Preparation of Gellan Gum-Inorganic Composite Film and Its Metal Ion Accumulation Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
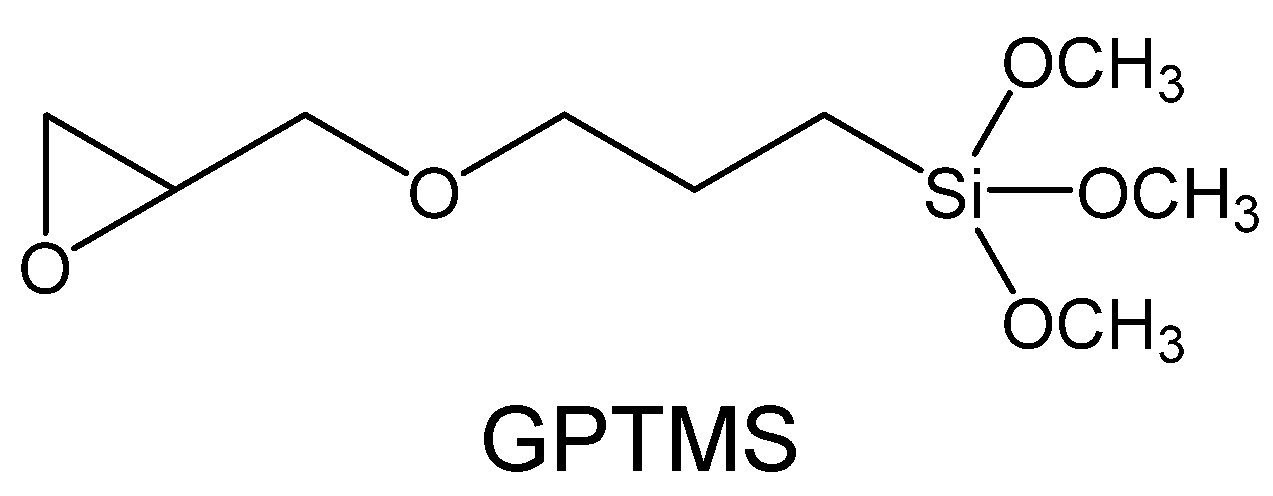
2.2. Preparation of Gellan Gum-GPTMS Composite Film
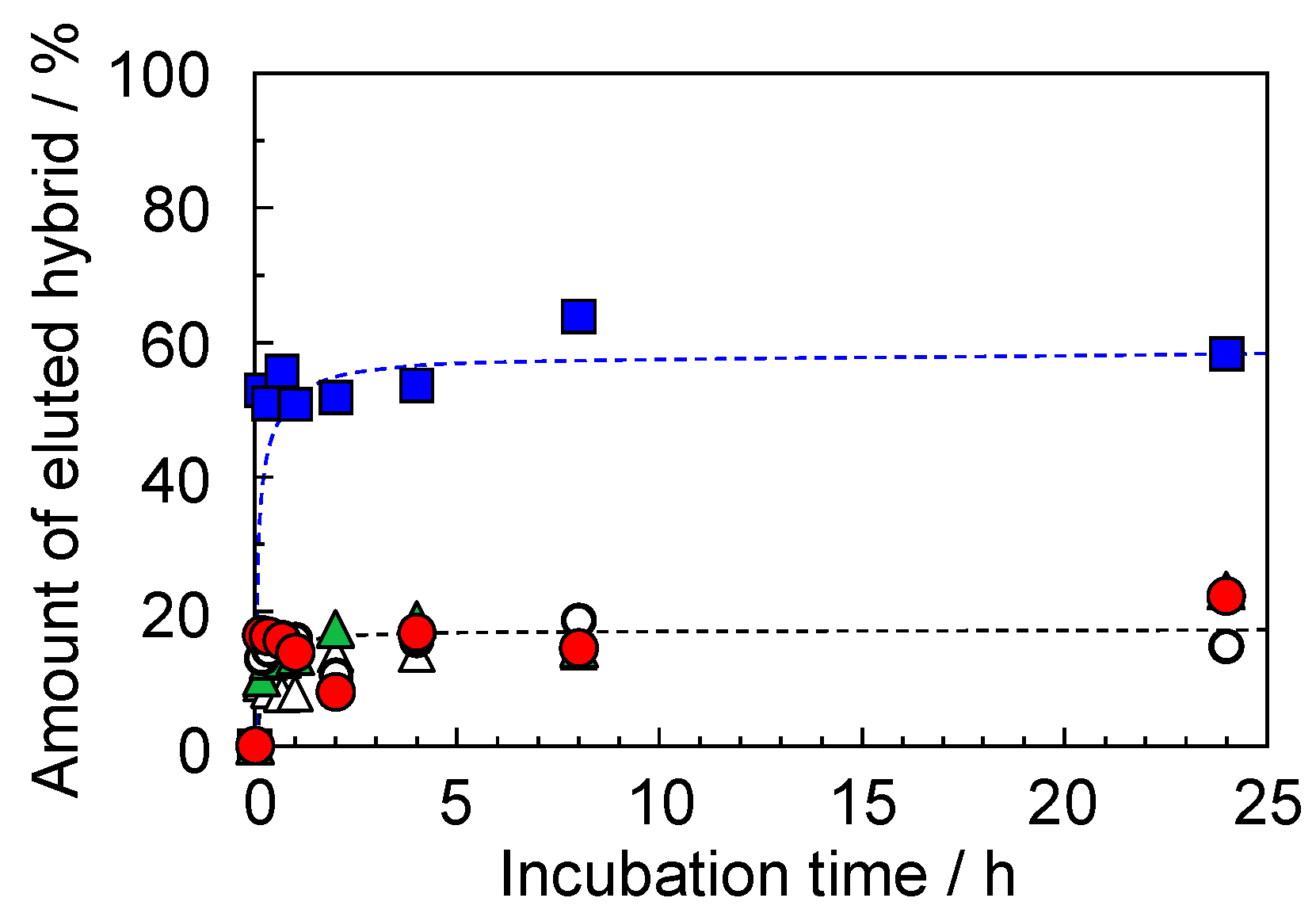
2.3. Water Stability of Gellan Gum-GPTMS Composite Film
2.4. Structural and Thermal Analyses of Gellan Gum-GPTMS Composite Film
2.5. Tensile Strength of Gellan Gum-GPTMS Composite Film
2.6. Accumulation of Metal Ions by Gellan Gam-GPTMS Composite Film
2.7. IR Measurements of Metal Ion-Accumulated Gellan Gum-GPTMS Composite Film
3. Results and Discussion
3.1. Preparation of Gellan Gum-GPTMS Composite Film
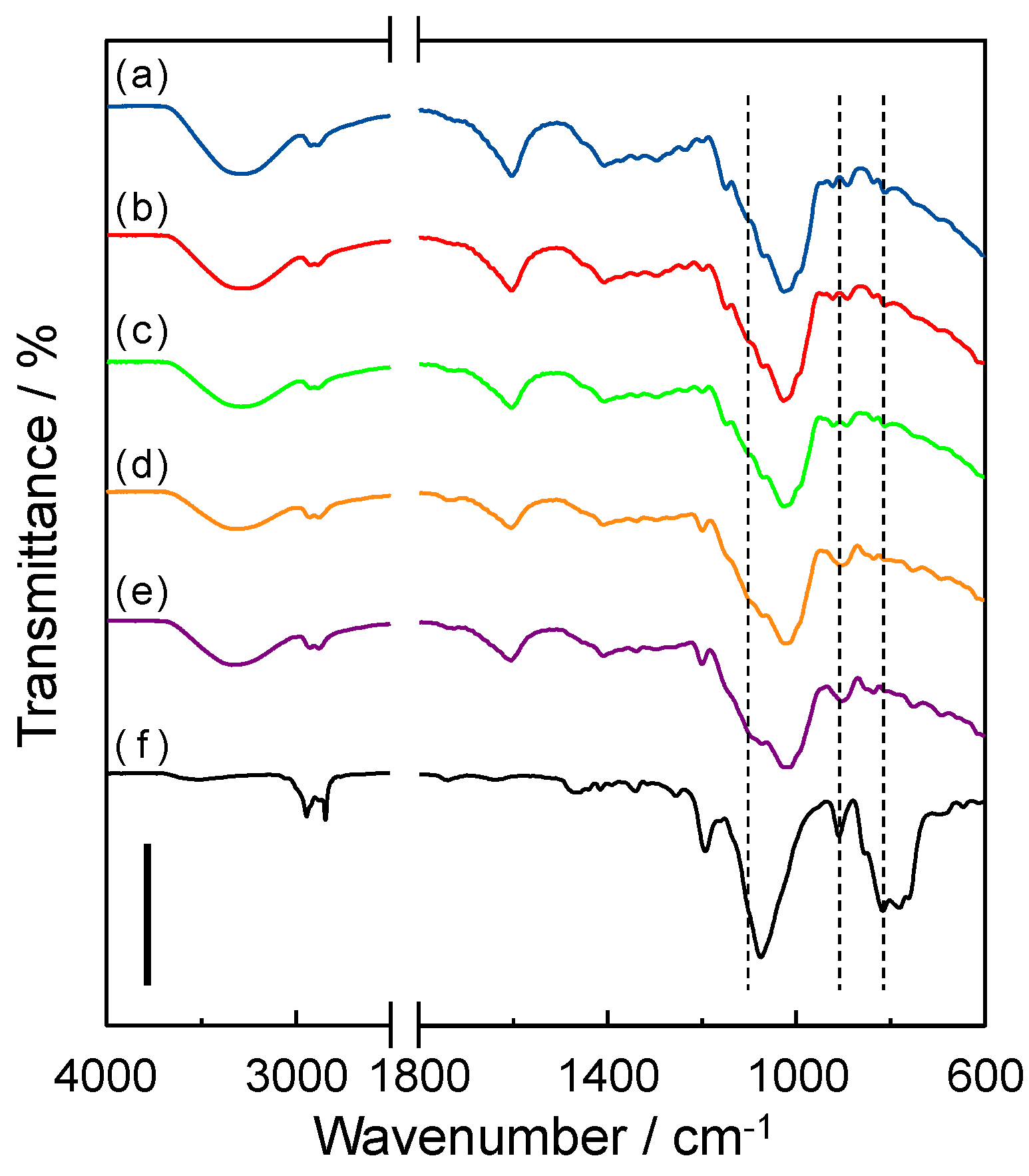
3.2. Molecular Structure of Gellan Gum-GPTMS Composite Film
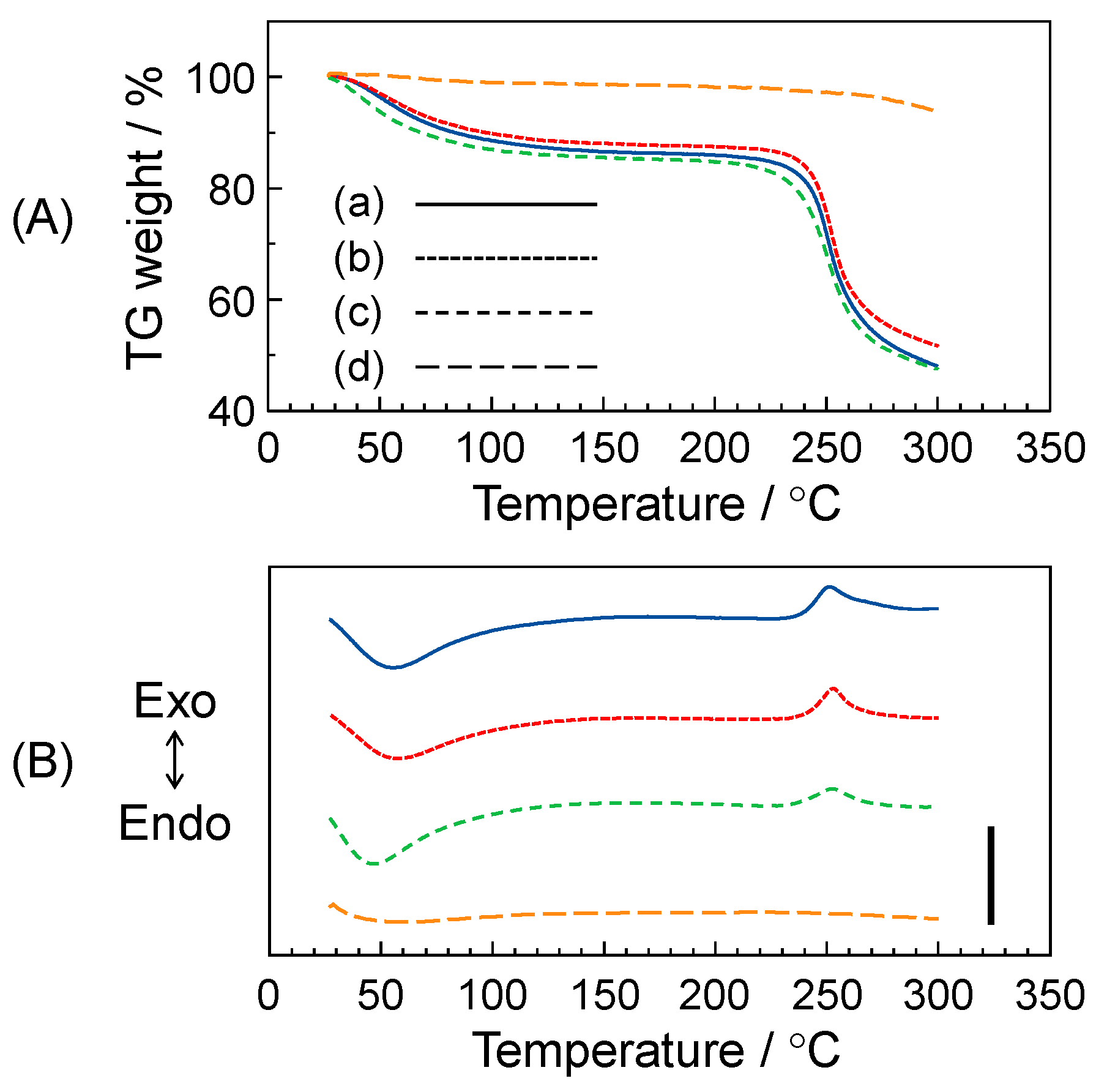
3.3. Thermal Stability of Gellan Gum-GPTMS Composite Film
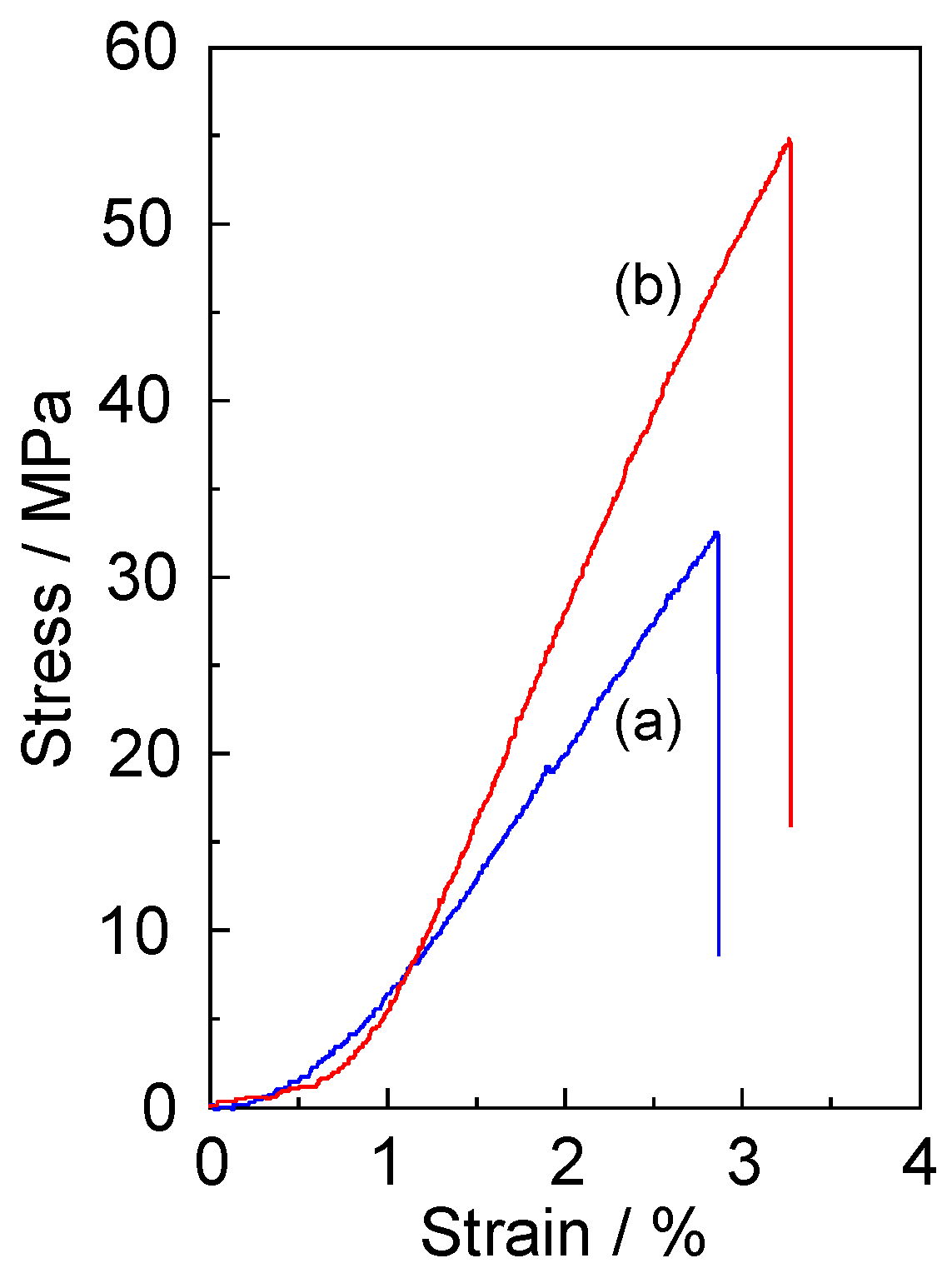
3.4. Mechanical Strength of Gellan Gum-GPTMS Composite Film
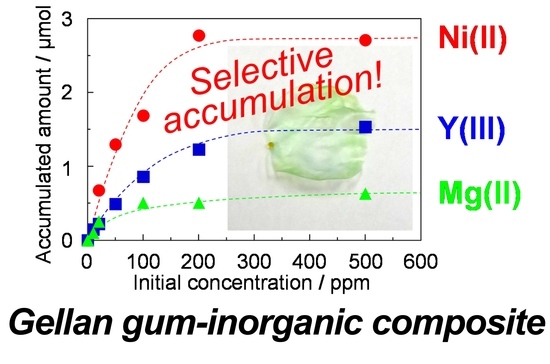
3.5. Accumulation of Metal Ions by the Gellan Gum-GPTMS Composite Film
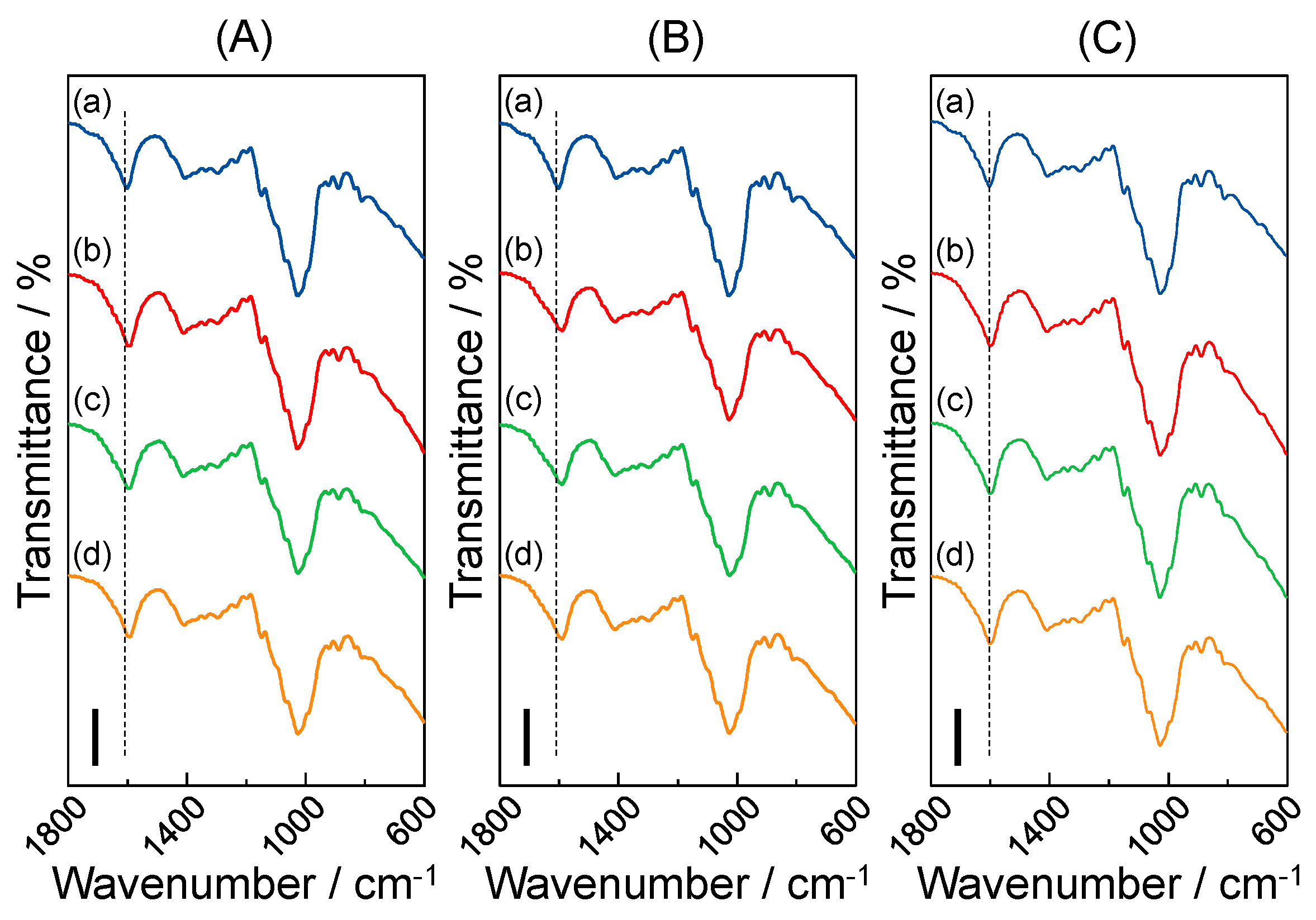
3.6. IR Measurements of the Metal Ion-Accumulated Gellan Gum-GPTMS Composite Film
3.7. Accumulative Mechanism of Metal Ions by the Composite Film
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bajaj, I.B.; Survase, S.A.; Saudagar, P.S.; Singhal, R.S. Gellan gum: Fermentative production, downstream processing and applications. Food Technol. Biotechnol. 2007, 45, 341–354. [Google Scholar]
- O’Neill, M.A.; Selvendran, R.R.; Morris, V.J. Structure of the acidic extracellular gelling polysaccharide produced by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 123–133. [Google Scholar] [CrossRef]
- Hadeler, B.; Scholz, S.; Reski, R. Gelrite and agar differently influence cytokinin-sensitivity of a moss. J. Plant Physiol. 1995, 146, 369–371. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Nadzir, M.M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical applications of bacterial exopolysaccharides: A review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef]
- Rudko, M.; Urbaniak, T.; Musiał, W. Recent developments in ion-sensitive systems for pharmaceutical applications. Polymers 2021, 13, 1641. [Google Scholar] [CrossRef]
- Kanesaka, S.; Watanabe, T.; Matsukawa, S. Binding effect of Cu2+ as a trigger on the sol-to-gel and coil-to-helix transition processes of polysaccharide, gellan gum. Biomacromolecules 2004, 5, 863–868. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A review on latest innovations in natural gums based hydrogels: Preparations & applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar]
- Erigoni, A.; Diaz, U. Porous silica-based organic-inorganic hybrid catalysts: A review. Catalysts 2021, 11, 79. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J. Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Sol-gel derived organic–inorganic hybrid materials: Synthesis, characterizations and applications. J. Sol-Gel Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Silane Coupling Agents, 2nd ed.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Arakaki, A.; Shimizu, K.; Oda, M.; Sakamoto, T.; Nishimura, T.; Kato, T. Biomineralization-inspired synthesis of functional organic/inorganic hybrid materials: Organic molecular control of self-organization of hybrids. Org. Biomol. Chem. 2015, 13, 974–989. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Aono, H. DNA-inorganic hybrid material as selective absorbent for harmful compounds. Polymer 2008, 49, 4658–4665. [Google Scholar] [CrossRef]
- Monton, M.R.N.; Forsberg, E.M.; Brennan, J.D. Tailoring sol-gel-derived silica materials for optical biosensing. Chem. Mater. 2012, 24, 796–811. [Google Scholar] [CrossRef]
- Nunes, S.C.; Bermudez, V.Z.; Silva, M.M.; Smith, M.J.; Morales, E.; Carlos, L.D.; Ferreira, R.A.S.; Rocha, J. Sol-gel derived Li+-doped poly(ε-caprolactone)/siloxane biohybrid electrolytes. J. Solid State Electrochem. 2006, 10, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Ogino, T. Anhydrous proton conductor consisting of pectin-inorganic composite material. J. Appl. Polym. Sci. 2015, 132, 42433–42439. [Google Scholar] [CrossRef]
- Coradin, T.; Livage, J. Aqueous silicates in biological sol-gel applications: New perspectives for old precursors. Acc. Chem. Res. 2007, 40, 819–826. [Google Scholar] [CrossRef]
- Yamada, M.; Abe, K. Selective accumulation of rare earth metal and heavy metal ions by DNA-inorganic hybrid material. Polym. J. 2014, 46, 366–371. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P.; Thachil, E.T. Toughening of epoxy resins. Des. Monomers Polym. 2006, 9, 129–152. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Shi, L.; Gunasekaran, S. Preparation of pectin–ZnO nanocomposite. Nanoscale Res. Lett. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theophanides, T. Infrared Spectroscopy—Materials Science, Engineering and Technology; IntechOpen: London, UK, 2012. [Google Scholar]
- Ma, S.; Liu, W.; Wei, Z.; Li, H. Mechanical and thermal properties and morphology of epoxy resins modified by a silicon compound. J. Macromol. Sci. A 2010, 47, 1084–1090. [Google Scholar] [CrossRef]
- Yamada, M.; Shiiba, S. Preparation of pectin-inorganic composite material as accumulative material of metal ions. J. Appl. Polym. Sci. 2015, 132, 42056–42063. [Google Scholar] [CrossRef]
- Lázaro, N.; Sevilla, A.L.; Morales, S.; Marqués, A.M. Heavy metal biosorption by gellan gum gel beads. Water Res. 2003, 37, 2118–2126. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.; Tong, T.; Epsztein, R.; Sun, M.; Verduzco, R.; Ma, J.; Elimelech, M. Selective removal of divalent cations by polyelectrolyte multilayer nanofiltration membrane: Role of polyelectrolyte charge, ion size, and ionic strength. J. Membr. Sci. 2018, 559, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, W.W.; Irmer, G. On the hydration of the rare earth ions in aqueous solution. J. Solut. Chem. 2020, 49, 316–331. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist-Reis, P.; Lamble, K.; Pattanaik, S.; Persson, I.; Sandström, M. Hydration of the yttrium(III) ion in aqueous solution. An X-ray diffraction and XAFS structural study. J. Phys. Chem. B 2000, 104, 402–408. [Google Scholar] [CrossRef]
- Lindqvist-Reis, P.; Persson, I.; Sandström, M. The hydration of the scandium(III) ion in aqueous solution and crystalline hydrates studied by XAFS spectroscopy, large-angle X-ray scattering and crystallography. Dalton Trans. 2006, 3868–3878. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Gaus, P.L. Basic Inorganic Chemistry; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
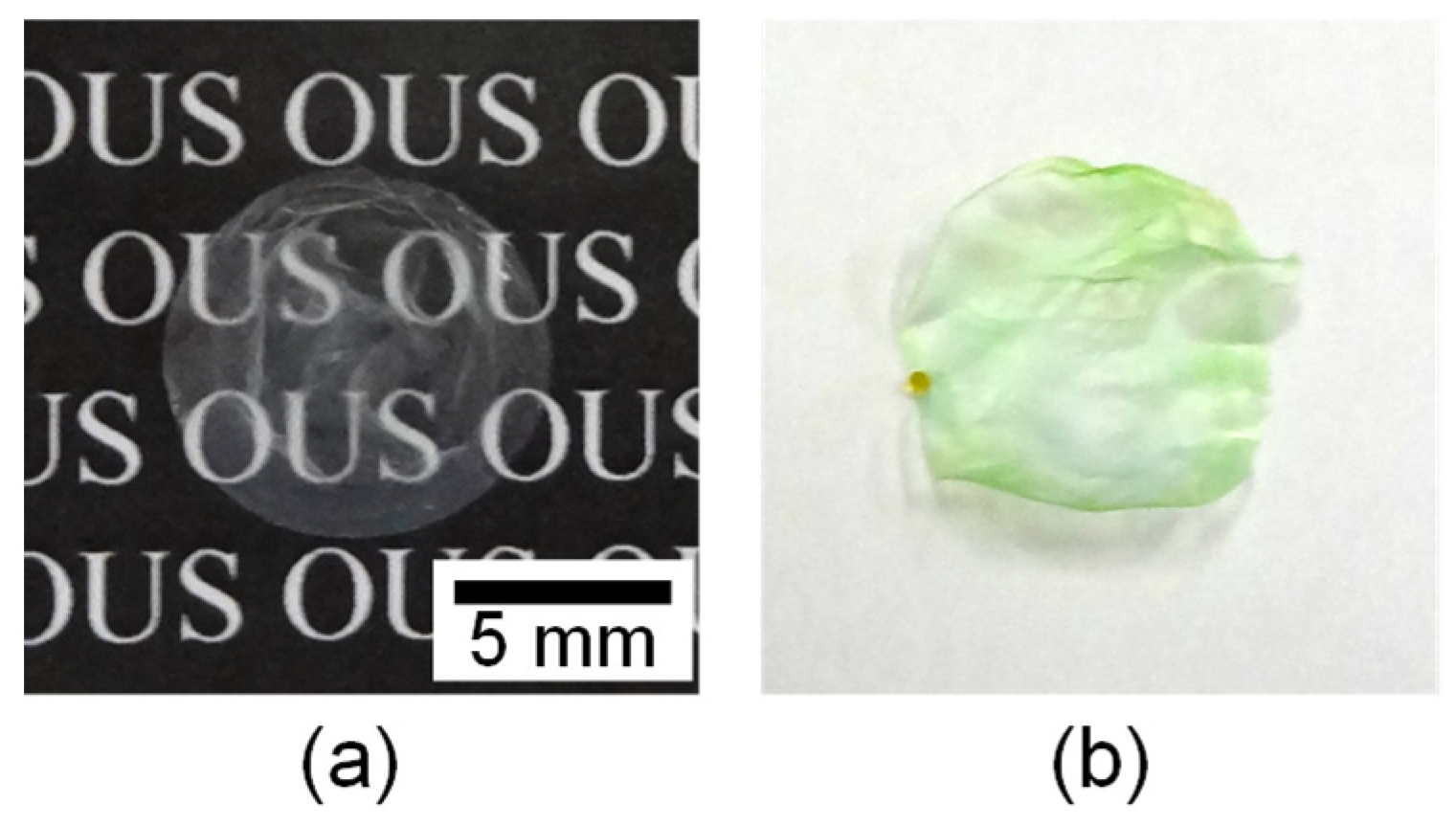


| Metal Ions | Accumulated Amounts/μmol | N Values | Group |
|---|---|---|---|
| Ni(II) | 2.7 | 3.4 | A |
| In(II) | 1.8 | 5.1 | B |
| La(III) | 1.6 | 5.8 | B |
| Zn(II) | 1.6 | 5.8 | B |
| Cu(II) | 1.5 | 6.2 | B |
| Y(III) | 1.5 | 6.2 | B |
| Ca(II) | 1.5 | 6.2 | B |
| Sc(III) | 0.9 | 10.3 | C |
| Al(III) | 0.8 | 11.6 | C |
| Mg(II) | 0.5 | 18.5 | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, M.; Kametani, Y. Preparation of Gellan Gum-Inorganic Composite Film and Its Metal Ion Accumulation Property. J. Compos. Sci. 2022, 6, 42. https://doi.org/10.3390/jcs6020042
Yamada M, Kametani Y. Preparation of Gellan Gum-Inorganic Composite Film and Its Metal Ion Accumulation Property. Journal of Composites Science. 2022; 6(2):42. https://doi.org/10.3390/jcs6020042
Chicago/Turabian StyleYamada, Masanori, and Yoshihiro Kametani. 2022. "Preparation of Gellan Gum-Inorganic Composite Film and Its Metal Ion Accumulation Property" Journal of Composites Science 6, no. 2: 42. https://doi.org/10.3390/jcs6020042
APA StyleYamada, M., & Kametani, Y. (2022). Preparation of Gellan Gum-Inorganic Composite Film and Its Metal Ion Accumulation Property. Journal of Composites Science, 6(2), 42. https://doi.org/10.3390/jcs6020042