A Versatile Strategy for the Fabrication of Poly(ethyl methacrylate) Composites
Abstract
:1. Introduction
2. Materials and Methods
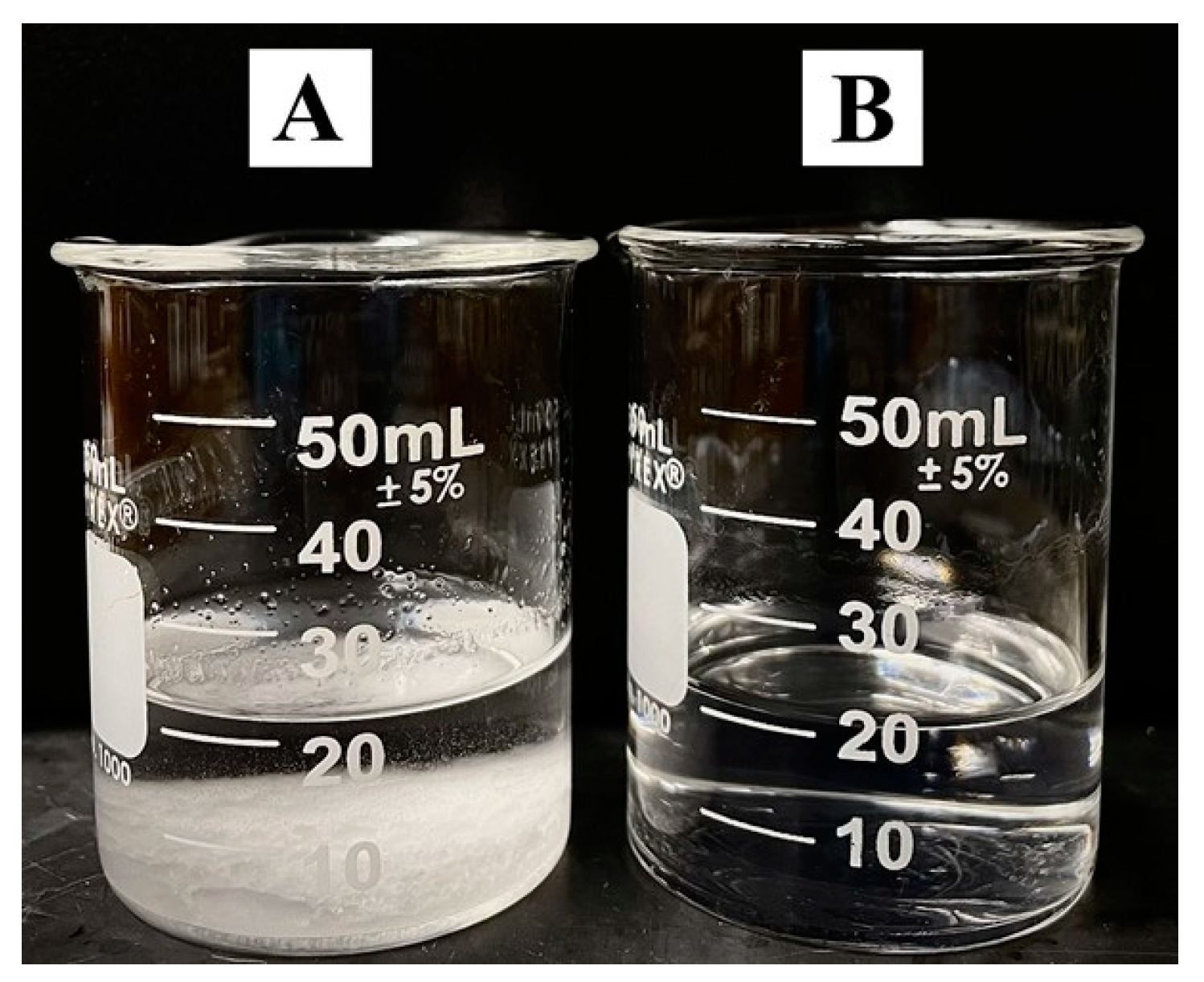
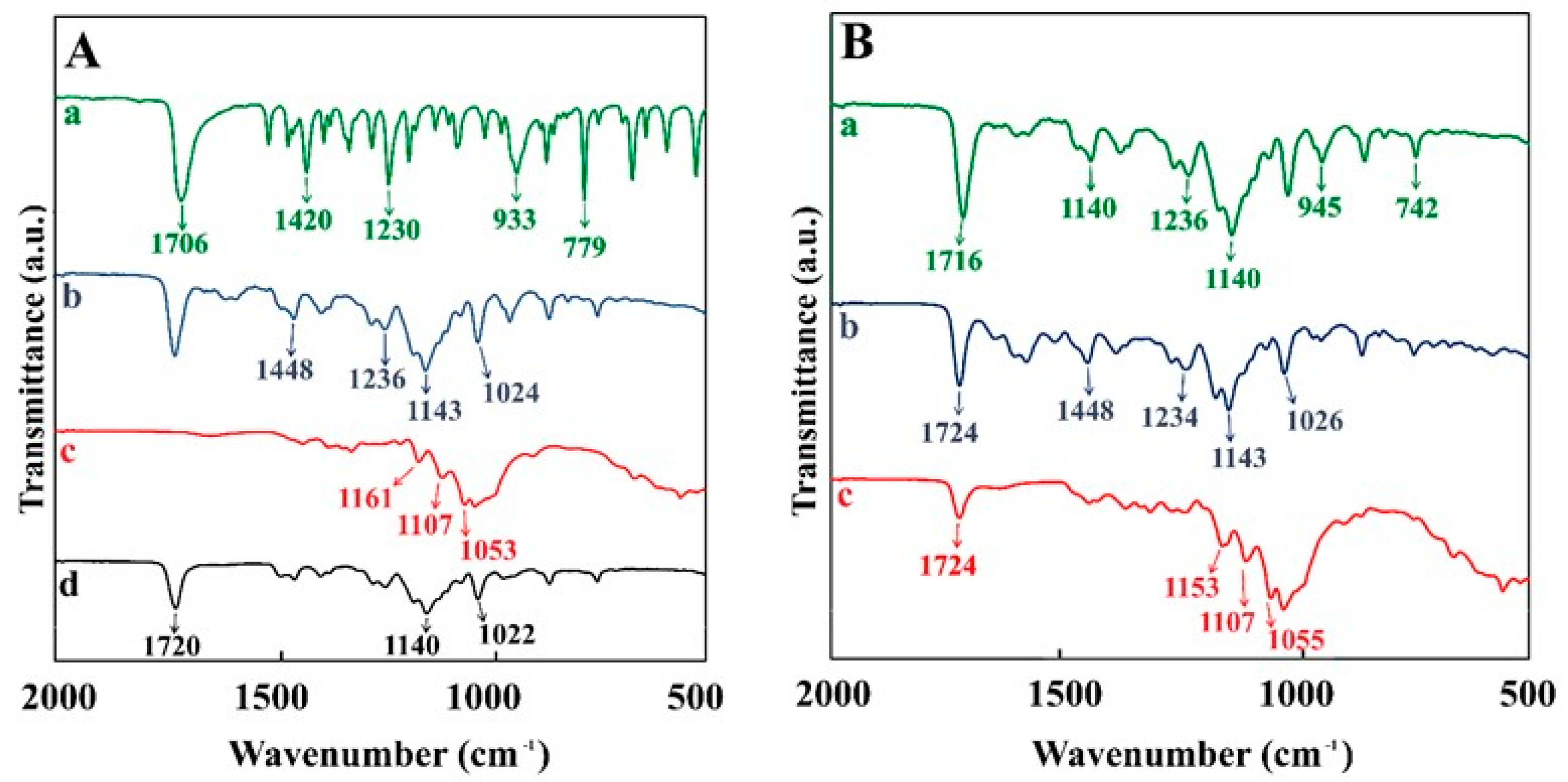
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohanty, F.; Swain, S.K. Effect of graphene platelets on the thermal and conducting properties of poly (ethyl methacrylate). Adv. Polym. Technol. 2018, 37, 1316–1322. [Google Scholar] [CrossRef]
- Abdelrazek, E. Influence of FeCl3 filler on the structure and physical properties of polyethyl-methacrylate films. Phys. B Condens. Matter 2007, 400, 26–32. [Google Scholar] [CrossRef]
- Armstrong, R.; Wright, J. Impedance studies of poly ethylmethacrylate coatings formed upon tin-free steel. Corros. Sci. 1992, 33, 1529–1539. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Silver Nanoparticles Decorated Polyethylmethacrylate/Graphene Oxide Composite: As Packaging Material. Polym. Compos. 2019, 40, E1199–E1207. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Nano silver embedded starch hybrid graphene oxide sandwiched poly (ethylmethacrylate) for packaging application. Nano-Struct. Nano-Objects 2019, 18, 100300. [Google Scholar] [CrossRef]
- Li, J.; Tan, S.; Ding, S.; Li, H.; Yang, L.; Zhang, Z. High-field antiferroelectric behaviour and minimized energy loss in poly (vinylidene-co-trifluoroethylene)-graft-poly (ethyl methacrylate) for energy storage application. J. Mater. Chem. 2012, 22, 23468–23476. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Mathew, C.M.; Rajendran, S. Highly porous lithium-ion conducting solvent-free poly (vinylidene fluoride-co-hexafluoropropylene)/poly (ethyl methacrylate) based polymer blend electrolytes for Li battery applications. Electrochim. Acta 2013, 93, 230–235. [Google Scholar] [CrossRef]
- Ramesh, S.; Uma, O.; Shanti, R.; Yi, L.J.; Ramesh, K. Preparation and characterization of poly (ethyl methacrylate) based polymer electrolytes doped with 1-butyl-3-methylimidazolium trifluoromethanesulfonate. Measurement 2014, 48, 263–273. [Google Scholar] [CrossRef]
- Sari, A.; Karlı, A.; Alkan, C.; Karaipekli, A. Polyethyl methacrylate (PEMA)/fatty acids blends as novel phase change materials for thermal energy storage. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 1813–1819. [Google Scholar] [CrossRef]
- Zakaria, N.; Isa, M.; Mohamed, N.; Subban, R. Characterization of polyvinyl chloride/polyethyl methacrylate polymer blend for use as polymer host in polymer electrolytes. J. Appl. Polym. Sci. 2012, 126, E419–E424. [Google Scholar] [CrossRef]
- Arnold, J.C.; Venditti, N.P. Prediction of the long-term creep behaviour of hydroxyapatite-filled polyethylmethacrylate bone cements. J. Mater. Sci. Mater. Med. 2007, 18, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.; Di Silvio, L.; Harper, E.; Bonfield, W. In vitro adhesion and biocompatability of osteoblast-like cells to poly (methylmethacrylate) and poly (ethylmethacrylate) bone cements. J. Mater. Sci. Mater. Med. 2002, 13, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jun, J.K.; Jeong, H.M. Shape memory and physical properties of poly (ethyl methacrylate)/Na-MMT nanocomposites prepared by macroazoinitiator intercalated in Na-MMT. Compos. Sci. Technol. 2008, 68, 1919–1926. [Google Scholar] [CrossRef]
- Barry, J.; Gidda, H.; Scotchford, C.; Howdle, S. Porous methacrylate scaffolds: Supercritical fluid fabrication and in vitro chondrocyte responses. Biomaterials 2004, 25, 3559–3568. [Google Scholar] [CrossRef]
- Hutcheon, G.; Messiou, C.; Wyre, R.; Davies, M.; Downes, S. Water absorption and surface properties of novel poly (ethylmethacrylate) polymer systems for use in bone and cartilage repair. Biomaterials 2001, 22, 667–676. [Google Scholar] [CrossRef]
- Barry, J.J.; Silva, M.M.; Cartmell, S.H.; Guldberg, R.E.; Scotchford, C.A.; Howdle, S.M. Porous methacrylate tissue engineering scaffolds: Using carbon dioxide to control porosity and interconnectivity. J. Mater. Sci. 2006, 41, 4197–4204. [Google Scholar] [CrossRef]
- Özcan, M.; Hotza, D.; Fredel, M.C.; Cruz, A.; Volpato, C.A.M. Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry. J. Compos. Sci. 2021, 5, 78. [Google Scholar] [CrossRef]
- Moura, N.K.; Siqueira, I.A.W.B.; Machado, J.P.B.; Kido, H.W.; Avanzi, I.R.; Rennó, A.C.M.; Trichês, E.S.; Passador, F.R. Production and Characterization of Porous Polymeric Membranes of PLA/PCL Blends with the Addition of Hydroxyapatite. J. Compos. Sci. 2019, 3, 45. [Google Scholar] [CrossRef] [Green Version]
- Grandfield, K.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite–silica–chitosan coatings. Mater. Charact. 2008, 59, 61–67. [Google Scholar] [CrossRef]
- Balla, E.D.; Bikiaris, N.D.; Nanaki, S.G.; Papoulia, C.; Chrissafis, K.; Klonos, P.A.; Kyritsis, A.; Kostoglou, M.; Zamboulis, A.; Papageorgiou, G.Z. Chloramphenicol Loaded Sponges Based on PVA/Nanocellulose Nanocomposites for Topical Wound Delivery. J. Compos. Sci. 2021, 5, 208. [Google Scholar] [CrossRef]
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. J. Compos. Sci. 2020, 4, 152. [Google Scholar] [CrossRef]
- Anton-Sales, I.; Roig-Sanchez, S.; Traeger, K.; Weis, C.; Laromaine, A.; Turon, P.; Roig, A. In vivo soft tissue reinforcement with bacterial nanocellulose. Biomater. Sci. 2021, 9, 3040–3050. [Google Scholar] [CrossRef] [PubMed]
- Echeverry-Rendon, M.; Reece, L.M.; Pastrana, F.; Arias, S.L.; Shetty, A.R.; Pavón, J.J.; Allain, J.P. Bacterial Nanocellulose Magnetically Functionalized for Neuro-Endovascular Treatment. Macromol. Biosci. 2017, 17, 1600382. [Google Scholar] [CrossRef] [PubMed]
- Bharimalla, A.; Deshmukh, S.; Vigneshwaran, N.; Patil, P.; Prasad, V. Nanocellulose-polymer composites for applications in food packaging: Current status, future prospects and challenges. Polym.- Plast. Technol. Eng. 2017, 56, 805–823. [Google Scholar] [CrossRef]
- Koppolu, R.; Lahti, J.; Abitbol, T.; Swerin, A.; Kuusipalo, J.; Toivakka, M. Continuous processing of nanocellulose and polylactic acid into multilayer barrier coatings. ACS Appl. Mater. Interfaces 2019, 11, 11920–11927. [Google Scholar] [CrossRef] [PubMed]
- Wesling, B.N.; Dias, G.M.; Müller, D.; Serpa, R.B.; Hotza, D.; Rambo, C.R. Enhanced Electrochemical Performance of Nanocellulose/PPy· CuCl 2 Electrodes for All-Cellulose-Based Supercapacitors. J. Electron. Mater. 2020, 49, 1036–1042. [Google Scholar] [CrossRef]
- Brett, C.J.; Ohm, W.; Fricke, B.R.; Alexakis, A.E.; Laarmann, T.; Körstgens, V.; Müller-Buschbaum, P.; Söderberg, L.D.; Roth, S.V. Nanocellulose-Assisted Thermally Induced Growth of Silver Nanoparticles for Optical Applications. ACS Appl. Mater. Interfaces 2021, 13, 27696–27704. [Google Scholar] [CrossRef]
- Herrera, M.A.; Sirviö, J.A.; Mathew, A.P.; Oksman, K. Environmental friendly and sustainable gas barrier on porous materials: Nanocellulose coatings prepared using spin-and dip-coating. Mater. Des. 2016, 93, 19–25. [Google Scholar] [CrossRef]
- Lang, A.W.; Österholm, A.M.; Reynolds, J.R. Paper-based electrochromic devices enabled by nanocellulose-coated substrates. Adv. Funct. Mater. 2019, 29, 1903487. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Arazi, R.; Mirjalili, B.B.F.; Azad, S. Synthesis and application of Fe3O4@ nanocellulose/TiCl as a nanofiller for high performance of quasisolid-based dye-sensitized solar cells. Int. J. Energy Res. 2019, 43, 4483–4494. [Google Scholar] [CrossRef]
- Feldman, D. Poly(Vinyl Alcohol) Recent Contributions to Engineering and Medicine. J. Compos. Sci. 2020, 4, 175. [Google Scholar] [CrossRef]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef] [PubMed]
- Deen, I.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite chitosan–halloysite nanotube–hydroxyapatite films. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 38–44. [Google Scholar] [CrossRef]
- Baker, K.; Sikkema, R.; Liang, W.; Zhitomirsky, I. Multifunctional Properties of Commercial Bile Salts for Advanced Materials Engineering. Adv. Eng. Mater. 2021, 23, 2001261. [Google Scholar] [CrossRef]
- Baker, K.; Sikkema, R.; Zhitomirsky, I. Application of bile acids for biomedical devices and sensors. Med. Devices Sens. 2020, 3, e10119. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- Aburideh, H.; Merzouk, N.K.; Naceur, M.W.; Tigrine, Z.; Tassalit, D.; Abbas, M. Thermal annealing effect on morphology and performance of polysulfone-cellulose acetate membranes: Application for water defluoridation technology. Cellul. Chem. Technol. 2019, 53, 583–597. [Google Scholar] [CrossRef]
- Zidan, T.; El-Menyawy, E. Thermal annealing-induced enhanced ordering and optical functions modification on poly (3-octylthiophene) films. Polym. Test. 2019, 75, 270–276. [Google Scholar] [CrossRef]
- Sikkema, R.; Keohan, B.; Zhitomirsky, I. Alginic Acid Polymer-Hydroxyapatite Composites for Bone Tissue Engineering. Polymers 2021, 13, 3070. [Google Scholar] [CrossRef]
- Sikkema, R.; Keohan, B.; Zhitomirsky, I. Hyaluronic-Acid-Based Organic-Inorganic Composites for Biomedical Applications. Materials 2021, 14, 4982. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, C.; Zhang, X.; Chang, J.; Dai, K. Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration. Acta Biomater. 2018, 66, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Mishra, S.; Sahoo, R.N.; Mallick, S. Infrared spectroscopy for analysis of co-processed ibuprofen and magnesium trisilicate at milling and freeze drying. Acta Chim. Slov. 2017, 64, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Jana, S. Spectroscopic characterization of chloramphenicol and tetracycline: An impact of biofield treatment. Pharm. Anal. Acta 2015, 6, 395. [Google Scholar]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Li, X.; Zhitomirsky, I. Deposition of poly (methyl methacrylate) and composites containing bioceramics and bioglass by dip coating using isopropanol-water co-solvent. Prog. Org. Coat. 2020, 148, 105883. [Google Scholar] [CrossRef]
- Jankovic, I.A.; Saponjic, Z.V.; Comor, M.I.; Nedeljkovic, J.M. Surface modification of colloidal TiO2 nanoparticles with bidentate benzene derivatives. J. Phys. Chem. C 2009, 113, 12645–12652. [Google Scholar] [CrossRef]
- Thitisomboon, W.; Gu, Q.; Weng, L.-T.; Gao, P. Surface confinement induced amorphization of polyethylene oxide in high-performance porous polyethylene films. Polymer 2021, 217, 123449. [Google Scholar] [CrossRef]
- Trifol, J.; Van Drongelen, M.; Clegg, F.; Plackett, D.; Szabo, P.; Daugaard, A. Impact of thermal processing or solvent casting upon crystallization of PLA nanocellulose and/or nanoclay composites. J. Appl. Polym. Sci. 2019, 136, 47486. [Google Scholar] [CrossRef] [Green Version]
- Mileva, D.; Tranchida, D.; Gahleitner, M. Designing polymer crystallinity: An industrial perspective. Polym. Cryst. 2018, 1, e10009. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, K.; Zhitomirsky, I. A Versatile Strategy for the Fabrication of Poly(ethyl methacrylate) Composites. J. Compos. Sci. 2022, 6, 40. https://doi.org/10.3390/jcs6020040
Baker K, Zhitomirsky I. A Versatile Strategy for the Fabrication of Poly(ethyl methacrylate) Composites. Journal of Composites Science. 2022; 6(2):40. https://doi.org/10.3390/jcs6020040
Chicago/Turabian StyleBaker, Kayla, and Igor Zhitomirsky. 2022. "A Versatile Strategy for the Fabrication of Poly(ethyl methacrylate) Composites" Journal of Composites Science 6, no. 2: 40. https://doi.org/10.3390/jcs6020040
APA StyleBaker, K., & Zhitomirsky, I. (2022). A Versatile Strategy for the Fabrication of Poly(ethyl methacrylate) Composites. Journal of Composites Science, 6(2), 40. https://doi.org/10.3390/jcs6020040





