In Vitro Electrochemical Corrosion Assessment of Magnesium Nanocomposites Reinforced with Samarium(III) Oxide and Silicon Dioxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. SEM Investigation
2.2. Microhardness Test
2.3. Electrochemical Corrosion Tests
2.3.1. Simulated Body Fluid (SBF) Preparation
2.3.2. Electrical Impedance Spectroscopy (EIS)
2.3.3. Potentiodynamic Polarization Test (PDP)
3. Results and Discussion
3.1. SEM Investigation
3.2. Microhardness
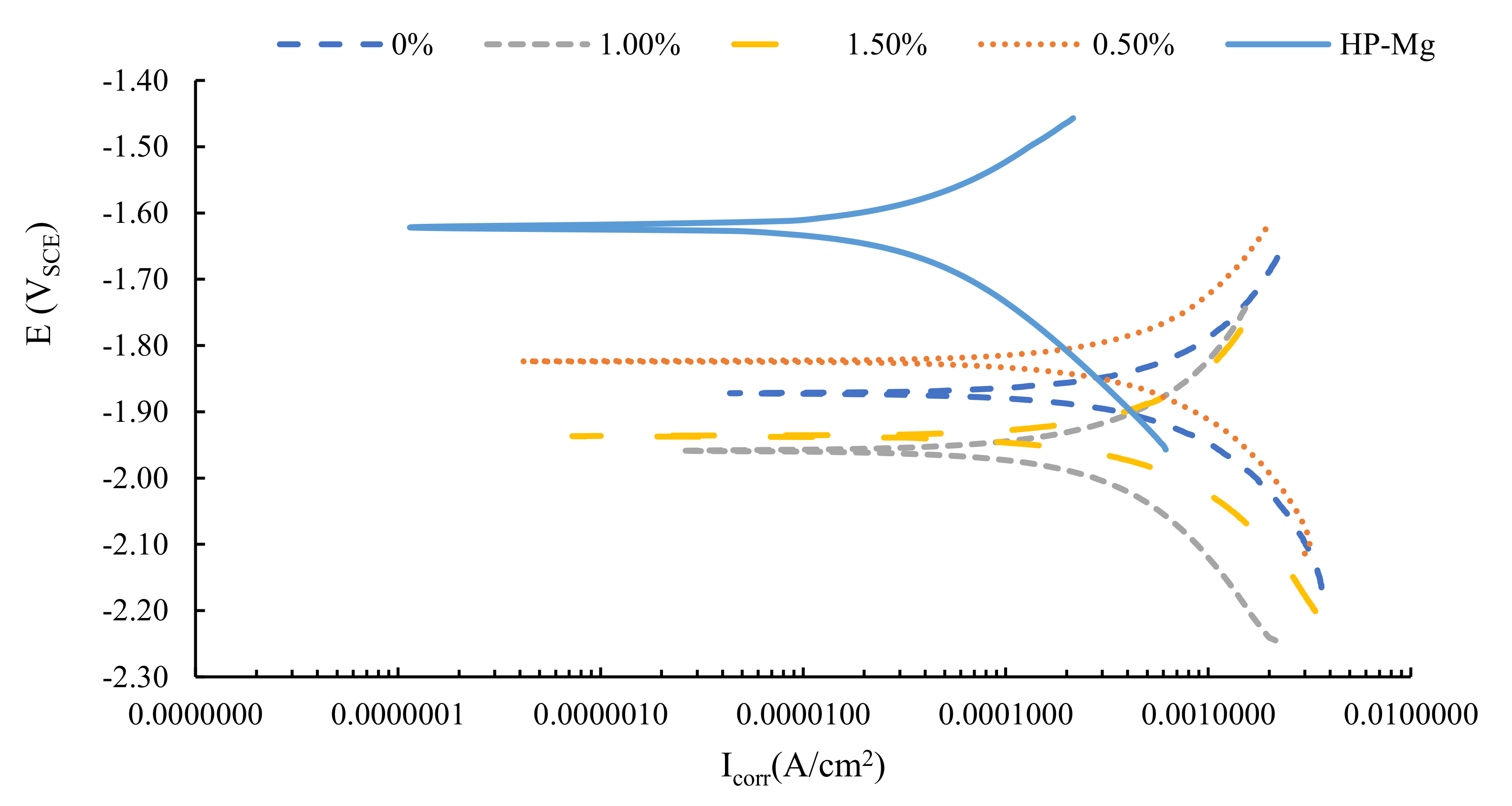
3.3. Electrochemical Corrosion Behavior
4. Conclusions
- The microhardness increased 31% for the 1.0 Vol% Sm2O3 nanocomposite and 12% for the 1.5 vol.% SiO2 nanocomposite compared to pure Mg.
- Both nanocomposites showed the highest corrosion resistance and lowest corrosion current density at the 1.0 Vol% content. Increasing the nanoparticles content above 1.0% Vol resulted in a reduction in corrosion resistance due to localized agglomerations of the nanoparticles at higher contents.
- The corrosion resistance of the pure magnesium samples was less than that for the HP-Mg. These pure magnesium samples were manufactured using the same fabrication methods used to make the nanocomposites.
- Therefore, a tribocorrosion behavior of the developed nanocomposites is hypothesized to be better than HP-Mg due to the improved wear resistance. Future work on tribocorrosion behavior is required to assess this hypothesis.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bicsák, Á.; Abel, D.; Tack, L.; Smponias, V.; Hassfeld, S.; Bonitz, L. Complications after osteosynthesis of craniofacial fractures—An analysis from the years 2015–2017. Oral Maxillofac. Surg. 2021, 25, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Planell, J.; Navarro, M. Challenges of bone repair. In Bone Repair Biomaterials; Woodhead Publishing: Sawston, UK, 2009; pp. 3–24. [Google Scholar]
- Johansson, B.; Grepe, A.; Wannfors, K.; Hirsch, J.M. A clinical study of changes in the volume of bone grafts in the atrophic maxilla. Dento Maxillo Facial Radiol. 2001, 30, 157–161. [Google Scholar] [CrossRef]
- Ibrahim, H.; Esfahani, S.N.; Poorganji, B.; Dean, D.; Elahinia, M. Resorbable bone fixation alloys, forming, and post-fabrication treatments. Mater. Sci. Eng. C 2017, 70, 870–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.-M.; Fujihara, K. Stiffness and strength design of composite bone plates. Compos. Sci. Technol. 2005, 65, 73–85. [Google Scholar] [CrossRef]
- Rahmanian, R.; Shayesteh Moghaddam, N.; Haberland, C.; Dean, D.; Miller, M.; Elahinia, M. Load bearing and stiffness tailored NiTi implants produced by additive manufacturing: A simulation study. In Proceedings of the International Society for Optical Engineering, San Diego, CA, USA, 5–7 July 1995. [Google Scholar]
- Abdalla, M.; Joplin, A.; Elahinia, M.; Ibrahim, H. Corrosion Modeling of Magnesium and Its Alloys for Biomedical Applications. Corros. Mater. Degrad. 2020, 1, 219–248. [Google Scholar] [CrossRef]
- Abdalla, M.; Ibrahim, H. A Physical Approach to Simulate the Corrosion of Ceramic-Coated Magnesium Implants. Appl. Sci. 2021, 11, 6724. [Google Scholar] [CrossRef]
- Lesz, S.; Hrapkowicz, B.; Karolus, M.; Gołombek, K. Characteristics of the Mg-Zn-Ca-Gd alloy after mechanical alloying. Materials 2021, 14, 226. [Google Scholar] [CrossRef]
- Cesarz-Andraczke, K.; Kazek-Kęsik, A. PEO layers on Mg-based metallic glass to control hydrogen evolution rate. Bull. Pol. Acad. Sci. Tech. Sci. 2020, 68, 119–124. [Google Scholar]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Persaud-Sharma, D.; McGoron, A. Biodegradable magnesium alloys: A review of material development and applications. J. Biomim. Biomater. Tissue Eng. 2011, 12, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Datta, M.K.; Chou, D.-T.; Hong, D.; Saha, P.; Chung, S.J.; Lee, B.; Sirinterlikci, A.; Ramanathan, M.; Roy, A.; Kumta, P.N. Structure and thermal stability of biodegradable Mg-Zn-Ca based amorphous alloys synthesized by mechanical alloying. Mater. Sci. Eng. B 2011, 176, 1637–1643. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Goodfellow. Magnesium-Material Properties. Available online: http://www.goodfellow.com/E/Magnesium.html (accessed on 7 March 2021).
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Nakatsugawa, I.; Kamado, S.; Kojima, Y.; Ninomiya, R.; Kubota, K. Corrosion of magnesium alloys containing rare earth elements. Corros. Rev. 1998, 16, 139–158. [Google Scholar] [CrossRef]
- Ibrahim, H.; Klarner, A.D.; Poorganji, B.; Dean, D.; Luo, A.A.; Elahinia, M. Microstructural, mechanical and corrosion characteristics of heat-treated Mg-1.2 Zn-0.5 Ca (wt%) alloy for use as resorbable bone fixation material. J. Mech. Behav. Biomed. Mater. 2017, 69, 203–212. [Google Scholar] [CrossRef]
- Chmielewska, A.; MacDonald, T.; Ibrahim, H.; McManus, T.; Lindemann, J.L.; Smith, P.; Rong, L.; Luo, A.; Advincula, R.; Swieszkowski, W. Biocompatibility of a novel heat-treated and ceramic-coated magnesium alloy (Mg-1.2 Zn-0.5 Ca-0.5 Mn) for resorbable skeletal fixation devices. MRS Commun. 2020, 10, 467–474. [Google Scholar] [CrossRef]
- Ibrahim, H.; Luo, A.; Dean, D.; Elahinia, M. Effect of Zn content and aging temperature on the in-vitro properties of heat-treated and Ca/P ceramic-coated Mg-0.5% Ca-x% Zn alloys. Mater. Sci. Eng. C 2019, 103, 109700. [Google Scholar] [CrossRef]
- Huang, Y.; Gan, W.; Kainer, K.U.; Hort, N. Role of multi-microalloying by rare earth elements in ductilization of magnesium alloys. J. Magnes. Alloy. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Evans, C. Toxicology and pharmacology of the lanthanides. In Biochemistry of the Lanthanides; Springer: Boston, MA, USA, 1990; pp. 339–389. [Google Scholar]
- Evans, C. Past, Present, and Possible Future Clinical Applications of the Lanthanides. In Biochemistry of the Lanthanides; Springer: Boston, MA, USA, 1990; pp. 391–425. [Google Scholar]
- Jing, H.; Wu, X.; Liu, Y.; Lu, M.; Yang, K.; Yao, Z.; Ke, W. Antibacterial property of Ce-bearing stainless steels. J. Mater. Sci. 2007, 42, 5118–5122. [Google Scholar] [CrossRef]
- Yuan, J.; Li, W.; Wang, C. Effect of the La alloying addition on the antibacterial capability of 316L stainless steel. Mater. Sci. Eng. C 2013, 33, 446–452. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Dargusch, M.; Wen, C. A review of the physiological impact of rare earth elements and their uses in biomedical Mg alloys. Acta Biomater. 2021, 130, 80–97. [Google Scholar] [CrossRef]
- Kujur, M.S.; Mallick, A.; Manakari, V.; Parande, G.; Tun, K.S.; Gupta, M. Significantly enhancing the ignition/compression/damping response of monolithic magnesium by addition of Sm2O3 nanoparticles. Metals 2017, 7, 357. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.; Gotman, I.; Unger, R.; Gutmanas, E. Bioresorbable β-TCP-FeAg nanocomposites for load bearing bone implants: High pressure processing, properties and cell compatibility. Mater. Sci. Eng. C 2017, 78, 88–95. [Google Scholar] [CrossRef]
- Prasadh, S.; Manakari, V.; Parande, G.; Wong, R.C.W.; Gupta, M. Hollow silica reinforced magnesium nanocomposites with enhanced mechanical and biological properties with computational modeling analysis for mandibular reconstruction. Int. J. Oral Sci. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Ali, M.; Hussein, M.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Kujur, M.S.; Manakari, V.; Parande, G.; Prasadh, S.; Wong, R.; Mallick, A.; Gupta, M. Effect of samarium oxide nanoparticles on degradation and invitro biocompatibility of magnesium. Mater. Today Commun. 2021, 26, 102171. [Google Scholar] [CrossRef]
- Herath, H.; Di Silvio, L.; Evans, J. In vitro evaluation of samarium (III) oxide as a bone substituting material. J. Biomed. Mater. Res. Part A 2010, 94, 130–136. [Google Scholar] [CrossRef]
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work. 2013, 4, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Liu, Y.; Li, Y. Adverse effects of amorphous silica nanoparticles: Focus on human cardiovascular health. J. Hazard. Mater. 2021, 406, 124626. [Google Scholar] [CrossRef]
- Haghshenas, M.; Gupta, M. Magnesium nanocomposites reinforced with rare earth element nanoparticles: Nanoindentation-driven response. Nanocomposites 2020, 6, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Kishimoto, K.; Miyaji, F.; Kokubo, T.; Yao, T.; Suetsugu, Y.; Tanaka, J.; Nakamura, T. Composition and structure of the apatite formed on PET substrates in SBF modified with various ionic activity products. J. Biomed. Mater. Res. 1999, 46, 228–235. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Meratian, M. Microstructure, mechanical properties and bio-corrosion evaluation of biodegradable AZ91-FA nanocomposites for biomedical applications. Mater. Sci. Eng. A 2010, 527, 6938–6944. [Google Scholar] [CrossRef]
- Lin, G.; Liu, D.; Chen, M.; You, C.; Li, Z.; Wang, Y.; Li, W. Preparation and characterization of biodegradable Mg-Zn-Ca/MgO nanocomposites for biomedical applications. Mater. Charact. 2018, 144, 120–130. [Google Scholar] [CrossRef]
- Haghshenas, M.; Muhammad, M.; Hasannaeimi, V.; Mukherjee, S.; Gupta, M. Ambient and non-ambient temperature depth-sensing indentation of Mg-Sm2O3 nanocomposites. Int. J. Adv. Manuf. Technol. 2019, 105, 2947–2956. [Google Scholar] [CrossRef]
- Feliu, S. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Kirkland, N.; Birbilis, N.; Staiger, M. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Manakari, V.; Kannan, S.; Parande, G.; Doddamani, M.; Columbus, S.; Vincent, S.; Gupta, M. In-vitro degradation of hollow silica reinforced magnesium syntactic foams in different simulated body fluids for biomedical applications. Metals 2020, 10, 1583. [Google Scholar] [CrossRef]
- Kannan, M.B.; Khakbaz, H.; Yamamoto, A. Understanding the influence of HEPES buffer concentration on the biodegradation of pure magnesium: An electrochemical study. Mater. Chem. Phys. 2017, 197, 47–56. [Google Scholar] [CrossRef]







| Chemical | Concentration (g/L) |
|---|---|
| NaCl | 5.403 |
| NaHCO3 | 0.504 |
| Na2CO3 | 0.426 |
| KCl | 0.225 |
| K2HPO4·3H2O | 0.23 |
| MgCl2·6H2O | 0.311 |
| HEPES | 17.892 |
| CaCl2 | 0.293 |
| Na2SO4 | 0.072 |
| 1 M NaOH (mL) | 15 |
| EIS Test Results | PDP Test Results | ||||
|---|---|---|---|---|---|
| Material | Rp (Ohm/cm2) | Rt (Ohm/cm2) | Rtot (Ohm/cm2) | Ecorr (V) | icorr (µA/cm2) |
| HP-Mg (as rolled) | 544 | 87 | 631 | −1.62 | 48 |
| Pure Mg | 174 | 83 | 256 | −1.87 | 770 |
| Mg-0.5% Sm2O3 | 184 | 151 | 334 | −1.82 | 623 |
| Mg-1% Sm2O3 | 339 | 48 | 387 | −1.96 | 490 |
| Mg-1.5% Sm2O3 | 135 | 23 | 158 | −1.94 | 812 |
| EIS Test Results | PDP Test Results | ||||
|---|---|---|---|---|---|
| Material | Rp (Ohm/cm2) | Rt (Ohm/cm2) | Rtot (Ohm/cm2) | Ecorr (V) | icorr (µA/cm2) |
| HP-Mg (as rolled) | 544 | 87 | 631 | −1.62 | 48 |
| Pure Mg | 174 | 83 | 256 | −1.87 | 770 |
| Mg-0.5% SiO2 | 237 | 82 | 318 | −1.748 | 320 |
| Mg-1% SiO2 | 312 | 104 | 416 | −1.702 | 153 |
| Mg-1.5% SiO2 | 263 | 89 | 352 | −1.747 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, M.; Sims, A.; Mehanny, S.; Haghshenas, M.; Gupta, M.; Ibrahim, H. In Vitro Electrochemical Corrosion Assessment of Magnesium Nanocomposites Reinforced with Samarium(III) Oxide and Silicon Dioxide Nanoparticles. J. Compos. Sci. 2022, 6, 154. https://doi.org/10.3390/jcs6060154
Abdalla M, Sims A, Mehanny S, Haghshenas M, Gupta M, Ibrahim H. In Vitro Electrochemical Corrosion Assessment of Magnesium Nanocomposites Reinforced with Samarium(III) Oxide and Silicon Dioxide Nanoparticles. Journal of Composites Science. 2022; 6(6):154. https://doi.org/10.3390/jcs6060154
Chicago/Turabian StyleAbdalla, Moataz, Austin Sims, Sherif Mehanny, Meysam Haghshenas, Manoj Gupta, and Hamdy Ibrahim. 2022. "In Vitro Electrochemical Corrosion Assessment of Magnesium Nanocomposites Reinforced with Samarium(III) Oxide and Silicon Dioxide Nanoparticles" Journal of Composites Science 6, no. 6: 154. https://doi.org/10.3390/jcs6060154
APA StyleAbdalla, M., Sims, A., Mehanny, S., Haghshenas, M., Gupta, M., & Ibrahim, H. (2022). In Vitro Electrochemical Corrosion Assessment of Magnesium Nanocomposites Reinforced with Samarium(III) Oxide and Silicon Dioxide Nanoparticles. Journal of Composites Science, 6(6), 154. https://doi.org/10.3390/jcs6060154









