Poly(Lactic Acid) (PLA)-Based Nanocomposites: Impact of Vermiculite, Silver, and Graphene Oxide on Thermal Stability, Isothermal Crystallization, and Local Mechanical Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Preparation
2.2.1. Preparation of HDTMA and HDP-Modified Vermiculites (VMT + HDP and VMT + HDTMA)
2.2.2. Preparation of VMT + Ag
2.2.3. Preparation GO and GO + Ag
2.2.4. Preparation of Reinforced PLA Nanocomposite
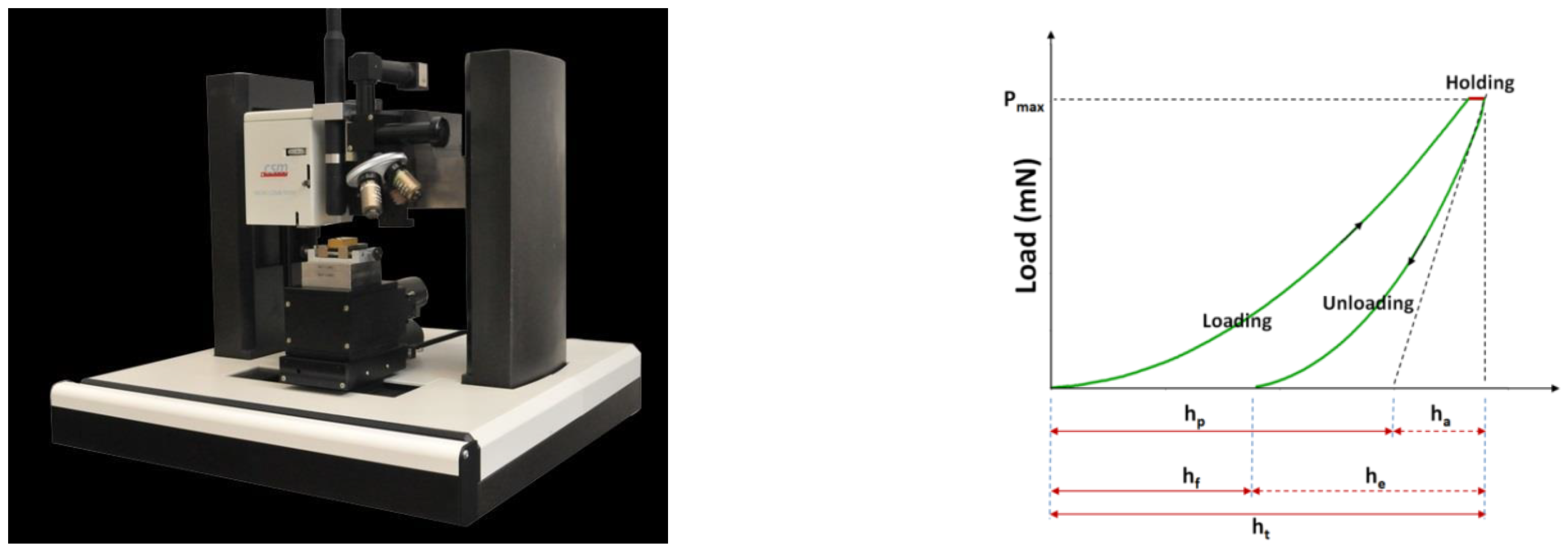
3. Methods of Characterizations
4. Results and Discussion
4.1. Fillers Characterization by X-ray Diffraction
4.2. Morphological and Structural Characteristics of Reinforced PLA Nanocomposite
4.3. Thermal Stability by Thermogravimetric Analysis
4.4. Thermal Properties by DSC
4.5. Mechanical Response
The Print Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Sharif, A.; Mondal, S.; Hoque, M.E. Polylactic acid (PLA)-based nanocomposites: Processing and properties. In Bio-Based Polymers and Nanocomposites; Springer: Cham, Switzerland, 2019; pp. 233–254. [Google Scholar]
- Bhardwaj, R.; Mohanty, A.K. Modification of Brittle Polylactide by Novel Hyperbranched Polymer-Based Nanostructures. Biomacromolecules 2007, 8, 2476–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Hyon, S.; Kim, C. Distributed virtual negative-sequence impedance control for accurate imbalance power sharing in islanded microgrids. Sustain. Energy Grids Netw. 2018, 16, 28–36. [Google Scholar] [CrossRef]
- Heidari, B.S.; Oliaei, E.; Shayesteh, H.; Davachi, S.M.; Hejazi, I.; Seyfi, J.; Bahrami, M.; Rashedi, H. Simulation of mechanical behavior and optimization of simulated injection molding process for PLA based antibacterial composite and nanocomposite bone screws using central composite design. J. Mech. Behav. Biomed. Mater. 2017, 65, 160–176. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Expression of normal stress difference and relaxation modulus for ternary nanocomposites containing biodegradable polymers and carbon nanotubes by storAge and loss modulus data. Compos. Part B Eng. 2019, 158, 162–168. [Google Scholar] [CrossRef]
- Škrlová, K.; Malachová, K.; Muñoz-Bonilla, A.; Měřinská, D.; Rybková, Z.; Fernández-García, M.; Plachá, D. Biocompatible polymer materials with antimicrobial properties for preparation of stents. Nanomaterials 2019, 9, 1548. [Google Scholar] [CrossRef] [Green Version]
- JAgadish, R.S.; Raj, B.; Asha, M.R. Blending of low-density polyethylene with vanillin for improved barrier and aroma-releasing properties in food packAging. J. Appl. Polym. Sci. 2009, 113, 3732–3741. [Google Scholar] [CrossRef]
- Luo, Y.-B.; Wang, X.-L.; Wang, Y.-Z. Effect of TiO2 nanoparticles on the long-term hydrolytic degradation behavior of PLA. Polym. Degrad. Stab. 2012, 97, 721–728. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Abbate, C.; Arena, M.; Ferreri, L. Effect of sepiolite on the biodegradation of poly (lactic acid) and polycaprolactone. Polym. Degrad. Stab. 2010, 95, 2049–2056. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly (lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Pyda, M.; Wunderlich, B. Reversing and nonreversing heat capacity of poly (lactic acid) in the glass transition region by TMDSC. Macromolecules 2005, 38, 10472–10479. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Hejna, A.; Biskup, R.; Szulc, J.; Michałowski, S.; Piasecki, A.; Kloziński, A. The Effect of Surface Treatment with Isocyanate and Aromatic Carbodiimide of Thermally Expanded Vermiculite Used as a Functional Filler for Polylactide-Based Composites. Polymers 2021, 13, 890. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Franciszczak, P.; Meljon, A. High performance hybrid PP and PLA biocomposites reinforced with short man-made cellulose fibres and softwood flour. Compos. Part A Appl. Sci. Manuf. 2015, 74, 132–139. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Jiménez, A.; Kenny, J.M. Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites. J. Food Eng. 2013, 118, 117–124. [Google Scholar] [CrossRef]
- Martino, V.P.; Jimenez, A.; Ruseckaite, R.A.; Averous, L. Structure and properties of clay nano-biocomposites based on poly (lactic acid) plasticized with polyadipates. Polym. Adv. Technol. 2011, 22, 2206–2213. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Ahmed, I.; Parsons, A.J.; Scotchford, C.A.; Walker, G.S.; Thielemans, W.; Rudd, C.D. Physico-chemical and mechanical properties of nanocomposites prepared using cellulose nanowhiskers and poly(lactic acid). J. Mater. Sci. 2012, 47, 2675–2686. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; del Carmen Garrigós, M.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stab. 2014, 108, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Andrzejewski, J.; Cheng, J.; Anstey, A.; Mohanty, A.K.; Misra, M. Development of Toughened Blends of Poly(lactic acid) and Poly(butylene adipate-co-terephthalate) for 3D Printing Applications: Compatibilization Methods and Material Performance Evaluation. ACS Sustain. Chem. Eng. 2020, 8, 6576–6589. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Piorkowska, E.; Bojda, J. The influence of matrix crystallinity, filler grain size and modification on properties of PLA/calcium carbonate composites. Polym. Test. 2017, 62, 203–209. [Google Scholar] [CrossRef]
- Wen, B.; Ma, L.; Zou, W.; Zheng, X. Enhanced thermal conductivity of poly(lactic acid)/alumina composite by synergistic effect of tuning crystallization of poly(lactic acid) crystallization and filler content. J. Mater. Sci. Mater. Electron. 2020, 31, 6328–6338. [Google Scholar] [CrossRef]
- Tenn, N.; Follain, N.; Soulestin, J.; Crétois, R.; Bourbigot, S.; Marais, S. Effect of Nanoclay Hydration on Barrier Properties of PLA/Montmorillonite Based Nanocomposites. J. Phys. Chem. C 2013, 117, 12117–12135. [Google Scholar] [CrossRef]
- Alves, J.L.; de Tarso Vieira e Rosa, P.; Realinho, V.; Antunes, M.; Velasco, J.I.; Morales, A.R. The effect of Brazilian organic-modified montmorillonites on the thermal stability and fire performance of organoclay-filled PLA nanocomposites. Appl. Clay Sci. 2020, 194, 105697. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Wan, G.; Li, B.; Zhao, G. Glass fiber reinforced PLA composite with enhanced mechanical properties, thermal behavior, and foaming ability. Polymer 2019, 181, 121803. [Google Scholar] [CrossRef]
- Pan, H.; Kong, J.; Chen, Y.; Zhang, H.; Dong, L. Improved heat resistance properties of poly(l-lactide)/basalt fiber biocomposites with high crystallinity under forming hybrid-crystalline morphology. Int. J. Biol. Macromol. 2019, 122, 848–856. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Sun, Y.; Zhang, M. Rheological properties and crystallization behavior of multi-walled carbon nanotube/poly (ε-caprolactone) composites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3137–3147. [Google Scholar] [CrossRef]
- Cacopardo, L.; Guazzelli, N.; Ahluwalia, A. Characterizing and Engineering Biomimetic Materials for Viscoelastic Mechanotransduction Studies. Tissue Eng. Part B Rev. 2021. ahead of print. [Google Scholar] [CrossRef]
- Khammassi, S.; Tarfaoui, M.; Lafdi, K. Study of mechanical performance of polymer nanocomposites reinforced with exfoliated graphite of different mesh sizes using micro-indentation. J. Compos. Mater. 2021, 55, 2617–2629. [Google Scholar] [CrossRef]
- Khammassi, S.; Tarfaoui, M. Micromechanical characterization of Carbon Black reinforced adhesive nanocomposite using micro indentation. Mater. Today Proc. 2021, 52, 222–226. [Google Scholar] [CrossRef]
- Khammassi, S.; Tarfaoui, M. Influence of exfoliated graphite filler size on the electrical, thermal, and mechanical polymer properties. J. Compos. Mater. 2020, 54, 3731–3741. [Google Scholar] [CrossRef]
- Khammassi, S.; Tarfaoui, M.; Qureshi, Y.; Benyahia, H. Mechanical properties of graphene nanoplatelets reinforced epikote 828 under dynamic compression. Mech. Mater. 2021, 158, 103873. [Google Scholar] [CrossRef]
- Valášková, M.; Hundáková, M.; Kutláková, K.M.; Seidlerová, J.; Čapková, P.; Pazdziora, E.; Matějová, K.; Heřmánek, M.; Klemm, V.; Rafaja, D. Preparation and characterization of antibacterial silver/vermiculites and silver/montmorillonites. Geochim. Cosmochim. Acta 2010, 74, 6287–6300. [Google Scholar] [CrossRef]
- Plachá, D.; Kovář, P.; Vaněk, J.; Mikeska, M.; Škrlová, K.; Dutko, O.; Řeháčková, L.; Slabotínský, J. Adsorption of nerve Agent simulants onto vermiculite structure: Experiments and modelling. J. Hazard. Mater. 2020, 382, 121001. [Google Scholar] [CrossRef]
- Plachá, D.; Jampilek, J. Graphenic materials for biomedical applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef] [Green Version]
- Plachá, D.; Martynková, G.S.; Rümmeli, M.H. Variations in the sorptive properties of organovermiculites modified with hexadecyltrimethylammonium and hexadecylpyridinium cations. J. Sci. Conf. Proc. 2010, 2, 36–41. [Google Scholar] [CrossRef]
- Škrlová, K.; Holišová, V.; Mikeska, M.; Rybková, Z.; Malachová, K.; Martynková, G.S.; Plachá, D. Polylactide composites suitable for medical devices. J. Nanosci. Nanotechnol. 2019, 19, 2506–2513. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Mechanical, structural and thermal properties of Ag–Cu and ZnO reinforced polylactide nanocomposite films. Int. J. Biol. Macromol. 2016, 86, 885–892. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Santoshi Kumari, A.; Veerabhadram, G.; Girija Mangatayaru, K. A novel green synthesis of silver nanoparticles using gum karaya: Characterization, antimicrobial and catalytic activity studies. J. Clust. Sci. 2014, 25, 409–422. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, J.; Wang, R.; Chen, H. Microwave-assisted Synthesis of Polylactide acid/vermiculite Nanocomposites via In-situ Polymerization. Polym. Mater. Sci. Eng 2009, 25, 162–164. [Google Scholar]
- Ye, H.; Hou, K.; Zhou, Q. Improve the thermal and mechanical properties of poly(L-lactide) by forming nanocomposites with pristine vermiculite. Chin. J. Polym. Sci. 2016, 34, 1–12. [Google Scholar] [CrossRef]
- Belaid, H.; NAgarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavaillès, V.; et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C 2020, 110, 110595. [Google Scholar] [CrossRef]
- Pinto, A.M.; Moreira, S.; Gonçalves, I.C.; Gama, F.M.; Mendes, A.M.; MAgalhães, F.D. Biocompatibility of poly(lactic acid) with incorporated graphene-based materials. Colloids Surf. B Biointerfaces 2013, 104, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, A.M.; Cabral, J.; Tanaka, D.A.P.; Mendes, A.M.; MAgalhães, F.D. Effect of incorporation of graphene oxide and graphene nanoplatelets on mechanical and gas permeability properties of poly(lactic acid) films. Polym. Int. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhu, K.; Sun, X.-N.; Liu, C.-G.; Wang, S.-B.; Chen, A.-Z. Cardiac Tissue Engineering on the Nanoscale. ACS Biomater. Sci. Eng. 2018, 4, 800–818. [Google Scholar] [CrossRef]
- Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K. Functionalization of Graphene Oxide and its Biomedical Applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 291–315. [Google Scholar] [CrossRef]
- PapAgeorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [Green Version]
- El Moumen, A.; Tarfaoui, M.; Lafdi, K. Mechanical characterization of carbon nanotubes based polymer composites using indentation tests. Compos. Part B Eng. 2017, 114, 1–7. [Google Scholar] [CrossRef]
- Wright-Charlesworth, D.D.; Miller, D.M.; Miskioglu, I.; King, J.A. Nanoindentation of injection molded PLA and self-reinforced composite PLA after in vitro conditioning for three months. J. Biomed. Mater. Res. Part A 2005, 74, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Voyiadjis, G.Z.; Samadi-Dooki, A.; Malekmotiei, L. Nanoindentation of high performance semicrystalline polymers: A case study on PEEK. Polym. Test. 2017, 61, 57–64. [Google Scholar] [CrossRef]
- Muzyka, R.; Kwoka, M.; Smędowski, Ł.; Díez, N.; Gryglewicz, G. Oxidation of graphite by different modified Hummers methods. New Carbon Mater. 2017, 32, 15–20. [Google Scholar] [CrossRef]
- Plachá, D.; Martynková, G.S.; Rümmeli, M.H. Preparation of organovermiculites using HDTMA: Structure and sorptive properties using naphthalene. J. Colloid Interface Sci. 2008, 327, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Tarfaoui, M.; Lafdi, K.; Beloufa, I.; Daloia, D.; Muhsan, A. Effect of graphene nano-additives on the local mechanical behavior of derived polymer nanocomposites. Polymers 2018, 10, 667. [Google Scholar] [CrossRef] [Green Version]












| Temperature (°C) | PLA | PLA + GO + Ag | PLA + VMT + Ag | PLA + VMT + HDTMA | PLA + VMT + HDP |
|---|---|---|---|---|---|
| Tg | 60.86 | 60.71 | 60.77 | 60.34 | 60.18 |
| Tc | 105.85 | 122.54 | 109.76 | 104.67 | 105.23 |
| Tm1 | 147.15 | - | 147.90 | 146.72 | 147.42 |
| Tm2 | 153.62 | 151.07 | 153.52 | 154.52 | 154.92 |
| Neat PLA | PLA VMT + Ag | PLA GO + Ag | PLA VMT + HDTMA | PLA VMT + VDP | ||
|---|---|---|---|---|---|---|
| E (GPa) | AverAge | 5.372 | 4.972 | 3.820 | 5.833 | 6.620 |
| SD | 0.161 | 0.286 | 0.0553 | 1.100 | 0.366 | |
| Hv (Vickers) | AverAge | 25.196 | 38.855 | 38.581 | 39.501 | 54.953 |
| SD | 18.349 | 3.987 | 1.891 | 0.526 | 7.452 | |
| S (mN/µm) | AverAge | 337.569 | 271.123 | 233.747 | 356.687 | 343.598 |
| SD | 3.099 | 15.004 | 11.450 | 69.461 | 4.554 | |
| D (µm) | AverAge | 15.330 | 14.300 | 14.810 | 14.580 | 12.960 |
| SD | 0.0566 | 0.594 | 0.584 | 0.329 | 0.650 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khammassi, S.; Tarfaoui, M.; Škrlová, K.; Měřínská, D.; Plachá, D.; Erchiqui, F. Poly(Lactic Acid) (PLA)-Based Nanocomposites: Impact of Vermiculite, Silver, and Graphene Oxide on Thermal Stability, Isothermal Crystallization, and Local Mechanical Behavior. J. Compos. Sci. 2022, 6, 112. https://doi.org/10.3390/jcs6040112
Khammassi S, Tarfaoui M, Škrlová K, Měřínská D, Plachá D, Erchiqui F. Poly(Lactic Acid) (PLA)-Based Nanocomposites: Impact of Vermiculite, Silver, and Graphene Oxide on Thermal Stability, Isothermal Crystallization, and Local Mechanical Behavior. Journal of Composites Science. 2022; 6(4):112. https://doi.org/10.3390/jcs6040112
Chicago/Turabian StyleKhammassi, Sabrine, Mostapha Tarfaoui, Kateřina Škrlová, Dagmar Měřínská, Daniela Plachá, and Fouad Erchiqui. 2022. "Poly(Lactic Acid) (PLA)-Based Nanocomposites: Impact of Vermiculite, Silver, and Graphene Oxide on Thermal Stability, Isothermal Crystallization, and Local Mechanical Behavior" Journal of Composites Science 6, no. 4: 112. https://doi.org/10.3390/jcs6040112
APA StyleKhammassi, S., Tarfaoui, M., Škrlová, K., Měřínská, D., Plachá, D., & Erchiqui, F. (2022). Poly(Lactic Acid) (PLA)-Based Nanocomposites: Impact of Vermiculite, Silver, and Graphene Oxide on Thermal Stability, Isothermal Crystallization, and Local Mechanical Behavior. Journal of Composites Science, 6(4), 112. https://doi.org/10.3390/jcs6040112







