Crafting Metal Surface Morphology to Prevent Formation of the Carbon–Steel Interfacial Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
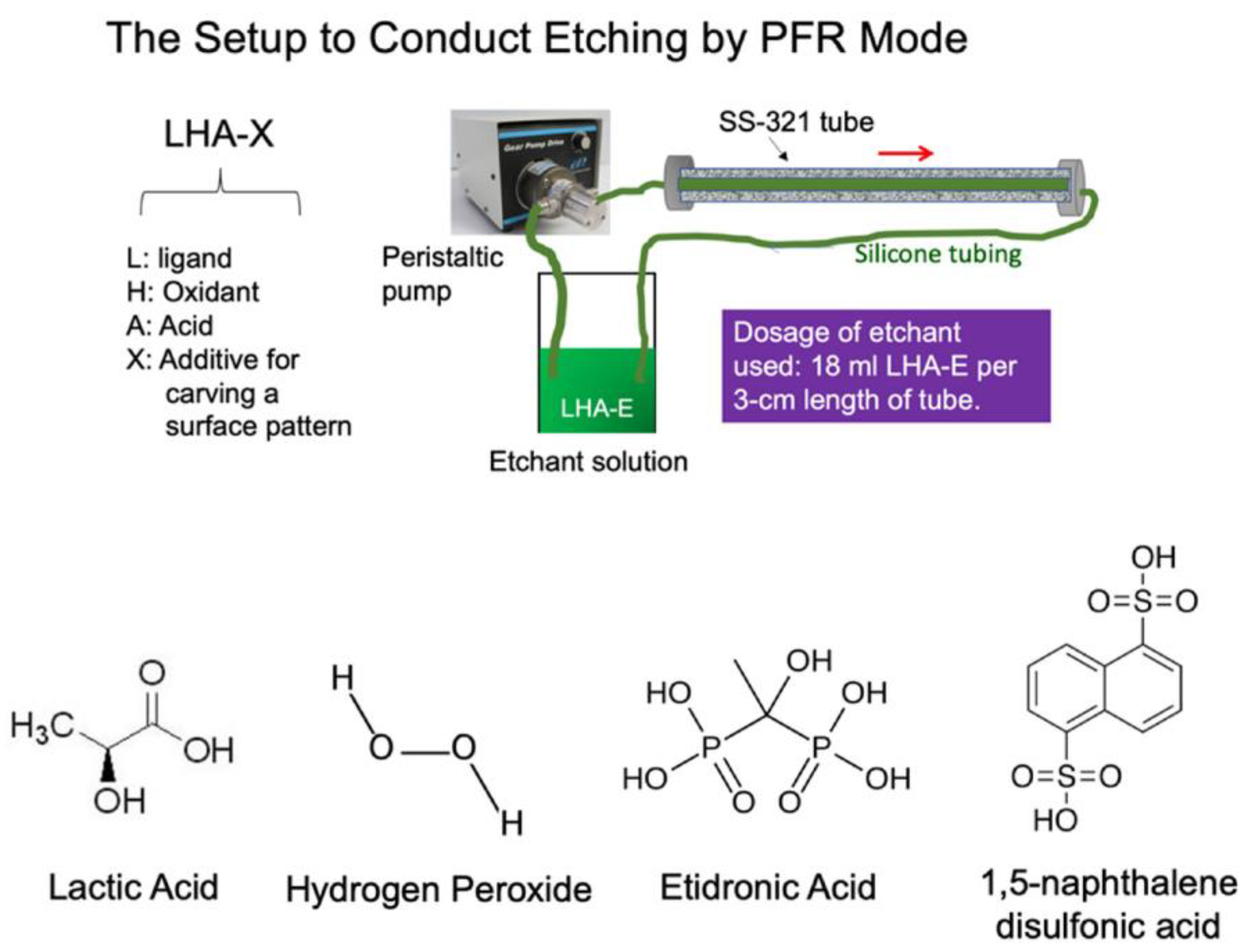
2.2. The Controlled Chemical Etching of the SS-321 Tube
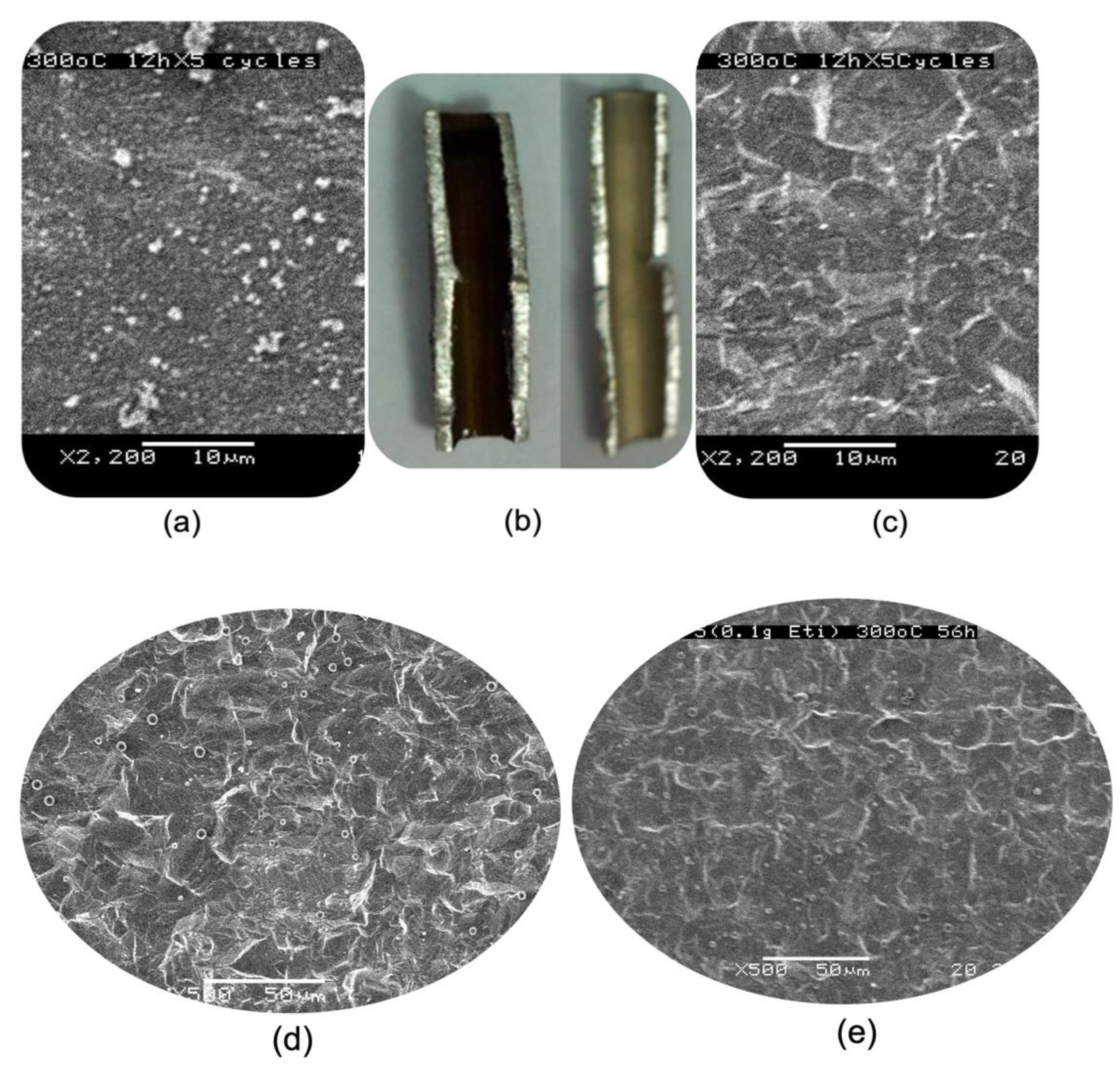
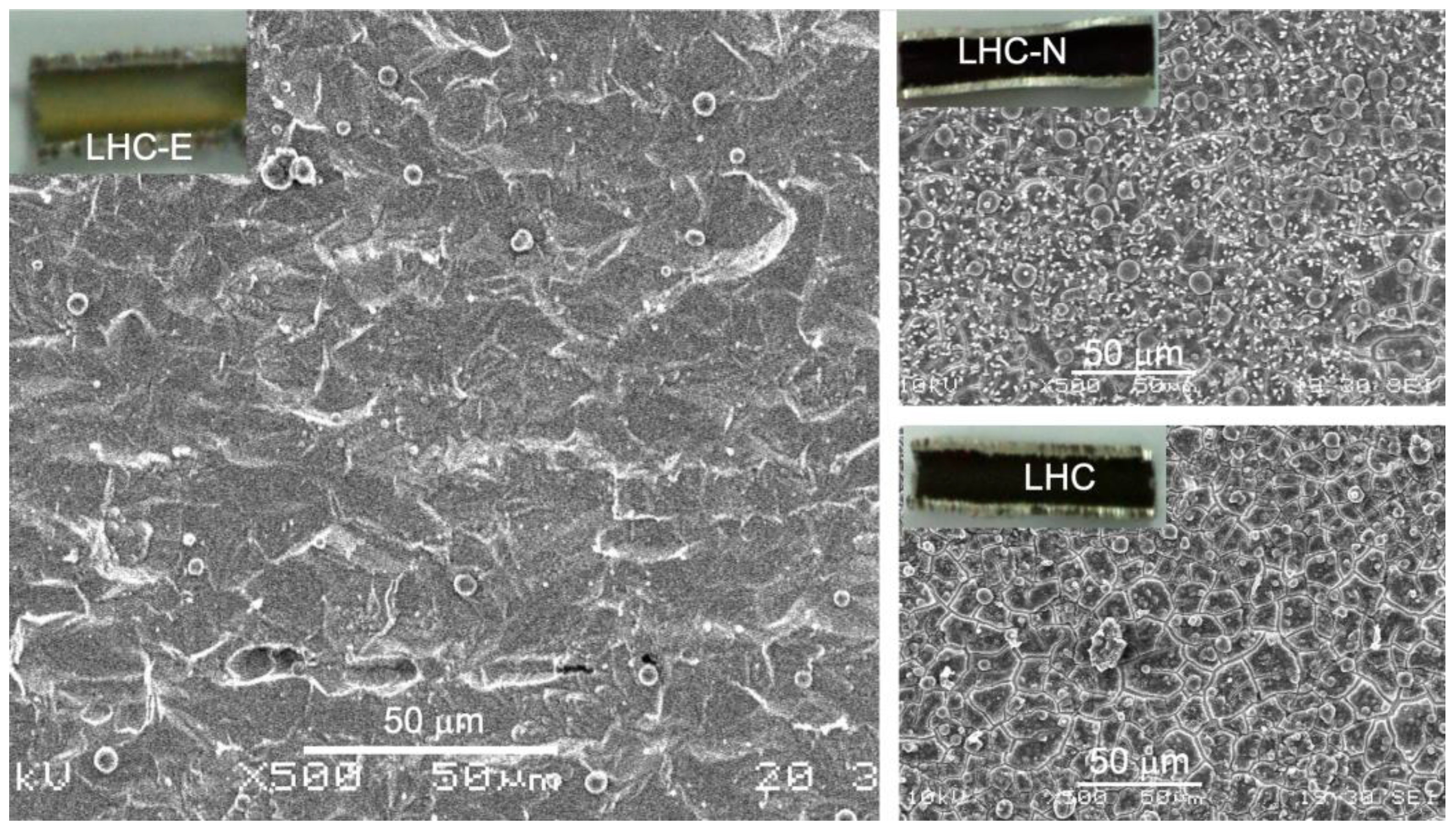
2.3. The Assessment of Coking Resistance of the Inner Surface of the SS-321 Tube
2.4. Characterization and Analysis
3. Results
3.1. Thermal Decomposition of the Designated Aerospace Lubricating Oil
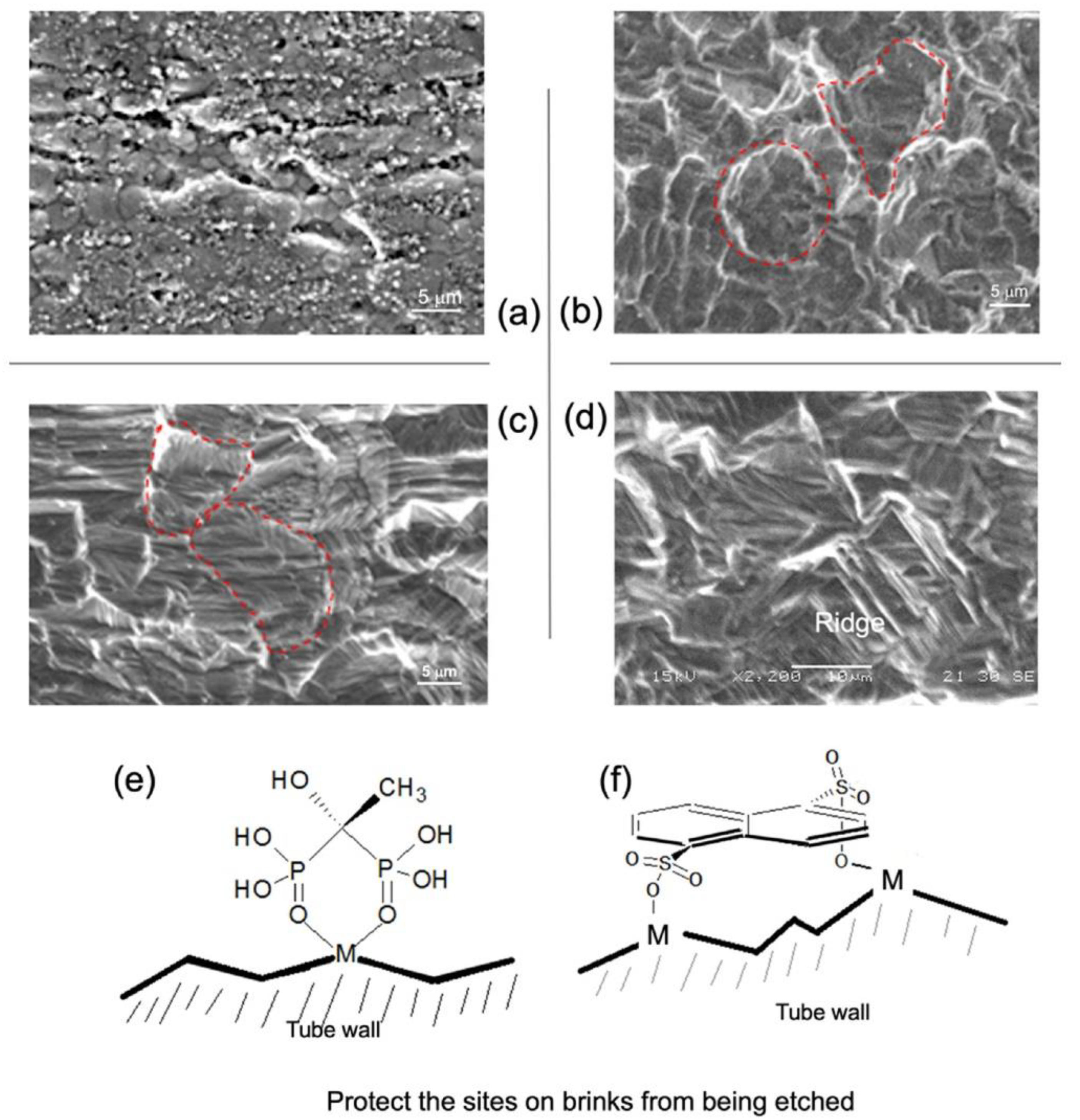
3.2. Restructuring the Inner Tube Surface by a Chemical Etching Means Deferring Coking
3.3. The Role of the Axial Shear Flow of the LHC-Based Etchant in the Attainment of the Surface Microstructures
4. Discussion
4.1. Coking Mechanism at the Interface between Oil and Steel
4.2. The Surface Chelating Effect on Crafting Microstructures and the Subsequent Leverage on Coking
5. Conclusions
- i.
- The coke formation in the SS-321 tube enclosing aerospace lubricant involves the construction of high-molecular-weight organic flocs and their entrapment on the inner surface of the crude tube, preferentially at tiny cracks, where the flocs are readily converted to carbonaceous grains, which by themselves also proliferate coke growth.
- ii.
- Formulation of an alternative type of etching solution consisting of the basic formula, lactic acid-HCl-H2O2-H2O (LHC), plus etidronic acid (E) or 1,5-naphthalene disulfonic acid (N) as a stabilizing agent.
- iii.
- Carrying out chemical etching using the three etchants, LHC, LHC-E, and LHC-N, through the axial flow to treat the SS-321 tube, which removes original defects and casts two specific topographic patterns at a micron-scale on the inner tube wall. In particular, LHC and LHC-E result in a grooved spherulite topographic pattern, whereas LHC-N has a ridge-like topographic pattern.
- iv.
- Examination of the leverages of surface roughness and topography on coking shows that removing micro defects and the oxide layer from the inner surface of the crude tube significantly enhances coke resistance. The roughness of the topography encourages the surface entrapment, but either topographic pattern counters the surface entrapment. The latter effect is attributed to micro-turbulence fields. Moreover, the ridge-like topography demonstrates a more extended anti-coking performance than the other pattern.
- v.
- The critical etching conditions affecting the anti-coking attributes include dosage per tube length, the optimal pumping rate, and etching duration concerning an etching recipe.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuznetsov, V.L.; Usol’Tseva, A.N.; Butenko, Y.V. Mechanism of Coking on Metal Catalyst Surfaces: I. Thermodynamic Analysis of Nucleation. Kinet. Catal. 2003, 44, 726–734. [Google Scholar] [CrossRef]
- Baker, R.T. Coking problems associated with hydrocarbon conversion processes. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 1996, 41. ISSN 0569-3772. [Google Scholar]
- Speight, J.G. The chemistry and physics of coking. Korean J. Chem. Eng. 1998, 15, 1–8. [Google Scholar] [CrossRef]
- Symoens, S.H.; Olahova, N.; Munñoz Gandarillas, A.E.; Karimi, H.; Djokic, M.R.; Reyniers, M.-F.; Marin, G.B.; Geem, K.M.V. State-of-the-art of coke formation during steam cracking: Anti-coking surface technologies. Ind. Eng. Chem. Res. 2018, 57, 16117–16136. [Google Scholar] [CrossRef]
- Saelee, T.; Lerdpongsiripaisarn, M.; Rittiruam, M.; Somdee, S.; Liu, A.; Praserthdam, S.; Praserthdam, P. Experimental and computational investigation on underlying factors promoting high coke resistance in NiCo bimetallic catalysts during dry reforming of methane. Sci. Rep. 2021, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.; Wilson, D.; Polley, G.; Pugh, S. Mitigation of Crude Oil Refinery Heat Exchanger Fouling Through Retrofits Based on Thermo-Hydraulic Fouling Models. Chem. Eng. Res. Des. 2004, 82, 53–71. [Google Scholar] [CrossRef]
- Chun, C.M.; Ramanarayanan, T.A.; Mumford, J.D. Relationship between coking and metal dusting. Mater. Corros. 1999, 50, 634–639. [Google Scholar] [CrossRef]
- Shubo, F.; Liming, S.; Qiangkun, L. A study on coke deposition and coking inhibitors during AGO pyrolysis in pulsed micro-reactor system. J. Anal. Appl. Pyrolysis 2002, 65, 301–312. [Google Scholar] [CrossRef]
- Kauffman, R.E.; Feng, A.; Karasek, K.R. Coke Formation from Aircraft Turbine Engine Oils: Part I—Deposit Analysis and Development of Laboratory Oil Coking Test. Tribol. Trans. 2000, 43, 823–829. [Google Scholar] [CrossRef]
- Altin, O.; Eser, S. Pre-oxidation of Inconel Alloys for Inhibition of Carbon Deposition from Heated Jet Fuel. Oxid. Met. 2006, 65, 75–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Albright, L.F. Pretreatments of Coils to Minimize Coke Formation in Ethylene Furnaces. Ind. Eng. Chem. Res. 2010, 49, 1991–1994. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Organic chemistry of coke formation. Appl. Catal. A Gen. 2001, 212, 83–96. [Google Scholar] [CrossRef]
- Andrésen, J.M.; Strohm, J.J.; Sun, L.; Song, C. Relationship between the Formation of Aromatic Compounds and Solid Deposition during Thermal Degradation of Jet Fuels in the Pyrolytic Regime. Energy Fuels 2001, 15, 714–723. [Google Scholar] [CrossRef]
- Kauffman, R.E.; Feng, A.S.; Karasek, K.R. Coke Formation from Aircraft Engine Oils: Part II—Effects of Oil Formulation and Surface Composition. Tribol. Trans. 2000, 43, 677–680. [Google Scholar] [CrossRef]
- Tang, S.; Luo, X.; Cai, C.; Wang, J.; Tang, A. Relationship between Coking Behavior in Hydrocarbon Fuel Pyrolysis and Surface Roughness. Energy Fuels 2018, 32, 1223–1229. [Google Scholar] [CrossRef]
- Ghazlani, M.A.; De Champlain, A.; Paquet, B.; Kalla, S.; Davenport, N. CFD Modelling of Heat Soak-Back Phenomenon Inside a Gas Turbine Combustor. In Proceedings of the ASME Turbo Expo: Turbine Technical Conference and Exposition, Montreal, QC, Canada, 15–19 June 2015. [Google Scholar] [CrossRef]
- Bazin, A.; De Champlain, A.; Paquet, B. Fuel nozzle coking analysis due to soak-back in a gas turbine combustor. Int. J. Energetic Mater. Chem. Propuls. 2015, 14, 163–175. [Google Scholar] [CrossRef]
- Boeve, S. Protecting Modern Jet Engines–More Time Flying with the Right Turbine Engine Oil, 14 January 2021. Available online: https://www.aviationpros.com/engines-components/aircraft-airframe-accessories/fuel-systems/article/21165652/protecting-modern-jet-engines-more-time-flying-with-the-right-turbine-engine-oil (accessed on 14 January 2021).
- Effect of Jet Engine Oil Coking, ExxonMobil. Available online: https://www.exxonmobil.com/en/aviation/knowledge-library/resources/engine-oil-coking (accessed on 15 November 2016).
- Lin, H.-C.; Chang, Y.-T.; Tsai, G.-L.; Wang, D.-M.; Hsieh, F.-C.; Jiang, J.-F. Oil Coking Prevention Using Electric Water Pump for Turbo-Charge Spark-Ignition Engines. Math. Probl. Eng. 2014, 2014, 498624. [Google Scholar] [CrossRef]
- Couch, K.A.; Gosling, C.D. Apparatus for Preventing Metal Catalyzed Coking. U.S. Patent 8,124,020B2, 11 October 2004. [Google Scholar]
- Terwijn, F.X.; Morii, K.; Takeuchi, Y.; Kato, Y.; Hayakawa, H.; Nishimura, N. Multi-Layered Anti-Coking Heat Resistant Metal Tube and Method for Manufacture Thereof. U.S. Patent 6,337,459 B1, 8 January 2002. [Google Scholar]
- Kisasoz, A.; Karaaslan, A.; Bayrak, Y. Effect of etching methods in metallographic studies of Duplex stainless steel 2205. Met. Sci. Heat Treat 2017, 58, 704–706. [Google Scholar] [CrossRef]
- Xin, F.; Ma, T.; Wang, Q. Spray Etching Rate Development of Stainless Steel in the Etchant for Printed Circuit Heat Exchanger Channels. Energy Procedia 2017, 105, 4828–4835. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, X.; Hong, L. Mineral-acid-free chemical polishing solutions for ferrous alloys. Appl. Surf. Sci. 2003, 218, 306–310. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Hočevar, M.; Golobič, I.; Gregorčič, P. Surface chemistry and morphology transition induced by critical heat flux incipience on laser-textured copper surfaces. Appl. Surf. Sci. 2019, 490, 220–230. [Google Scholar] [CrossRef]
- You, Y.; Zhang, B.; Tao, S.; Liang, Z.; Tang, B.; Zhou, R.; Yuan, D. Effect of Surface Microstructure on the Heat Dissipation Performance of Heat Sinks Used in Electronic Devices. Micromachines 2021, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Azih, C.; Yaras, M.I. Effects of spatial gradients in thermophysical properties on the topology of turbulence in heated channel flow of supercritical fluids. Phys. Fluids 2018, 30, 015108. [Google Scholar] [CrossRef]
- Megson, D.; Ortiz, X.; Jobst, K.J.; Reiner, E.J.; Mulder, M.F.; Balouet, J.-C. A comparison of fresh and used aircraft oil for the identification of toxic substances linked to aerotoxic syndrome. Chemosphere 2016, 158, 116–123. [Google Scholar] [CrossRef]
- Rao, P.N.; Kunzru, D. Fabrication of microchannels on stainless steel by wet chemical etching. J. Micromechanics Microengineering 2007, 17, N99–N106. [Google Scholar] [CrossRef]
- Portanova, R.; Lajunen, L.H.J.; Tolazzi, M.; Piispanen, J. Critical evaluation of stability constants for alpha-hydroxycarboxylic acid complexes with protons and metal ions and the accompanying enthalpy changes. Part II. Aliphatic 2-hydroxycarboxylic acids (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 495–540. [Google Scholar] [CrossRef]
- Gorman, J.E.; Clydesdale, F.M. Thermodynamic and Kinetic Stability Constants of Selected Carboxylic Acids and Iron. J. Food Sci. 1984, 49, 500–503. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef]
- Nicholas, J.F. The Terrace-Ledge-Kink (TLK) Model, Landolt-Börnstein-Group III Condensed Matter 24A. In Structure; Chiarotti, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; Chapter. 2.1.1.5.1. [Google Scholar] [CrossRef]





| Sample Tubes | Constituents of Etchant | Etching Duration (min) | Average Surface Roughness (μm) | |
|---|---|---|---|---|
| H2O/H2O2/HCl/lactic Acid c vol. (mL) | E or N d (wt %) | |||
| C (crude) a | - | - | - | 6.44 |
| LHC b | 1/3/9/5 | 0 | 30 | 5.24 |
| LHC-E/a | As above | 0.57 | 10 | 6.52 |
| LHC-E/b | As above | 0.57 | 20 | 6.87 |
| LHC-E/c | As above | 0.57 | 30 | 6.28 |
| LHC-E | As above | 1.14 | 30 | 8.91 |
| LHC-N | As above | 0.14 | 30 | 5.40 |
| H2O/H2O2/HCl/ vol. (mL) | ||||
| SHP | 9/1.35/9 | 30 | 1.46 | |
| Oil-Samples a | Retention Time (min) of LC Peaks c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.81 | 2.22 | 3.49 | 3.71 | 3.84 | 4.13 | 4.55 | 5.68 | 8.55 | ||
| Fresh | Relative peak intensity (%) b | 100 b. | 4.54 | 12.1 | ||||||
| C (crude) | 100 | 10 | 7.1 | 9.2 | 10 | 7.9 | 2.1 | 1.1 | 0.42 | |
| LHC-E/a | 100 | 8.1 | 6.7 | 9.4 | 7.6 | 7.2 | 2.3 | 0.9 | 0.45 | |
| LHC-E/b | 100 | 8.4 | 6.7 | 8.9 | 8.4 | 7.6 | 2.2 | 0.88 | 0.44 | |
| LHC-E/c | 100 | 8.5 | 6.2 | 8.5 | 7.9 | 7.4 | 1.7 | 1.1 | 0.56 | |
| LHC-E | 100 | 8.9 | 5 | 8.9 | 8.9 | 5.6 | 1.7 | 1.1 | 0.56 | |
| Etchant No. | Mean Particle Size (μm) | Standard Deviation (μm) |
|---|---|---|
| C | 10.9 | 10.5 |
| LHC-E/a | 0.49 | 0.04 |
| LHC-E/b | 0.63 | 0.18 |
| LHC-E/c | 0.47 | 0.03 |
| LHC-E | 1.13 | 0.21 |
| C | LHC | LHC-N | LHC-E |
|---|---|---|---|
| 0.708% | 0.044% | 0.028% | 0.288% |
| Property | Crude Tube | LHC-E Sample Tube |
|---|---|---|
| Wall thickness (mm) | 0.88 | 0.82 |
| Tensile strength (MPa) | 679.4 | 687.4 |
| Modulus of elasticity (GPa) | 104.7 | 102.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Tay, S.W.; Hong, L. Crafting Metal Surface Morphology to Prevent Formation of the Carbon–Steel Interfacial Composite. J. Compos. Sci. 2022, 6, 266. https://doi.org/10.3390/jcs6090266
Zheng Y, Tay SW, Hong L. Crafting Metal Surface Morphology to Prevent Formation of the Carbon–Steel Interfacial Composite. Journal of Composites Science. 2022; 6(9):266. https://doi.org/10.3390/jcs6090266
Chicago/Turabian StyleZheng, Yuanhuan, Siok Wei Tay, and Liang Hong. 2022. "Crafting Metal Surface Morphology to Prevent Formation of the Carbon–Steel Interfacial Composite" Journal of Composites Science 6, no. 9: 266. https://doi.org/10.3390/jcs6090266
APA StyleZheng, Y., Tay, S. W., & Hong, L. (2022). Crafting Metal Surface Morphology to Prevent Formation of the Carbon–Steel Interfacial Composite. Journal of Composites Science, 6(9), 266. https://doi.org/10.3390/jcs6090266






