Improved Wear and Corrosion Resistance in TiC-Reinforced SUS304 Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterization
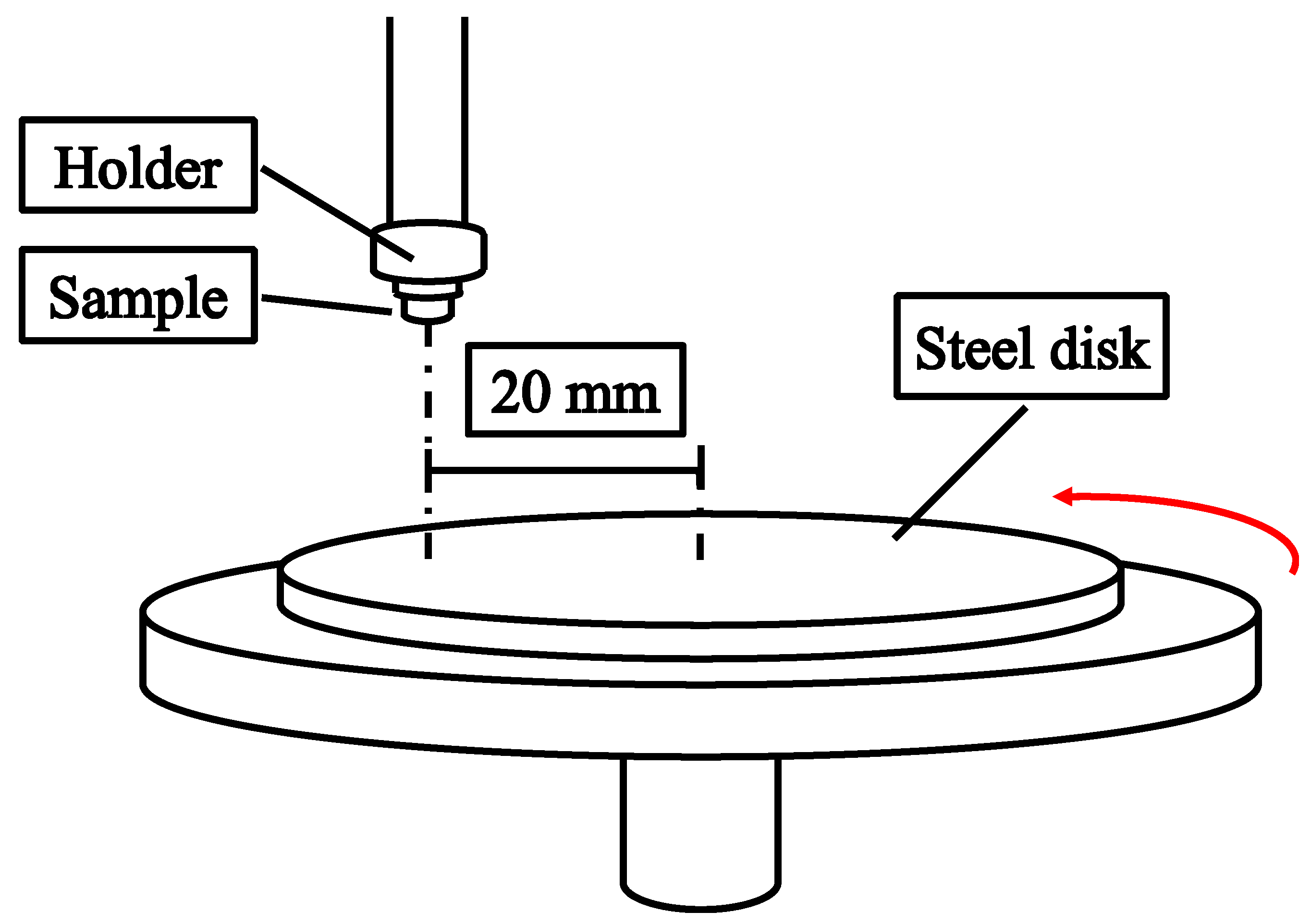
2.2. Tribological and Mechanical Testing
2.3. Electrochemical Measurements in 3.5 wt % NaCl Solution
2.4. Sinking Corrosion Test in 1.5 M Four Corrosive Solutions
3. Results
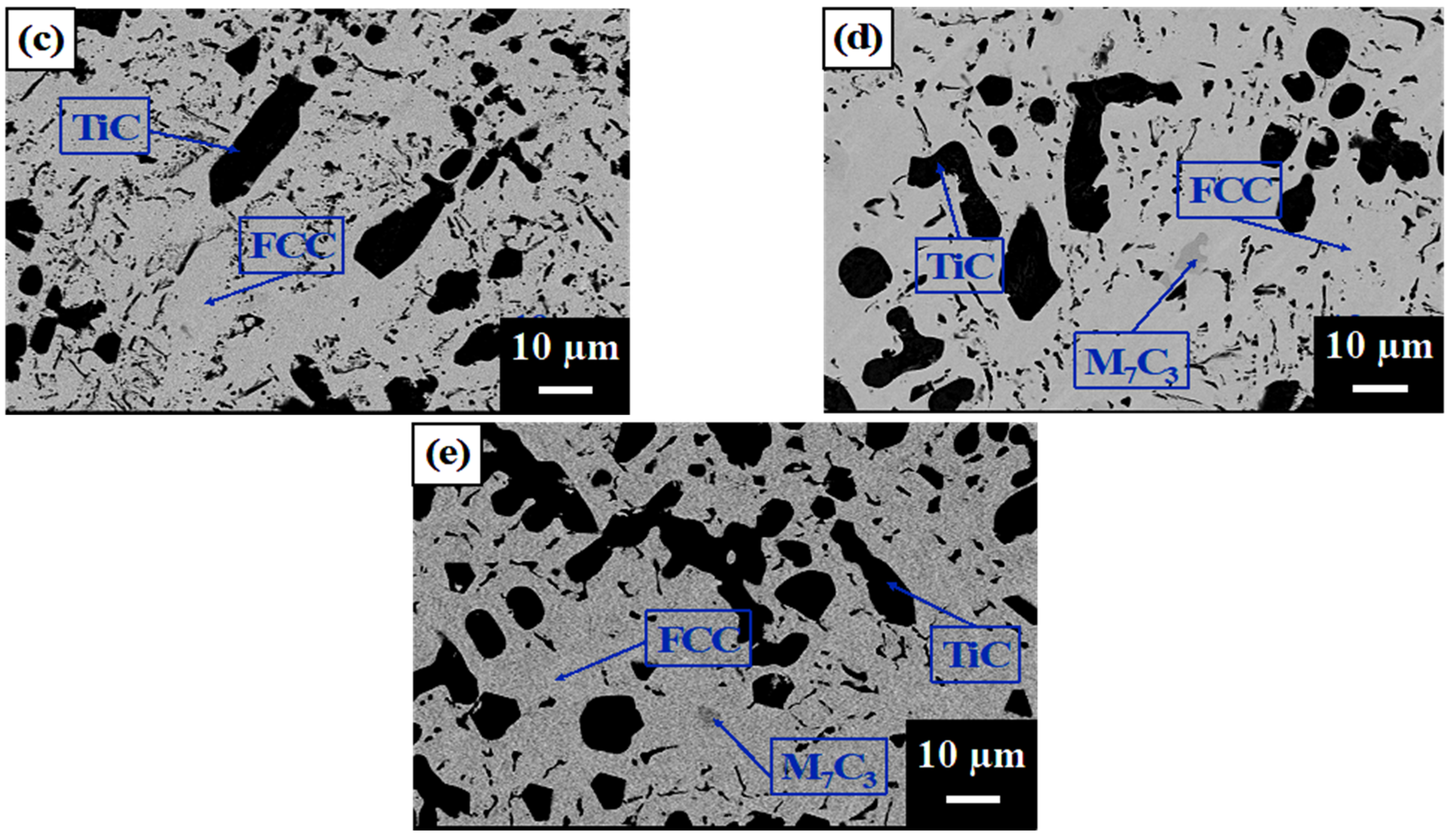
3.1. Microstructure, Phases, and Compositions
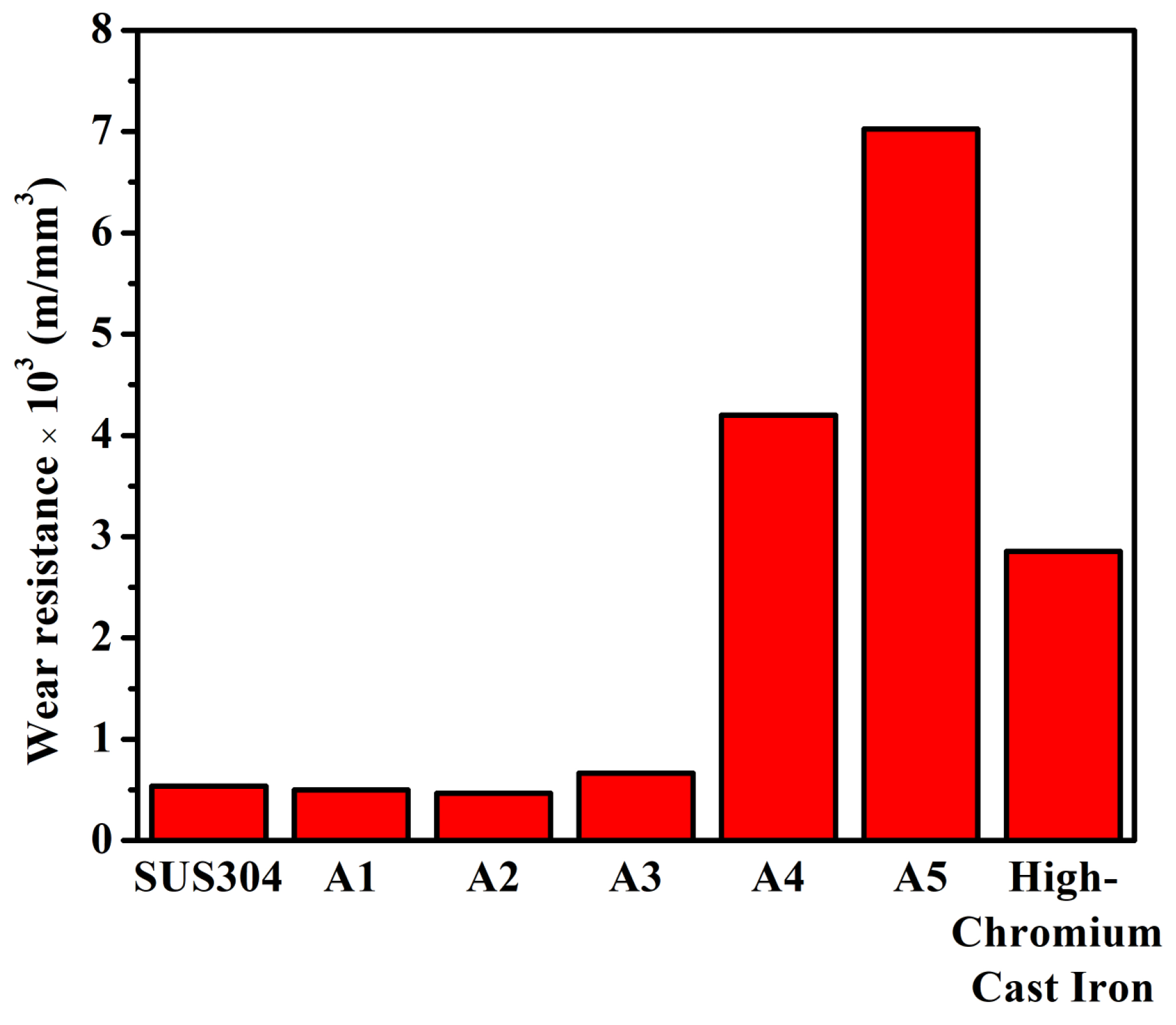
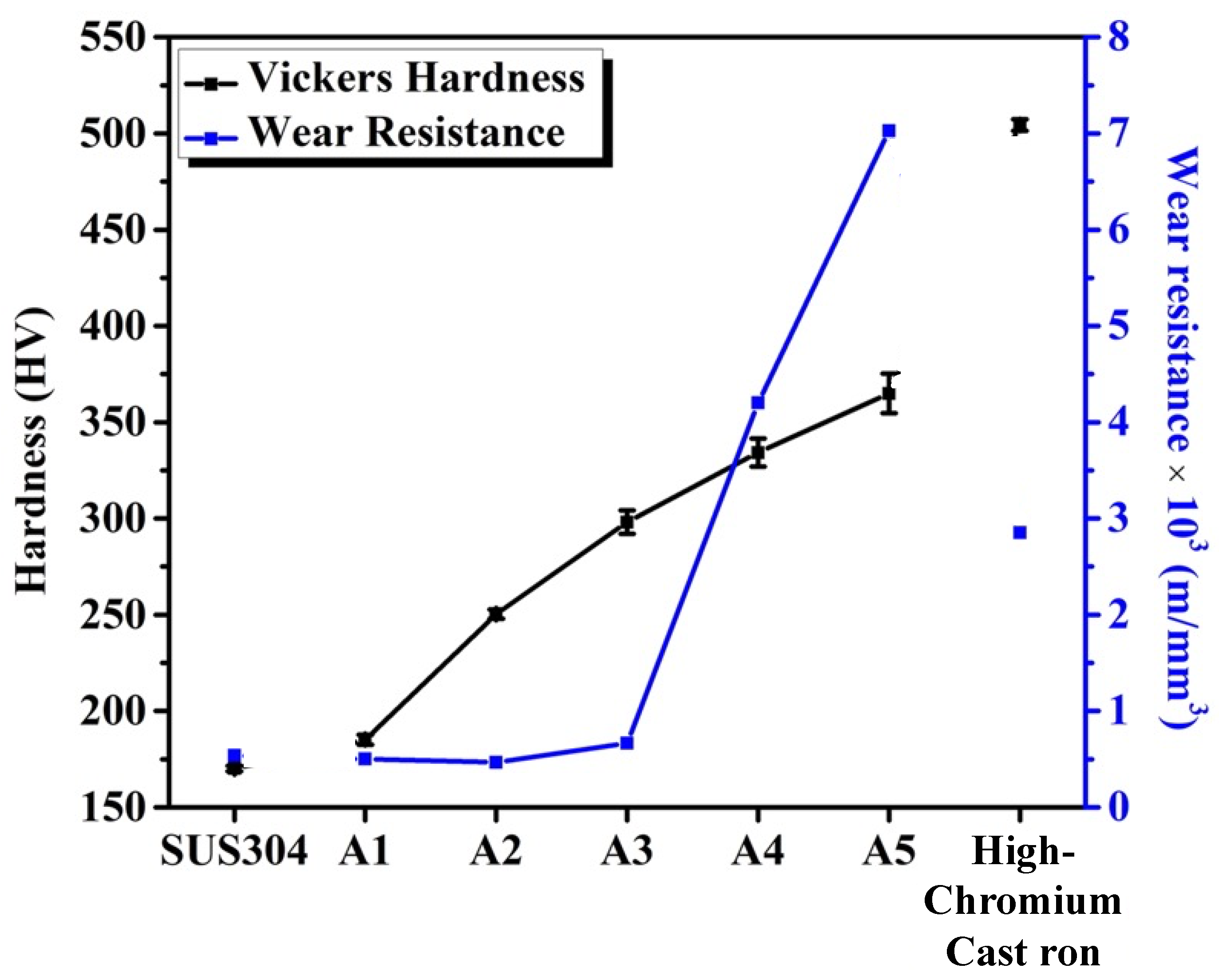
3.2. Tribological and Mechanical Properties
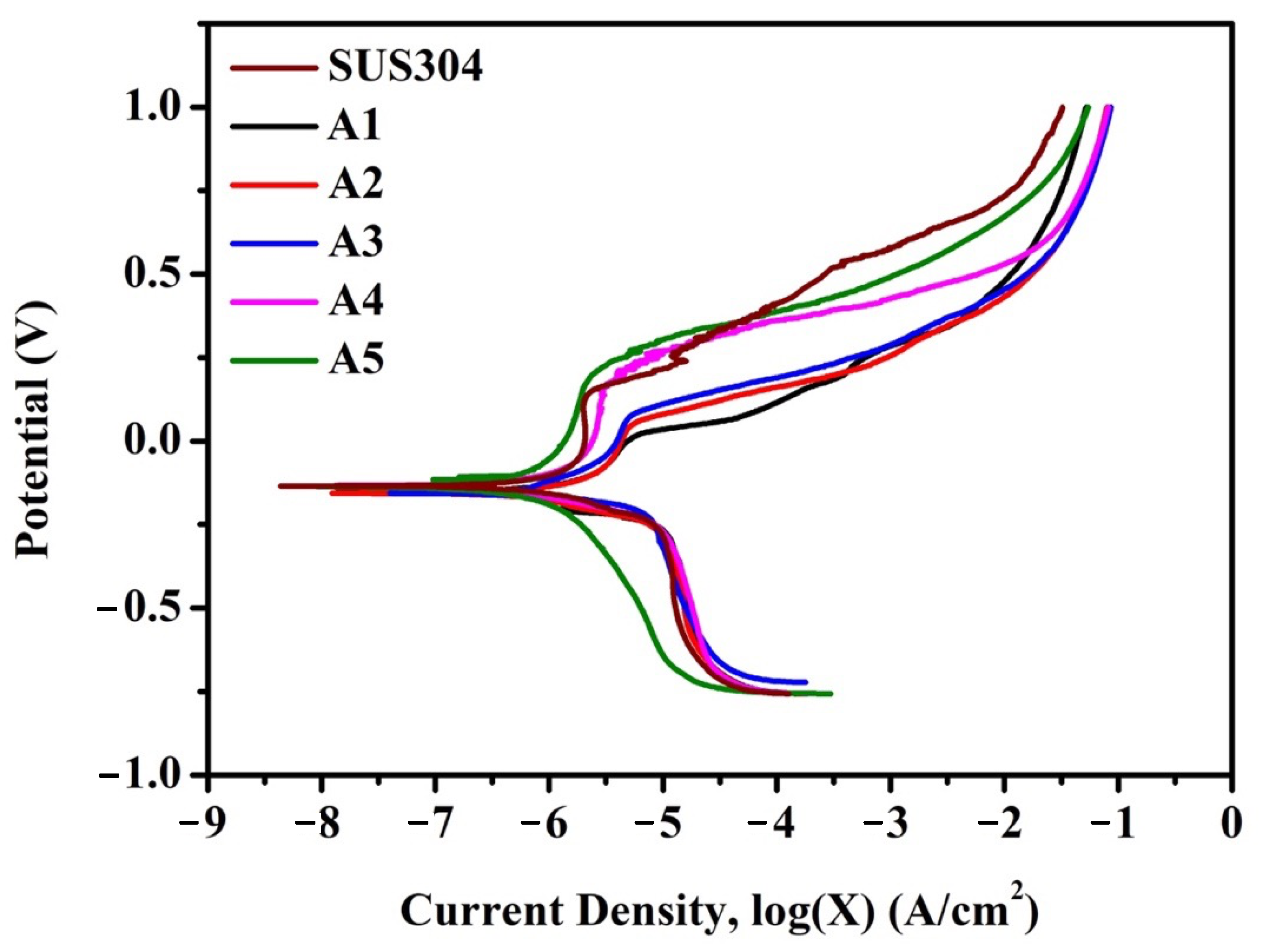
3.3. Electrochemical Analysis for Corrosion Resistance in 3.5 wt % NaCl Solution
3.4. Mass Loss Analysis for Corrosion Resistance
4. Discussion
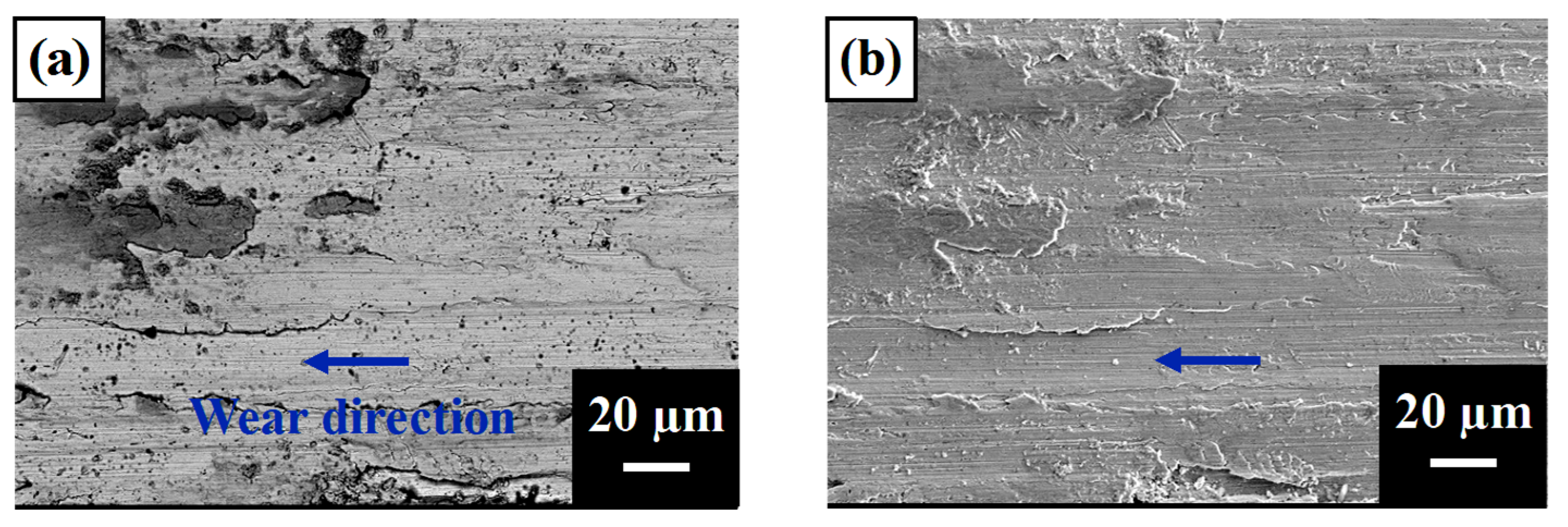
4.1. Morphology Analysis for Wear Behavior
4.2. Composition Analysis for Corrosion Behavior
5. Conclusions
- All alloys contained a TiC phase and an austenite matrix (FCC) in which A1 had a eutectic structure between the FCC dendrites, and A2, A3, A4, and A5 contained proeutectic dendritic TiC, dendritic FCC enveloping the TiC dendrite, and a eutectic structure. The volume fraction of the TiC dendrites increased, but that of the eutectic phase decreased with an increasing TiC content, and A4 and A5 had M7C3 carbides in addition to TiC. A second FCC phase with a slightly smaller lattice constant was identified as a (Fe and Ni) phase, which was the eutectic FCC phase with a higher Cr content than the proeutectic FCC.
- The hardness of the alloys increased from 185 HV (A1) to 365 HV (A5). The microstructural morphology affected the wear resistance during the adhesive wear tests. A1 and A2 had needle-like TiC with poor wear resistance, which was lower than that of SUS304. A4 and A5, having more block-like TiC and M7C3 carbides, showed good wear resistance. Notably, A5 exhibited the best wear resistance, followed by A4. Both A4 and A5 performed considerably better than high-chromium cast iron.
- A4 and A5 exhibited better corrosion resistance in every aspect than SUS304, with A5 exhibiting the best performance. Apart from the improved corrosion resistance owing to the increased Cr and Ni contents of the FCC matrix, the increased volume fraction of chemically stable TiC and M7C3 also positively contributed to the improved corrosion resistance, as the exposed area fraction of the alloy matrix correspondingly reduced when the carbide content increased. A5 showed excellent corrosion resistance in all the acid solutions. A4 exhibited good corrosion resistance in all the acid solutions, except in the H2SO4 solution.
- A5 exhibited the best wear and corrosion resistance, thus, demonstrating great potential to be applied in wear and corrosive environments, such as rotary shafts, rotors, bearings, and structural parts in food, as well as in the chemical and optoelectronic industries. Apart from its poor performance in the sulfuric acid solution, A4 also exhibited good wear and corrosion resistance. Therefore, this could be used as a low-cost alternative to A5.
- Overall, this study provides a conceptual design for improved SUS304 stainless steel with enhanced mechanical and anti-corrosive properties. In particular, A4 and A5 alloys could be applied for commercial use. Furthermore, this study investigated the microstructure and properties of alloys and revealed the effect of incorporating carbides in 304 stainless steel. Thus, similar alloy designs could be applied to improve other Fe–Cr–Ni stainless steel systems.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high entropy alloys. Ann. Chim. Sci. Mat. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Yeh, J.W.; Lin, S.J. Breakthrough applications of high-entropy materials. J. Mater. Res. 2018, 33, 3129–3137. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Garcia Filho, F.D.C.; Ritchie, R.O.; Meyers, M.A.; Monteiro, S.N. Cantor-derived medium-entropy alloys: Bridging the gap between traditional metallic and high-entropy alloys. J. Mater. Res. Technol. 2022, 17, 1868–1895. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A brief review of high-entropy films. Mater. Chem. Phys. 2018, 210, 12–19. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Wu, C.F.; Arifin, D.E.S.; Wang, C.A.; Ruan, J.J. Coalescence and split of high-entropy polymer lamellar cocrystals. Polymer 2018, 138, 188–202. [Google Scholar] [CrossRef]
- Ghahabi, E.; Shajari, Y.; Razavi, M.; Mobasherpour, I.; Fard, S.A.T. Effect of iron content on the wear behavior and adhesion strength of TiC-Fe nanocomposite coatings on low carbon steel produced by air plasma spray. Ceram. Int. 2020, 46, 2670–2676. [Google Scholar] [CrossRef]
- Huang, L.; Pan, Y.F.; Zhang, J.X.; Liu, A.J.; Du, Y.; Luo, F.H. Densification, microstructure and mechanical performance of TiC/Fe composites by spark plasma sintering. J. Mater. Res. Technol. JMRT 2020, 9, 6116–6124. [Google Scholar] [CrossRef]
- Ni, Z.; Sun, Y.; Xue, F.; Bai, J.; Lu, Y. Microstructure and properties of austenitic stainless steel reinforced with in situ TiC particulate. Mater. Des. 2011, 32, 1462–1467. [Google Scholar] [CrossRef]
- Das, K.; Bandyopadhyay, T.K.; Chatterjee, S. Synthesis and characterization of austenitic Steel matrix composite reinforced with in-situ TiC particles. J. Mater. Sci. 2005, 40, 5007–5010. [Google Scholar] [CrossRef]
- prava Dalai, R.; Das, S.; Das, K. Development of TiC reinforced austenitic manganese steel. Can. Metall. Q. 2014, 53, 317–325. [Google Scholar] [CrossRef]
- Au, C.; Benedetto, A.; Aschner, M. Manganese transport in eukaryotes: The role of DMT1. Neurotoxicology 2008, 29, 569–576. [Google Scholar] [CrossRef]
- Squitti, R.; Gorgone, G.; Panetta, V.; Lucchini, R.; Bucossi, S.; Albini, E.; Alessio, L.; Alberici, A.; Melgari, J.M.; Benussi, L.; et al. Implications of metal exposure and liver function in Parkinsonian patients resident in the vicinities of ferroalloy plants. J. Neural. Transm. 2009, 116, 1281–1287. [Google Scholar] [CrossRef]
- Perl, D.P.; Olanow, C.W. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007, 66, 675–682. [Google Scholar] [CrossRef]
- Zhang, P.; Lokuta, K.M.; Turner, D.E.; Liu, B. Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: Differential involvement of microglia and astroglia. J. Neurochem. 2010, 112, 434–443. [Google Scholar] [CrossRef]
- Pulkkinen, H.; Apajalahti, H.; Papula, S.; Talonen, J.; Hänninen, H. Pitting Corrosion Resistance of Mn-Alloyed Austenitic Stainless Steels. Steel Res. Int. 2014, 85, 324–335. [Google Scholar] [CrossRef]
- Koyama, S.; Tsuneto, T. Direct Bonding of SUS304 Stainless Steel by Metal Salt Generation Bonding Technique with Formic Acid. Procedia Mater. Sci. 2016, 12, 95–99. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Wang, R.G.; Kato, M.; Nakasa, K. Observation by atomic force microscope of corrosion product during pitting corrosion on SUS304 stainless steel. Scr. Mater. 2005, 52, 227–230. [Google Scholar] [CrossRef]
- Kattamis, T.Z.; Suganuma, T. Solidification Processing and Tribological Behavior of Particulate Tic-Ferrous Matrix Composites. Mater. Sci. Eng. A 1990, 128, 241–252. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhang, X.Y.; Li, F.C.; Zeng, G.T. Study on an Fe-TiC surface composite produced in situ. Mater. Des. 1999, 20, 233–236. [Google Scholar] [CrossRef]
- Blatsi, C.; Patsidis, A.C.; Psarras, G.C. Dielectric Properties and Energy Storage of Hybrid/Boron Nitride/Titanium Carbide/Epoxy Nanocomposites. J. Compos. Sci. 2022, 6, 259. [Google Scholar] [CrossRef]
- Doǧan, Ö.N.; Hawk, J.A.; Tylczak, J.H.; Wilson, R.D.; Govier, R.D. Wear of titanium carbide reinforced metal matrix composites. Wear 1999, 225, 758–769. [Google Scholar] [CrossRef]
- Galgali, R.K.; Ray, H.S.; Chakrabarti, A.K. Wear characteristics of TiC reinforced cast iron composites Part 1—Adhesive wear. Mater. Sci. Technol. 2013, 14, 810–815. [Google Scholar] [CrossRef]
- Zhi, X.H.; Xing, J.D.; Fu, H.G.; Gao, Y.M. Effect of titanium on the as-cast microstructure of hypereutectic high chromium cast iron. Mater. Charact. 2008, 59, 1221–1226. [Google Scholar] [CrossRef]
- Bedolla-Jacuinde, A.; Correa, R.; Quezada, J.G.; Maldonado, C. Effect of titanium on the as-cast microstructure of a 16%chromium white iron. Mater. Sci. Eng. A 2005, 398, 297–308. [Google Scholar] [CrossRef]
- Matsuo, T.T.; Kiminami, C.S.; Fo, W.B.; Bolfarini, C. Sliding wear of spray-formed high-chromium white cast iron alloys. Wear 2005, 259, 445–452. [Google Scholar] [CrossRef]
- Wu, Q.L.; Sun, Y.S.; Yang, C.D.; Xue, F.; Song, F.M. Microstructure and mechanical properties of common straight carbon steels strengthened by TiC dispersion. Mater. Trans. 2006, 47, 2393–2398. [Google Scholar] [CrossRef]
- Tang, X.H.; Chung, R.; Li, D.Y.; Hinckley, B.; Dolman, K. Variations in microstructure of high chromium cast irons and resultant changes in resistance to wear, corrosion and corrosive wear. Wear 2009, 267, 116–121. [Google Scholar] [CrossRef]
- Yu, S.K.; Sasaguri, N.; Matsubara, Y. Effects of retained austenite on abrasion wear resistance and hardness of hypoeutectic high Cr white cast iron. Int. J. Cast Metals Res. 1999, 11, 561–566. [Google Scholar] [CrossRef]
- Kouřil, M.; Novák, P.; Bojko, M. Limitations of the linear polarization method to determine stainless steel corrosion rate in concrete environment. Cem. Concr. Compos. 2006, 28, 220–225. [Google Scholar] [CrossRef]
- Izquierdo, J.; Martin-Ruiz, L.; Fernandez-Perez, B.M.; Rodriguez-Raposo, R.; Santana, J.J.; Souto, R.M. Scanning microelectrochemical characterization of the effect of polarization on the localized corrosion of 304 stainless steel in chloride solution. J. Electroanal. Chem. 2014, 728, 148–157. [Google Scholar] [CrossRef]
- Atabaki, M.M.; Jafari, S.; Abdollah-pour, H. Abrasive Wear Behavior of High Chromium Cast Iron and Hadfield Steel—A Comparison. J. Iron Steel Res. Int. 2012, 19, 43–50. [Google Scholar] [CrossRef]
- Chotěborský, R.; Hrabě, P.; Müller, M.; Savková, J.; Jirka, M. Abrasive wear of high chromium Fe-Cr-C hardfacing alloys. Res. Agric. Eng. 2008, 54, 192–198. [Google Scholar] [CrossRef]
- De Carvalho, M.; Xavier, R.; Pontes, C.; Morone, C.; Boccalini, M.; Sinatora, A. Microstructure, mechanical properties and wear resistance of high speed steel rolls for hot rolling mills. Iron Steelmak. 2002, 29, 27–32. [Google Scholar]
- Guo, J.; Ai, L.; Wang, T.; Feng, Y.; Wan, D.; Yang, Q. Microstructure evolution and micro-mechanical behavior of secondary carbides at grain boundary in a Fe–Cr–W–Mo–V–C alloy. Mater. Sci. Eng. A 2018, 715, 359–369. [Google Scholar] [CrossRef]
- Talib, N.; Nasir, R.M.; Rahim, E.A. Tribological behaviour of modified jatropha oil by mixing hexagonal boron nitride nanoparticles as a bio-based lubricant for machining processes. J. Clean. Prod. 2017, 147, 360–378. [Google Scholar] [CrossRef]
- Singh, Y.; Garg, R.; Kumar, S. RETRACTED ARTICLE: Friction and Wear Characterization of Modified Jatropha Oil (Jatropha Curcas) Using Pin on Disc Tribometer. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 44, 2018–2029. [Google Scholar] [CrossRef]
- Yoon, E.S.; Kong, H.S.; Kwon, O.K.; Oh, J.E. Evaluation of frictional characteristics for a pin-on-disk apparatus with different dynamic parameters. Wear 1997, 203, 341–349. [Google Scholar] [CrossRef]
- Mahdavian, S.M.; Mai, Y.W.; Cotterell, B. Friction Metallic Transfer and Debris Analysis of Sliding Surfaces. Wear 1982, 82, 221–232. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Das, K. Corrosion Behaviour of TiC-Reinforced Hadfield Manganese Austenitic Steel Matrix In-Situ Composites. Open J. Met. 2015, 5, 11–17. [Google Scholar] [CrossRef]
- Okamoto, G. Passive film of 18-8 stainless steel structure and its function. Corr. Sci. 1973, 13, 471–489. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Peissl, S.; Mori, G.; Leitner, H.; Ebner, R.; Eglsäer, S. Influence of chromium, molybdenum and cobalt on the corrosion behaviour of high carbon steels in dependence of heat treatment. Mater. Corr. 2006, 57, 759–765. [Google Scholar] [CrossRef]
- Hegde, A.; Patil, A.; Tambrallimath, V. Corrosion behaviour of sintered austenitic stainless steel composites. Int. J. Eng. Res. Technol. 2014, 3, 14–17. [Google Scholar] [CrossRef]
- Caliskan, N.; Akbas, E. Corrosion inhibition of austenitic stainless steel by some pyrimidine compounds in hydrochloric acid. Mater. Corros.–Werkst. Korros. 2012, 63, 231–237. [Google Scholar] [CrossRef]
- Asteman, H.; Spiegel, M. Investigation of the HCl (g) attack on pre-oxidized pure Fe, Cr, Ni and commercial 304 steel at 400 °C. Corr. Sci. 2007, 49, 3626–3637. [Google Scholar] [CrossRef]
- Hermas, A.A.; Morad, M.S. A comparative study on the corrosion behaviour of 304 austenitic stainless steel in sulfamic and sulfuric acid solutions. Corr. Sci. 2008, 50, 2710–2717. [Google Scholar] [CrossRef]
- Peled, P.; Harush, S.; Itzhak, D. The effect of Ni addition on the corrosion behaviour of sintered stainless steel in H2SO4. Corr. Sci. 1988, 28, 327–329. [Google Scholar] [CrossRef]
- Wang, Z.; Paschalidou, E.-M.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Mechanisms of Cr and Mo Enrichments in the Passive Oxide Film on 316L Austenitic Stainless Steel. Front. Mater. 2019, 6, 232. [Google Scholar] [CrossRef]
- Liu, X.L.; Jiang, Y.; Zhang, H.B.; He, Y.H. Corrosion behavior of porous Ti3SiC2 in nitric acid and aqua regia. Trans. Nonferrous Met. Soc. China 2017, 27, 584–590. [Google Scholar] [CrossRef]






| Alloys | Elements (at.%) | |||||
|---|---|---|---|---|---|---|
| Fe | Cr | Ni | Ti | C | Configurational Entropy | |
| A1 | 69.13 | 18.06 | 7.11 | 2.85 | 2.85 | 0.95 R |
| A2 | 65.01 | 16.98 | 6.69 | 5.66 | 5.66 | 1.09 R |
| A3 | 60.95 | 15.92 | 6.27 | 8.43 | 8.42 | 1.19 R |
| A4 | 56.95 | 14.88 | 5.86 | 11.16 | 11.15 | 1.26 R |
| A5 | 53.01 | 13.85 | 5.45 | 13.85 | 13.84 | 1.32 R |
| Alloys | Elements (at.%) | |||||
|---|---|---|---|---|---|---|
| Fe | Cr | Ni | Ti | C | ||
| A1 | Black phase (TiC) | 0.6 | 0.6 | 0.1 | 48.4 | 49.3 |
| Light-gray phase (FCC) | 76.1 | 16.2 | 6.0 | 1.3 | 0.4 | |
| A2 | Black phase (TiC) | 0.4 | 0.7 | 0.1 | 47.9 | 51.0 |
| Light-gray phase (FCC) | 70.8 | 16.7 | 6.8 | 0.9 | 4.0 | |
| A3 | Black phase (TiC) | 0.5 | 0.9 | 0.1 | 48.1 | 50.0 |
| Light-gray phase (FCC) | 70.8 | 16.9 | 7.5 | 1.5 | 3.1 | |
| A4 | Black phase (TiC) | 1.0 | 0.9 | 0.1 | 51.3 | 46.5 |
| Gray phase (M7C3) | 43.3 | 11.3 | 4.2 | 15.9 | 24.2 | |
| Light-gray phase (FCC) | 71.7 | 17.8 | 7.1 | 0.9 | 2.3 | |
| A5 | Black phase (TiC) | 0.5 | 0.8 | 0.1 | 47.2 | 49.6 |
| Gray phase (M7C3) | 29.4 | 48.5 | 1.5 | 0.7 | 19.9 | |
| Light-gray phase (FCC) | 69.5 | 20.1 | 7.0 | 0.5 | 2.9 | |
| Samples | Ecorr (V) | jcorr × 10−8 (A/cm2) | Etr (V) | jpass × 10−6 (A/cm2) |
|---|---|---|---|---|
| SUS304 | −0.134 | 4325 | 0.128 | 2070 |
| A1 | −0.152 | 5333 | −0.002 | 3573 |
| A2 | −0.156 | 2568 | 0.042 | 3890 |
| A3 | −0.157 | 2080 | 0.064 | 4083 |
| A4 | −0.131 | 4808 | 0.166 | 2612 |
| A5 | −0.135 | 1047 | 0.178 | 1702 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-J.; Yeh, J.-W. Improved Wear and Corrosion Resistance in TiC-Reinforced SUS304 Stainless Steel. J. Compos. Sci. 2023, 7, 34. https://doi.org/10.3390/jcs7010034
Lu C-J, Yeh J-W. Improved Wear and Corrosion Resistance in TiC-Reinforced SUS304 Stainless Steel. Journal of Composites Science. 2023; 7(1):34. https://doi.org/10.3390/jcs7010034
Chicago/Turabian StyleLu, Chieh-Jung, and Jien-Wei Yeh. 2023. "Improved Wear and Corrosion Resistance in TiC-Reinforced SUS304 Stainless Steel" Journal of Composites Science 7, no. 1: 34. https://doi.org/10.3390/jcs7010034
APA StyleLu, C.-J., & Yeh, J.-W. (2023). Improved Wear and Corrosion Resistance in TiC-Reinforced SUS304 Stainless Steel. Journal of Composites Science, 7(1), 34. https://doi.org/10.3390/jcs7010034





