Conductive Oxides for Formulating Mitigated-Sensitivity Energetic Composite Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Al/ITO Energetic Composite
2.2. Characterization Techniques
3. Results and Discussion
3.1. Individual Ingredients and Energetic Mixture Characteristics
3.2. Sensitivity Properties of the Al/ITO Energetic Composites
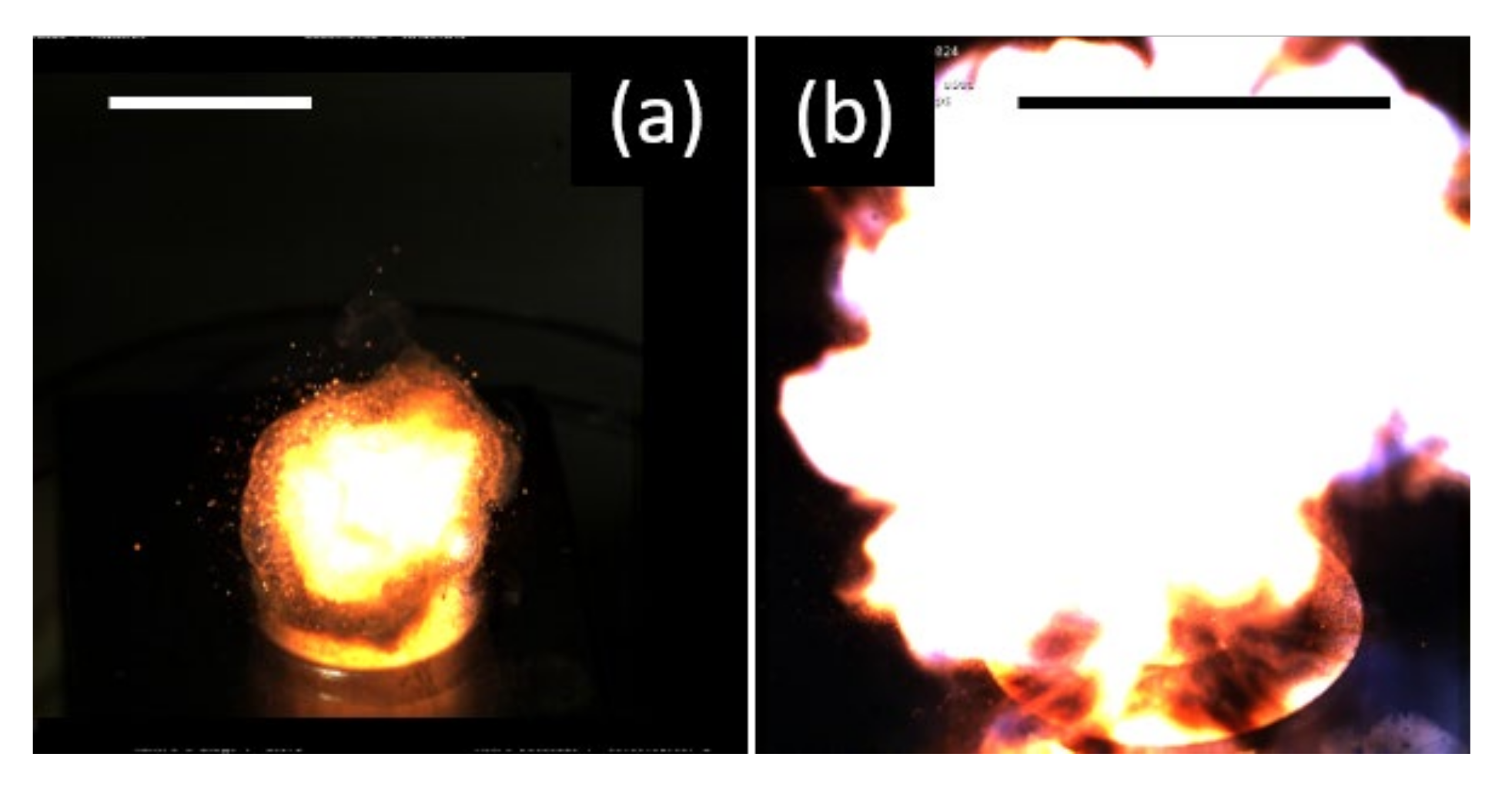
3.3. Ignition Ability of the Al/ITO Energetic Composites
3.4. Combustion Speed of the Al/ITO Energetic Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhavan, J. The Chemistry of Explosives, 2nd ed.; Royal Society of Chemistry: London, UK, 2004. [Google Scholar]
- Fischer, S.H.; Grubelich, M.C. Theoretical energy release of thermites, intermetallics, and combustible metals. In Proceedings of the 24th International Pyrotechnics Seminar, Monterey, CA, USA, 27–31 July 1998; pp. 231–286. [Google Scholar]
- Pantoya, M.L.; Granier, J.J. Combustion Behaviour of Highly Energetic Thermites: Nano versus Micron Composites. Propellants Explos. Pyrotech. 2005, 30, 53–62. [Google Scholar] [CrossRef]
- Pantoya, M.L.; Levitas, V.I.; Granier, J.J.; Henderson, J.B. Effect of bulk density on reaction propagation in nanothermites and micronthermites. J. Propuls. Power 2009, 25, 65–470. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, J.P. High Energy Materials, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- No. 4489; NATO Standardization Agreement (STANAG) on Explosives, Impact Sensitivity Tests. 1st ed. NATO Standardization Agency: Brussels, Belgium, 1999.
- No. 4487; NATO Standardization Agreement (STANAG) on Explosive, Friction Sensitivity Tests. 1st ed. NATO Standardization Agency: Brussels, Belgium, 2002.
- United Nations. Recommendations on the Transport of Dangerous Goods: Manual of Tests and Criteria, 4th revised ed.; United Nations: New York, NY, USA; Geneva, Switzerland, 2007. [Google Scholar]
- Talawar, M.B.; Agrawal, A.P.; Anniyappan, M.; Wani, D.S.; Bansode, M.K.; Gore, G.M. Primary explosives: Electrostatic discharge initiation, additive effect and its relation to thermal and explosive characteristics. J. Hazard. Mater. 2006, 137, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Greason, W.D. Electrostatic discharge characteristics for the human body and circuit packs. J. Electrost. 2003, 59, 285–300. [Google Scholar] [CrossRef]
- Moureaux, P.; Poyard, J.L. Phénomènes Electrostatiques—Risques Associés et Prévention; INRS: Quebec City, QC, Canada, 2019; ED 6354. [Google Scholar]
- Gibot, P.; Miesch, Q.; Bach, A.; Schnell, F.; Gadiou, R.; Spitzer, D. Mechanical desensitization of an Al/WO3 nanothermite by means of carbonaceous coatings derived from carbohydrates. C J. Carbon Res. 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Gibot, P.; Goetz, V. Aluminium/Tin (IV) oxide thermite composite: Sensitivities and reaction propagation. J. Energ. Mater. 2020, 38, 295–308. [Google Scholar] [CrossRef]
- Puszynski, J.A.; Bulian, C.J.; Swiatkiewicz, J.J. Processing and ignition characteristics of aluminum-bismuth trioxide nanothermite system. J. Propuls. Power 2007, 23, 698. [Google Scholar] [CrossRef]
- Poper, K.H.; Collins, E.S.; Pantoya, M.L.; Daniels, M.A. Controlling the electrostatic discharge ignition sensitivity of composite energetic materials using carbon nanotube additives. J. Electrost. 2014, 72, 428–432. [Google Scholar] [CrossRef]
- Siegert, B.; Comet, M.; Muller, O.; Pourroy, G.; Spitzer, D. Reduced-sensitivity nanothermites containing manganese oxide filled carbon nanofibers. J. Phys. Chem. C 2010, 114, 19562–19568. [Google Scholar] [CrossRef]
- Thiruvengadathan, R.; Chung, S.W.; Basuray, S.; Balasubramaniam, B.; Staley, C.S.; Gangopahyay, K.; Gangopadhyay, S. A versatile self-assembly approach toward high performance nanoenergetic composite using functionalized graphene. Langmuir 2014, 30, 6556–6564. [Google Scholar] [CrossRef]
- Bach, A.; Gibot, P.; Vidal, L.; Gadiou, R.; Spitzer, D. Modulation of the Reactivity of a WO3/Al Energetic Material with Graphitized Carbon Black as Additive. J. Energ. Mater. 2015, 33, 260–276. [Google Scholar] [CrossRef]
- Gibot, P.; Bach, A.; Vidal, L.; Schnell, F.; Gadiou, R.; Spitzer, D. Safer and performing energetic materials based on polyaniline-doped nanocomposites. J. Energ. Mater. 2017, 35, 136–147. [Google Scholar] [CrossRef]
- Gibot, P.; Goetz, V. SnO2–polyaniline composites for the desensitization of Al/SnO2 thermite composites. J. Appl. Polym. Sci. 2020, 137, 48947. [Google Scholar] [CrossRef]
- Foley, T.; Pacheco, A. Development of nanothermite composites with variable electrostatic discharge ignition thresholds. Propellants Explos. Pyrotech. 2007, 32, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Yana, N.; Qin, L.; Hao, H.; Hui, L.; Zhao, F.; Feng, H. Iron oxide/aluminum/graphene energetic nanocomposites synthesized by atomic layer deposition: Enhanced energy release and reduced electrostatic ignition hazard. Appl. Surf. Sci. 2017, 408, 51–59. [Google Scholar] [CrossRef]
- Li, S.; Tian, M.; Gao, Q. Nanometre-thin indium tin oxide for advanced high-performance electronics. Nat. Mater. 2019, 18, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, X.; Liu, C.; Ni, J.; Hou, G.; Zhao, Y.; Zhang, X. Highly transparent and conductive indium tin oxide thin films for solar cells grown by reactive thermal evaporation at low temperature. Appl. Phys. A Mater. Sci. Process. 2014, 117, 815–822. [Google Scholar] [CrossRef]
- Luis, A.; Nunes de Carvalho, C.; Lavareda, G.; Amaral, A.; Brogueira, P.; Godinho, M.H. ITO coated flexible transparent substrates for liquid crystal-based devices. Vacuum 2002, 64, 475–479. [Google Scholar] [CrossRef]
- Betz, U.; Olsson, M.K.; Marthy, J.; Escola, M.F.; Atamny, F. Thin films engineering of indium tin oxide: Large area at panel displays application. Surf. Coat. 2006, 200, 5751–5759. [Google Scholar] [CrossRef]
- Wan, Q.; Dattoli, E.N.; Fung, W.Y.; Guo, W.; Chen, Y.; Pan, X.; Lu, W. High-performance transparent conducting oxide nanowires. Nano Lett. 2006, 6, 2909–2915. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chang, H.; Wu, S.; Leng, Y.; Wang, L. Study on electromagnetic shielding of infrared/visible optical window. Mod. Appl. Sci. 2015, 9, 231–236. [Google Scholar]
- Kerkache, L.; Layadia, A.; Mosser, A. Effect of oxygen partial pressure on the structural and optical properties of dc sputtered ITO thin films. J. Alloy Compd. 2009, 485, 46–50. [Google Scholar] [CrossRef]
- Al-Dahoudi, N.; Bisht, H.; Gobbert, C.; Krajewski, T.; Aegerter, M. Transparent conducting, anti-static and antistatic—Anti-glare coatings on plastic substrates. Thin Solid Films 2001, 392, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Gibot, P. Templated synthesis of Cr2O3 material for energetic composites with high performance. Solid State Sci. 2019, 94, 162–167. [Google Scholar] [CrossRef]
- Martirosyan, K.S. Nanoenergetic gas-generator: Principles and applications. J. Mater. Chem. 2011, 21, 9400–9405. [Google Scholar] [CrossRef]
- Gibot, P.; Comet, M.; Eichhorn, A.; Schnell, F.; Muller, O.; Ciszek, F.; Boehrer, Y.; Spitzer, D. Highly insensitive/reactive thermite prepared from Cr2O3 nanoparticles. Propellants Explos. Pyrotech. 2011, 36, 80–87. [Google Scholar] [CrossRef]
- Gibot, P.; Comet, M.; Vidal, L.; Moitrier, F.; Lacroix, F.; Suma, Y.; Schnell, F.; Spitzer, D. Synthesis of WO3 nanoparticles for superthermites by the template method from silica spheres. Solid State Sci. 2011, 13, 908–914. [Google Scholar] [CrossRef]
- Gibot, P.; Puel, E. Study on indium (III) oxide/aluminum thermite energetic composites. J. Compos. Sci. 2021, 5, 166. [Google Scholar] [CrossRef]
- Gibot, P.; Oudot, F.; Lallemand, B.; Schnell, F.; Spitzer, D. Nanosized niobium (V) and tantalum (V) oxide ceramics as competitive oxidizers within aluminum-based nanothermites. Energ. Mater. Front. 2021, 2, 167–173. [Google Scholar] [CrossRef]
- Pichot, V.; Comet, M.; Miesch, J.; Spitzer, D. Nanodiamond for tuning the properties of energetic composites. J. Hazard. Mater. 2015, 300, 194–201. [Google Scholar] [CrossRef]
- Bezmelnitsyn, A.; Thiruvengadathan, R.; Barizuddin, S.; Tappmeyer, D.; Apperson, S.; Gangopadhyay, K.; Gangopadhyay, S. Modified nanoenergetic composites with tunable combustion characteristics for propellant applications. Propellants Explos. Pyrotech. 2010, 35, 384–394. [Google Scholar] [CrossRef]
- Sanders, V.E.; Asay, B.W.; Foley, T.J.; Tappan, B.C.; Pacheco, A.N.; Son, S.F. Reaction propagation of four nanoscale energetic composites (Al/MoO3, Al/WO3, Al/CuO and Bi2O3). J. Propuls. Power 2007, 23, 707–714. [Google Scholar] [CrossRef]


| Sensitivity Test | ESD (mJ) | Impact (J) | Friction (N) |
|---|---|---|---|
| Al/ITO_µm | 13.70 | >100 | >360 |
| Al/ITO_nm | <0.21 | >100 | 324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibot, P.; Puel, E.; Lallemand, B.; Oudot, F. Conductive Oxides for Formulating Mitigated-Sensitivity Energetic Composite Materials. J. Compos. Sci. 2022, 6, 174. https://doi.org/10.3390/jcs6060174
Gibot P, Puel E, Lallemand B, Oudot F. Conductive Oxides for Formulating Mitigated-Sensitivity Energetic Composite Materials. Journal of Composites Science. 2022; 6(6):174. https://doi.org/10.3390/jcs6060174
Chicago/Turabian StyleGibot, Pierre, Estelle Puel, Bastien Lallemand, and Franck Oudot. 2022. "Conductive Oxides for Formulating Mitigated-Sensitivity Energetic Composite Materials" Journal of Composites Science 6, no. 6: 174. https://doi.org/10.3390/jcs6060174
APA StyleGibot, P., Puel, E., Lallemand, B., & Oudot, F. (2022). Conductive Oxides for Formulating Mitigated-Sensitivity Energetic Composite Materials. Journal of Composites Science, 6(6), 174. https://doi.org/10.3390/jcs6060174






