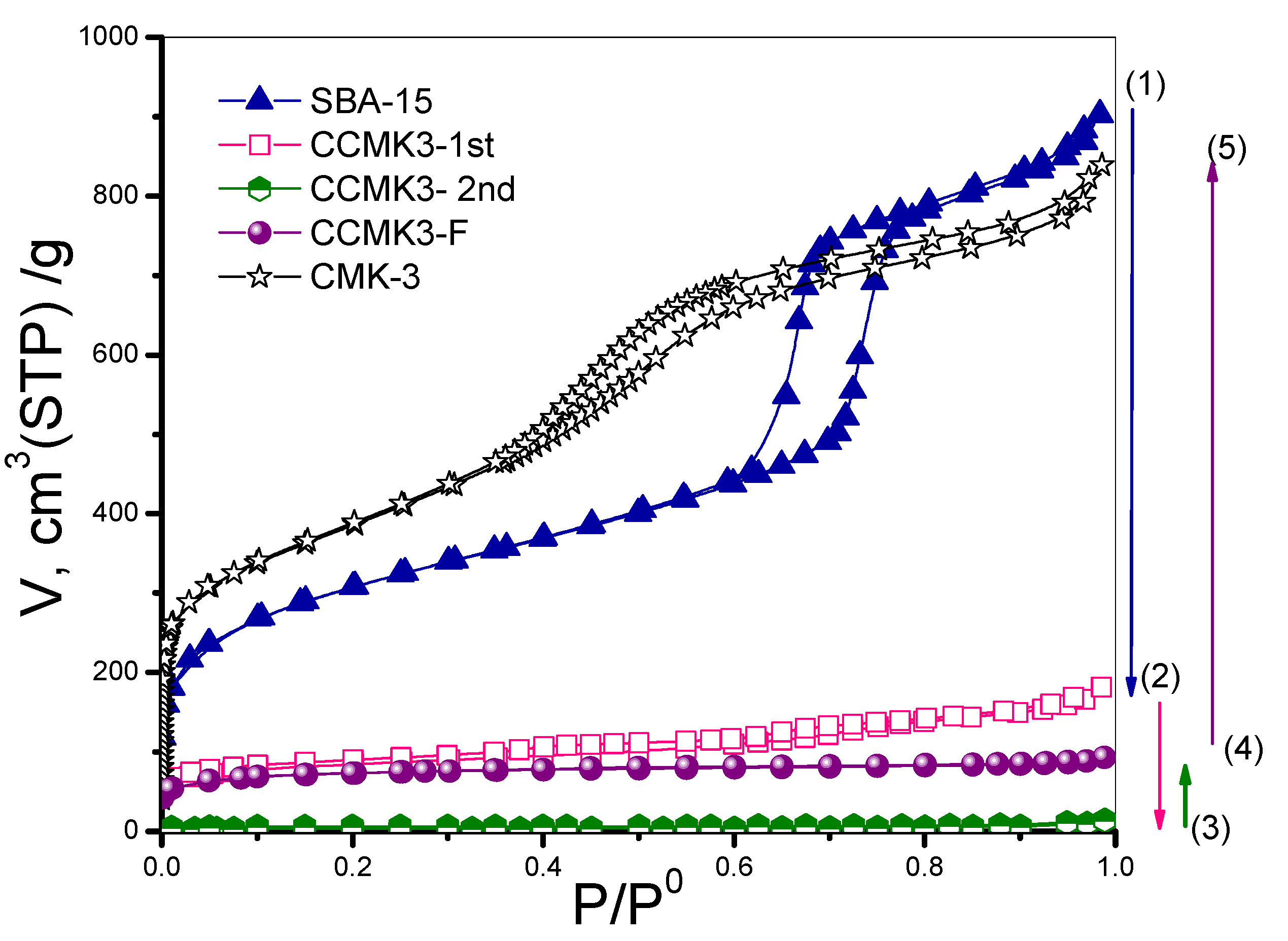
3.2. Textural Behavior for Each Synthesis Stage
Pore Size Distribution
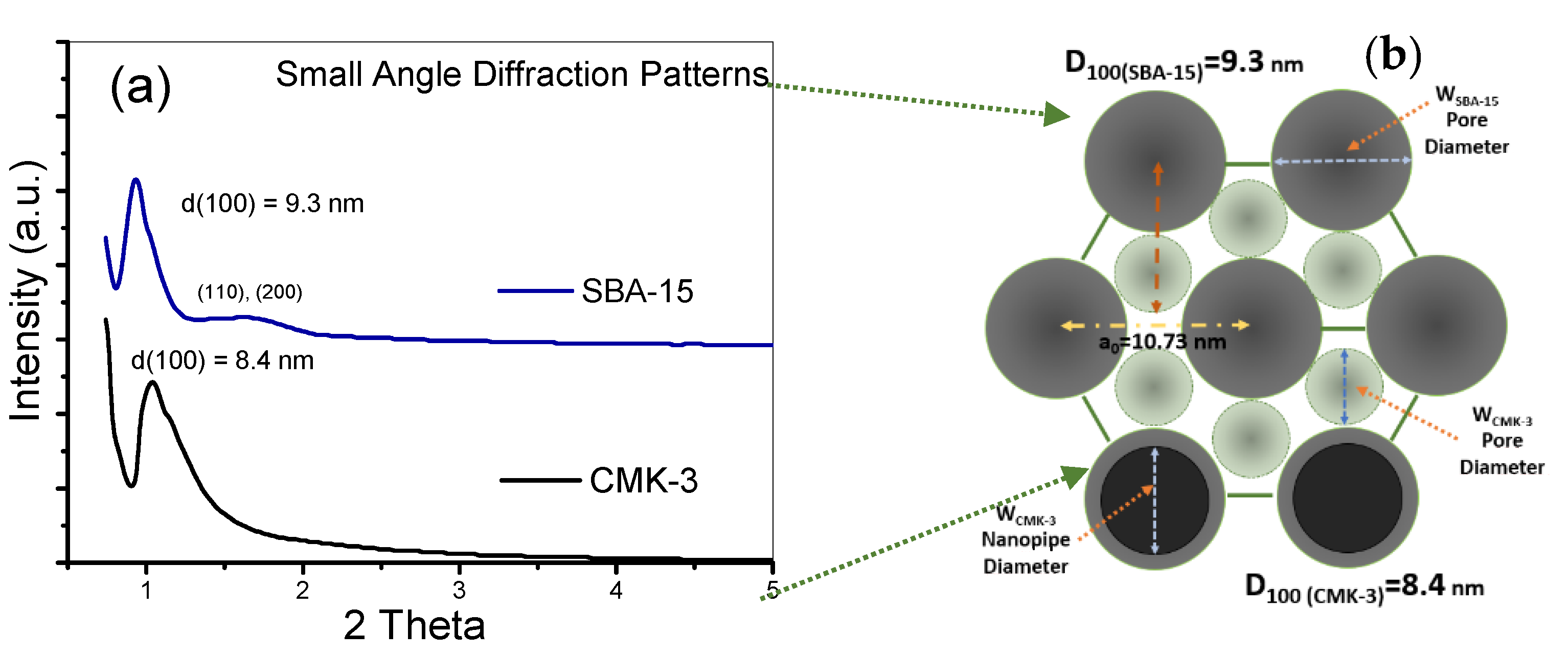
3.3. Additional Structural Characterization of SBA-15 Template, and CMK-3 Mesoporous Carbon
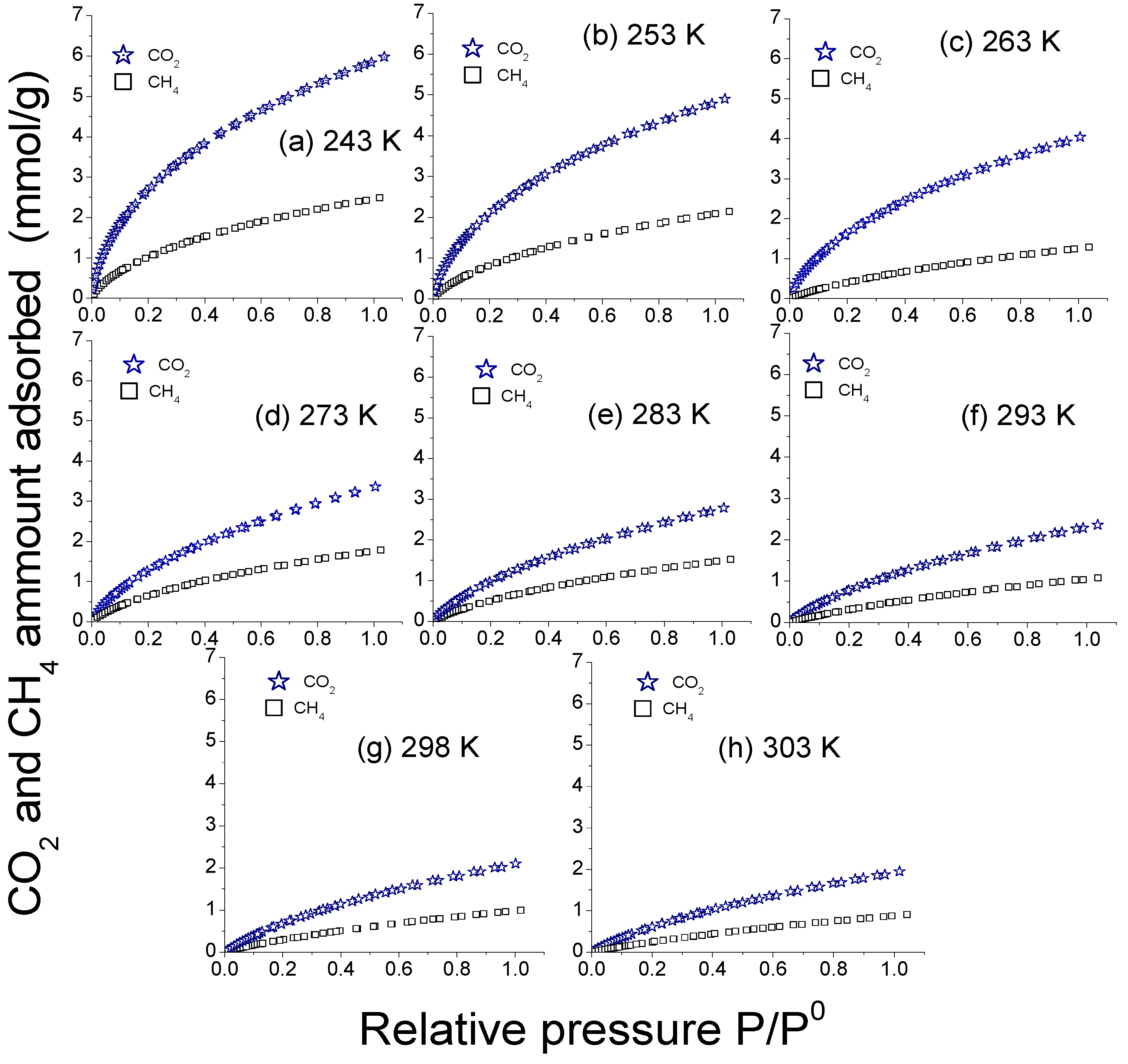
3.4. Evaluation of the CO2 and CH4 Adsorption
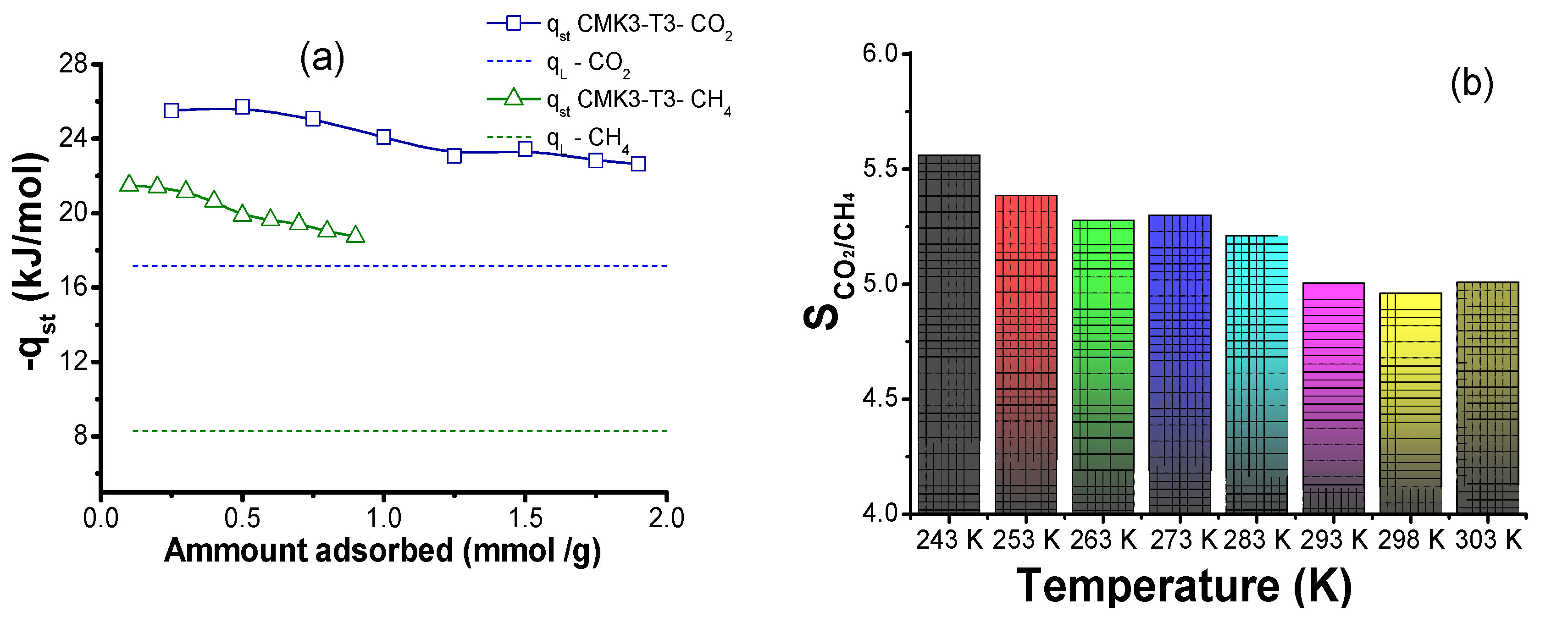
CO2 and CH4 Adsorption Selectivity and Isosteric Enthalpy Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marcos-Hernández, M.; Villagrán, D. 11—Mesoporous Composite Nanomaterials for Dye Removal and Other Applications. In Micro and Nano Technologies; Kyzas, G.Z., Mitropoulos, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–293. ISBN 978-0-12-814132-8. [Google Scholar]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Yuan, H.; Jin, B.; Meng, L.Y. Effect of the SBA-15 template and KOH activation method on CO2 adsorption by N-doped polypyrrole-based porous carbons. Carbon Lett. 2018, 28, 116–120. [Google Scholar] [CrossRef]
- Venosta, L.F.; Juárez, J.M.; Anunziata, O.A.; Bercoff, P.G.; Gómez Costa, M.B. Synthesis and characteristics of CMK-3 modified with magnetite nanoparticles for application in hydrogen storage. J. Nanopart. Res. 2020, 22, 227. [Google Scholar] [CrossRef]
- Montiel-Centeno, K.; Barrera, D.; Villarroel-Rocha, J.; Arroyo-Gómez, J.J.; Moreno, M.S.; Sapag, K. CMK-3 nanostructured carbon: Effect of temperature and time carbonization on textural properties and H2 storage. Chem. Eng. Commun. 2019, 206, 1581–1595. [Google Scholar] [CrossRef]
- Jarczewski, S.; Drozdek, M.; Michorczyk, P.; Cuadrado-Collados, C.; Gandara-Loe, J.; Silvestre-Albero, J.; Kuśtrowski, P. Oxidative dehydrogenation of ethylbenzene over CMK-1 and CMK-3 carbon replicas with various mesopore architectures. Microporous Mesoporous Mater. 2018, 271, 262–272. [Google Scholar] [CrossRef]
- Janus, P.; Janus, R.; Dudek, B.; Drozdek, M.; Silvestre-Albero, A.; Rodríguez-Reinoso, F.; Kuśtrowski, P. On mechanism of formation of SBA-15/furfuryl alcohol-derived mesoporous carbon replicas and its relationship with catalytic activity in oxidative dehydrogenation of ethylbenzene. Microporous Mesoporous Mater. 2020, 299, 110118. [Google Scholar] [CrossRef]
- Yun, L.; Gao, Z.; Cheng, X.; Li, P.; Wang, L.; Guo, N.; Luo, C.; Zhu, X.; Liu, B.; Wu, D.; et al. Effect of peroxydisulfate oxidation catalyzed with ordered mesoporous carbons on controlling ultrafiltration membrane fouling by algal organic matter. Chemosphere 2022, 303, 135037. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. CO2 adsorption by activated templated carbons. J. Colloid Interface Sci. 2012, 366, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.-C.; Jin, Z.; Lu, W.; Sun, Z.; Alemany, L.B.; Lomeda, J.R.; Tour, J.M. In situ Synthesis of Polymer-Modified Mesoporous Carbon CMK-3 Composites for CO2 Sequestration. ACS Appl. Mater. Interfaces 2011, 3, 4782–4786. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of New, Nanoporous Carbon with Hexagonally Ordered Mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, X.; Shao, Y.; Sun, K.; Jia, P.; Zhang, L.; Liu, Q.; Wang, Y.; Hu, S.; Xiang, J. Structural differences of the soluble oligomers and insoluble polymers from acid-catalyzed conversion of sugars with varied structures. Carbohydr. Polym. 2019, 216, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Solovyov, L.A.; Shmakov, A.N.; Zaikovskii, V.I.; Joo, S.H.; Ryoo, R. Detailed structure of the hexagonally packed mesostructured carbon material CMK-3. Carbon 2002, 40, 2477–2481. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.; Dai, S. Nano/Microporous Materials: Mesoporous and Surface-Functionalized Mesoporous Carbon. Encycl. Inorg. Chem. 2009.
- Olchowski, R.; Dobrowolski, R. Synthesis, properties and applications of CMK-3-type ordered mesoporous carbons. Ann. Univ. Mariae Curie-Sklodowska Sect. AA–Chem. 2018, 73, 11–30. [Google Scholar] [CrossRef]
- Zhou, J.; Su, W.; Sun, Y.; Deng, S.; Wang, X. Enhanced CO2 Sorption on Ordered Mesoporous Carbon CMK-3 in the Presence of Water. J. Chem. Eng. Data 2016, 61, 1348–1352. [Google Scholar] [CrossRef]
- Lakhi, K.S.; Cha, W.S.; Choy, J.-H.; Al-Ejji, M.; Abdullah, A.M.; Al-Enizi, A.M.; Vinu, A. Synthesis of mesoporous carbons with controlled morphology and pore diameters from SBA-15 prepared through the microwave-assisted process and their CO2 adsorption capacity. Microporous Mesoporous Mater. 2016, 233, 44–52. [Google Scholar] [CrossRef]
- Ojeda-López, R.; Vilarrasa-García, E.; Azevedo, D.C.; Felipe, C.; Cecilia, J.A.; Rodríguez-Castellón, E. CO2 selectivity in CO2:CH4 and CO2:N2 mixtures on carbon microfibers (CMFs) and carbon microspheres (CMSs). Fuel 2022, 324, 124242. [Google Scholar] [CrossRef]
- Varghese, A.M.; Karanikolos, G.N. CO2 capture adsorbents functionalized by amine—Bearing polymers: A review. Int. J. Greenh. Gas Control 2020, 96, 103005. [Google Scholar] [CrossRef]
- Alonso, A.; Moral-Vico, J.; Abo Markeb, A.; Busquets-Fité, M.; Komilis, D.; Puntes, V.; Sánchez, A.; Font, X. Critical review of existing nanomaterial adsorbents to capture carbon dioxide and methane. Sci. Total Environ. 2017, 595, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Ojeda-López, R.; Pérez-Hermosillo, I.J.; Marcos Esparza-Schulz, J.; Cervantes-Uribe, A.; Domínguez-Ortiz, A. SBA-15 materials: Calcination temperature influence on textural properties and total silanol ratio. Adsorption 2015, 21, 659–669. [Google Scholar] [CrossRef]
- Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M. Evidence for General Nature of Pore Interconnectivity in 2-Dimensional Hexagonal Mesoporous Silicas Prepared Using Block Copolymer Templates. J. Phys. Chem. B 2002, 106, 4640–4646. [Google Scholar] [CrossRef]
- Esparza, J.M.; Ojeda, M.L.; Campero, A.; Domínguez, A.; Kornhauser, I.; Rojas, F.; Vidales, A.M.; López, R.H.; Zgrablich, G. N2 sorption scanning behavior of SBA-15 porous substrates. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 35–45. [Google Scholar] [CrossRef]
- Li, L.; Song, H.; Chen, X. Ordered mesoporous carbons from the carbonization of sulfuric-acid-treated silica/triblock copolymer/sucrose composites. Microporous Mesoporous Mater. 2006, 94, 9–14. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information Sucrose. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sucrose (accessed on 4 August 2022).
- Farzin Nejad, N.; Shams, E.; Amini, M.K.; Bennett, J.C. Ordered mesoporous carbon CMK-5 as a potential sorbent for fuel desulfurization: Application to the removal of dibenzothiophene and comparison with CMK-3. Microporous Mesoporous Mater. 2013, 168, 239–246. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Kim, T.-W.; Ryoo, R. Synthesis and Characterization of Hexagonally Ordered Carbon Nanopipes. Chem. Mater. 2003, 15, 2815–2823. [Google Scholar] [CrossRef]
- Espinal, L. Porosity and Its Measurement. In Characterization of Materials; Wiley Online Library: Hoboken, NJ, USA, 2012; pp. 1–10. ISBN 9780471266969. [Google Scholar]
- Smith, D.J. Chapter 1: Characterization of Nanomaterials Using Transmission Electron Microscopy. In Nanocharacterisation (2); The Royal Society of Chemistry: London, UK, 2015; pp. 1–29. ISBN 978-1-84973-805-7. [Google Scholar]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-08-097035-6. [Google Scholar]
- Barrera, D.; Dávila, M.; Cornette, V.; de Oliveira, J.C.A.; López, R.H.; Sapag, K. Pore size distribution of ordered nanostructured carbon CMK-3 by means of experimental techniques and Monte Carlo simulations. Microporous Mesoporous Mater. 2013, 180, 71–78. [Google Scholar] [CrossRef]
- Yu, C.; Fan, J.; Tian, B.; Zhao, D.; Stucky, G.D. High-Yield Synthesis of Periodic Mesoporous Silica Rods and Their Replication to Mesoporous Carbon Rods. Adv. Mater. 2002, 14, 1742–1745. [Google Scholar] [CrossRef]
- Hiraide, S.; Yamada, M.; Kataoka, S.; Inagi, Y.; Endo, A. Time evolution of the framework structure of SBA-15 during the aging process. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123807. [Google Scholar] [CrossRef]
- Aguilar García, E.; Cruz, M.S.; Madeira, H.Y.; Huesca, R.H.; Cruz, M.A.P. Synthesis of Fe Catalysts Doped in SBA-15 by EISA Method: Characterization and Catalytic Studies in 2-Propanol Decomposition. Kinet. Catal. 2021, 62, S38–S47. [Google Scholar] [CrossRef]
- Barrera, D.; Villarroel-Rocha, J.; Marenco, L.; Oliva, M.I.; Sapag, K. Non-Hydrothermal Synthesis of Cylindrical Mesoporous Materials: Influence of the Surfactant/Silica Molar Ratio. Adsorpt. Sci. Technol. 2011, 29, 975–988. [Google Scholar] [CrossRef]
- Santos, S.M.L.; Cecilia, J.A.; Vilarrasa-García, E.; Silva Junior, I.J.; Rodríguez-Castellón, E.; Azevedo, D.C.S. The effect of structure modifying agents in the SBA-15 for its application in the biomolecules adsorption. Microporous Mesoporous Mater. 2016, 232, 53–64. [Google Scholar] [CrossRef]
- Chen, H.; Dou, B.; Song, Y.; Xu, Y.; Zhang, Y.; Wang, C.; Zhang, X.; Tan, C. Pyrolysis characteristics of sucrose biomass in a tubular reactor and a thermogravimetric analysis. Fuel 2012, 95, 425–430. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M. Physical adsorption characterization of ordered and amorphous mesoporous materials. In Nanoporous Materials: Science and Engineering; Series on Chemical Engineering; World Scientific: Singapore, 2004; Volume 4, pp. 317–364. ISBN 978-1-86094-210-5. [Google Scholar]
- Thommes, M.; Morell, J.; Cychosz, K.A.; Fröba, M. Combining Nitrogen, Argon, and Water Adsorption for Advanced Characterization of Ordered Mesoporous Carbons (CMKs) and Periodic Mesoporous Organosilicas (PMOs). Langmuir 2013, 29, 14893–14902. [Google Scholar] [CrossRef]
- Galarneau, A.; Villemot, F.; Rodriguez, J.; Fajula, F.; Coasne, B. Validity of the t-plot Method to Assess Microporosity in Hierarchical Micro/Mesoporous Materials. Langmuir 2014, 30, 13266–13274. [Google Scholar] [CrossRef]
- Lysenko, N.D.; Yaremov, P.S.; Shvets, A.V.; Il’in, V.G. Effect of the chemical and structural modification of CMK-3 mesoporous carbon molecular sieve on hydrogen adsorption. Theor. Exp. Chem. 2009, 45, 380–385. [Google Scholar] [CrossRef]
- Juárez, J.M.; Gómez Costa, M.B.; Anunziata, O.A. Synthesis and characterization of Pt-CMK-3 hybrid nanocomposite for hydrogen storage. Int. J. Energy Res. 2015, 39, 128–139. [Google Scholar] [CrossRef]
- Medina-Juárez, O.; García-Sánchez, M.Á.; Arellano-Sánchez, U.; Kornhauser-Straus, I.; Rojas-González, F. Optimal Surface Amino-Functionalization Following Thermo-Alkaline Treatment of Nanostructured Silica Adsorbents for Enhanced CO2 Adsorption. Materials 2016, 9, 898. [Google Scholar] [CrossRef] [Green Version]
- Vinu, A.; Srinivasu, P.; Takahashi, M.; Mori, T.; Balasubramanian, V.V.; Ariga, K. Controlling the textural parameters of mesoporous carbon materials. Microporous Mesoporous Mater. 2007, 100, 20–26. [Google Scholar] [CrossRef]
- Qiu, Y.; Huo, J.; Jia, F.; Shanks, B.H.; Li, W. N- and S-doped mesoporous carbon as metal-free cathode catalysts for direct biorenewable alcohol fuel cells. J. Mater. Chem. A 2016, 4, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Thu Hang, L.T.; Bich Thuy, H.T. Enhancement in Graphitization of Ordered Mesoporous Carbon by Assistance of Soybean Oil Surfactant. VNU J. Sci. Nat. Sci. Technol. 2020, 36, 17–23. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, X.; Chen, Y.; Huang, J.; Luo, H.; Deng, S. Adsorption of CO2, CH4, and N2 on Ordered mesoporous carbon: Approach for greenhouse gases capture and biogas upgrading. Environ. Sci. Technol. 2013, 47, 10. [Google Scholar] [CrossRef]
- Lakhi, K.S.; Baskar, A.V.; Zaidi, J.S.M.; Al-Deyab, S.S.; El-Newehy, M.; Choy, J.-H.; Vinu, A. Morphological control of mesoporous CN based hybrid materials and their excellent CO2 adsorption capacity. RSC Adv. 2015, 5, 40183–40192. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Rentschler, J.; Sethia, G.; Weinman, S.; Perrone, R.; Liu, K. Synthesis of T-type zeolite nanoparticles for the separation of CO2/N2 and CO2/CH4 by adsorption process. Chem. Eng. J. 2013, 230, 380–388. [Google Scholar] [CrossRef]
- Moura, P.A.S.; Bezerra, D.P.; Vilarrasa-Garcia, E.; Bastos-Neto, M.; Azevedo, D.C.S. Adsorption equilibria of CO2 and CH4 in cation-exchanged zeolites 13X. Adsorption 2016, 22, 71–80. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; De Weireld, G.; Sayari, A. Amine-Bearing Mesoporous Silica for CO2 and H2S Removal from Natural Gas and Biogas. Langmuir 2009, 25, 13275–13278. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Lee, J.Y.; Jang, H.T. Synthesis of mesoporous magnesium oxide: Its application to CO2 chemisorption. Int. J. Greenh. Gas Control 2010, 4, 51–56. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Enhanced CO2 capture in Fe3O4-graphene nanocomposite by physicochemical adsorption. J. Appl. Phys. 2014, 116, 64306. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Ramakrishna, V.R. The role of the isosteric heat of adsorption in the characterization of solid surfaces. Colloq. Int. du CNRS 1971, 201, 431. [Google Scholar]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids; McGraw-Hill Chemical Engineering Series; McGraw-Hill: New York, NY, USA, 1987; ISBN 9780070517998. [Google Scholar]



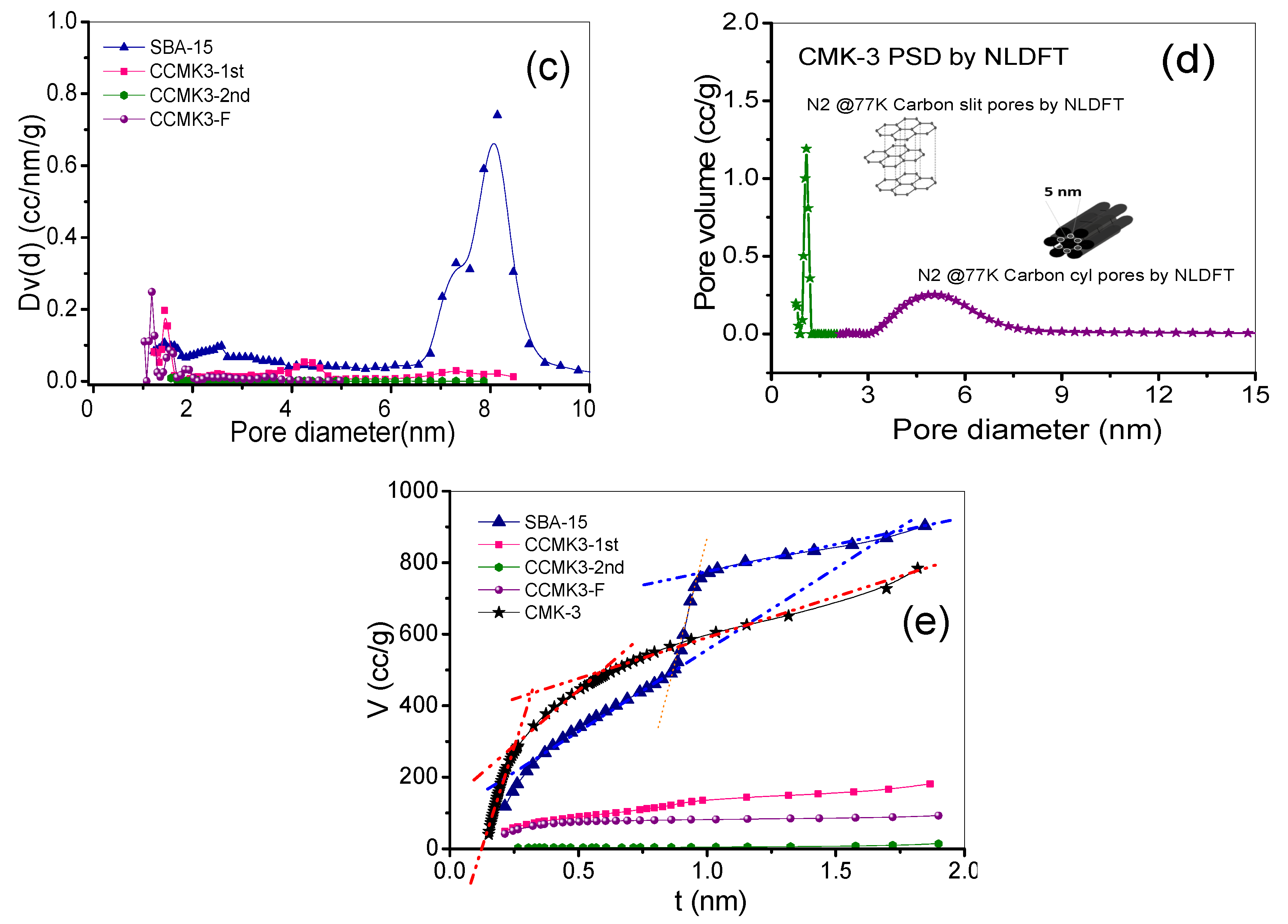

| SBA-15 | CCMK3-1st | CCMK3-2nd | CCMK3-F | CMK-3 | |
|---|---|---|---|---|---|
| AsBET (m2/g) | 1054 | 307 | 12.5 | 266.7 | 1350 |
| CBET | 502 | 844 | 128 | 1429 | 1121 |
| Vt (cm3/g) | 1.32 | 0.25 | 0.01 | 0.13 | 1.22 |
| Vmicro (cm3/g) | 0.12 | 0.08 | - | 0.09 | 0.16 |
| Vmeso | 1.20 | 0.17 | 0.01 | 0.04 | 1.06 |
| PSD BJH (nm) | 6.25 | 3.1, 5.7 | - | 3.4 | 3.4–3.7 |
| PSD DA (nm) | 1.7 | 1.4 | 1.7 | 1.4 | 1.4 |
| PSD NLDFT (nm) | 1.4, 8.1 | 1.4, 4.2 | 1.6 | 1.2 | 1.2, 5 |
| SBA-15 Mesopore Diameter (nm) | CMK-3 Mesopore Diameter (nm) | CMK-3 Nanopipe Diameter (nm) | |
|---|---|---|---|
| TEM Analysis (Histograms) | 6–8.5 | 3.5–5.5 | 5–7 |
| N2 Adsorption (NLDFT) | 8.1 | 5 | - |
| XRD data | 7.6 | 4.9 | 6.28 |
| Molecule Adsorbed | Kinetic Diameter, nm | Polarizability, 10−24 cm3 | Quadrupole, A3 |
|---|---|---|---|
| CO2 | 0.33 | 1.9 | 0.64 |
| CH4 | 0.38 | 2.6 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroz-Estrada, K.; Esparza-Schulz, M.; Felipe, C. A Better Understanding of the SBA-15 Pores Filling through Textural Changes in CMK-3 Carbon Synthesis and Its CO2:CH4 Adsorption Selectivity. J. Compos. Sci. 2022, 6, 344. https://doi.org/10.3390/jcs6110344
Quiroz-Estrada K, Esparza-Schulz M, Felipe C. A Better Understanding of the SBA-15 Pores Filling through Textural Changes in CMK-3 Carbon Synthesis and Its CO2:CH4 Adsorption Selectivity. Journal of Composites Science. 2022; 6(11):344. https://doi.org/10.3390/jcs6110344
Chicago/Turabian StyleQuiroz-Estrada, Karla, Marcos Esparza-Schulz, and Carlos Felipe. 2022. "A Better Understanding of the SBA-15 Pores Filling through Textural Changes in CMK-3 Carbon Synthesis and Its CO2:CH4 Adsorption Selectivity" Journal of Composites Science 6, no. 11: 344. https://doi.org/10.3390/jcs6110344
APA StyleQuiroz-Estrada, K., Esparza-Schulz, M., & Felipe, C. (2022). A Better Understanding of the SBA-15 Pores Filling through Textural Changes in CMK-3 Carbon Synthesis and Its CO2:CH4 Adsorption Selectivity. Journal of Composites Science, 6(11), 344. https://doi.org/10.3390/jcs6110344







