Study on the Impact of a Combination of Synthetic Wollastonite and 2-Mercaptobenzothiazole-Based Fillers on UHMWPE Polymeric Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Objects
- The precursor components in the model system were calcium sulfate CaSO4·2H2O (p.a.), silicon dioxide (pur.), and potassium hydrate (p.a.), which were mixed in a stoichiometric ratio.
- In the second case, borogypsum was processed in an autoclave by a potassium hydrate solution (p.a.) in a stoichiometric ratio. The base components of borogypsum were calcium sulfate dihydrate and amorphous silica.
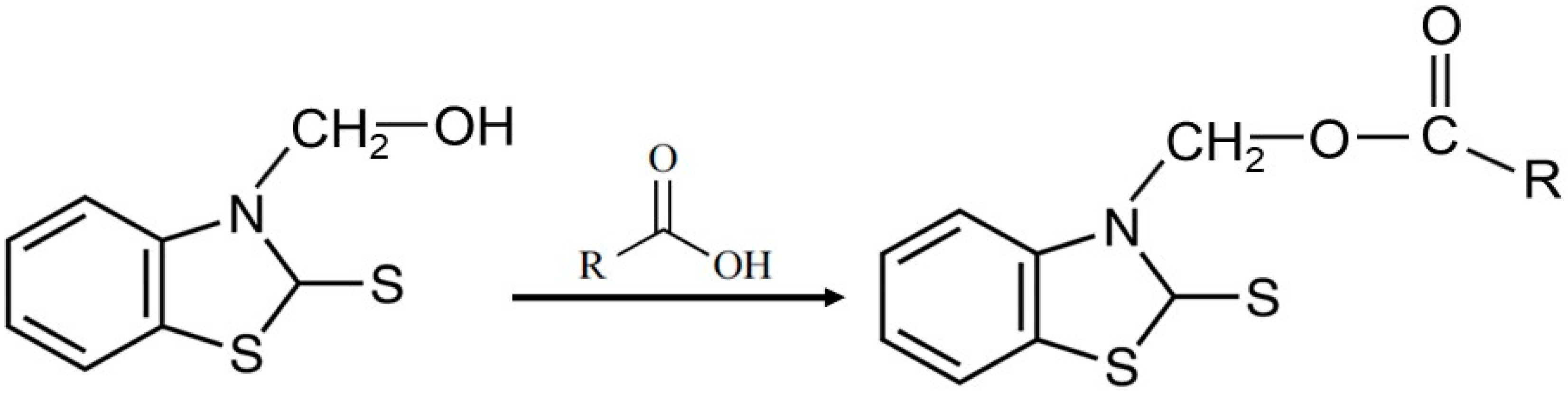
2.2. PCM Fabrication Technology
2.3. Research Methods
3. Results
3.1. Study of the Synthesized Wollastonite Particles
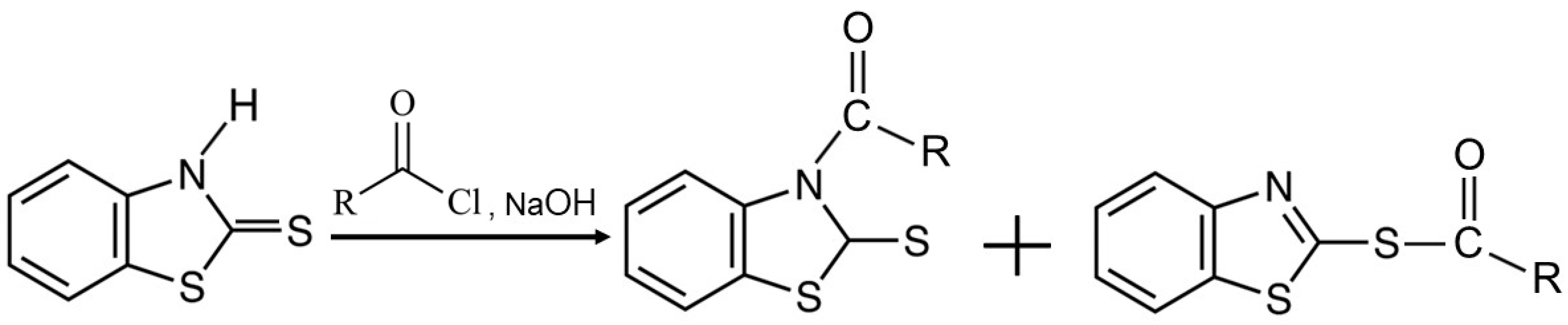
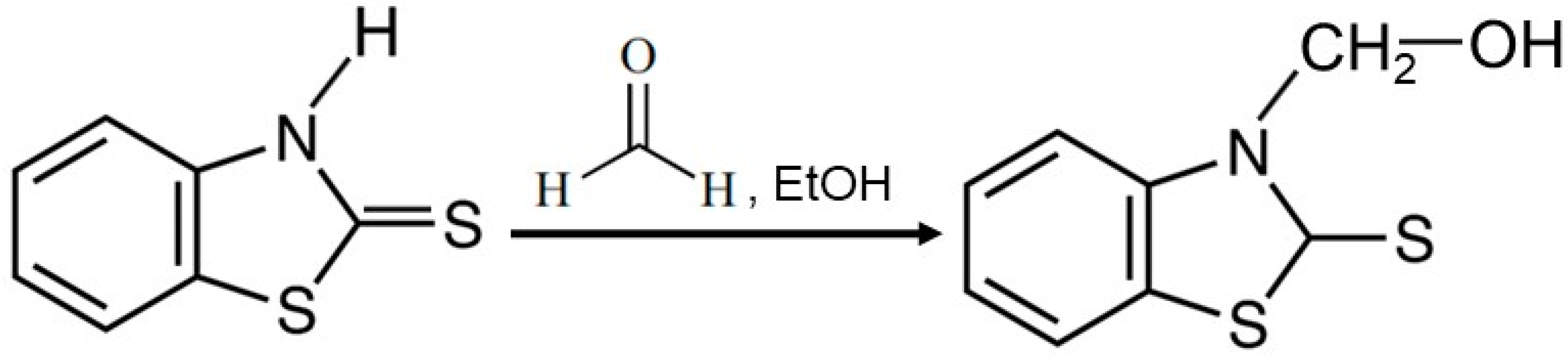
3.2. Characterization of MBT
3.3. Stress–Strain Properties of the PCM
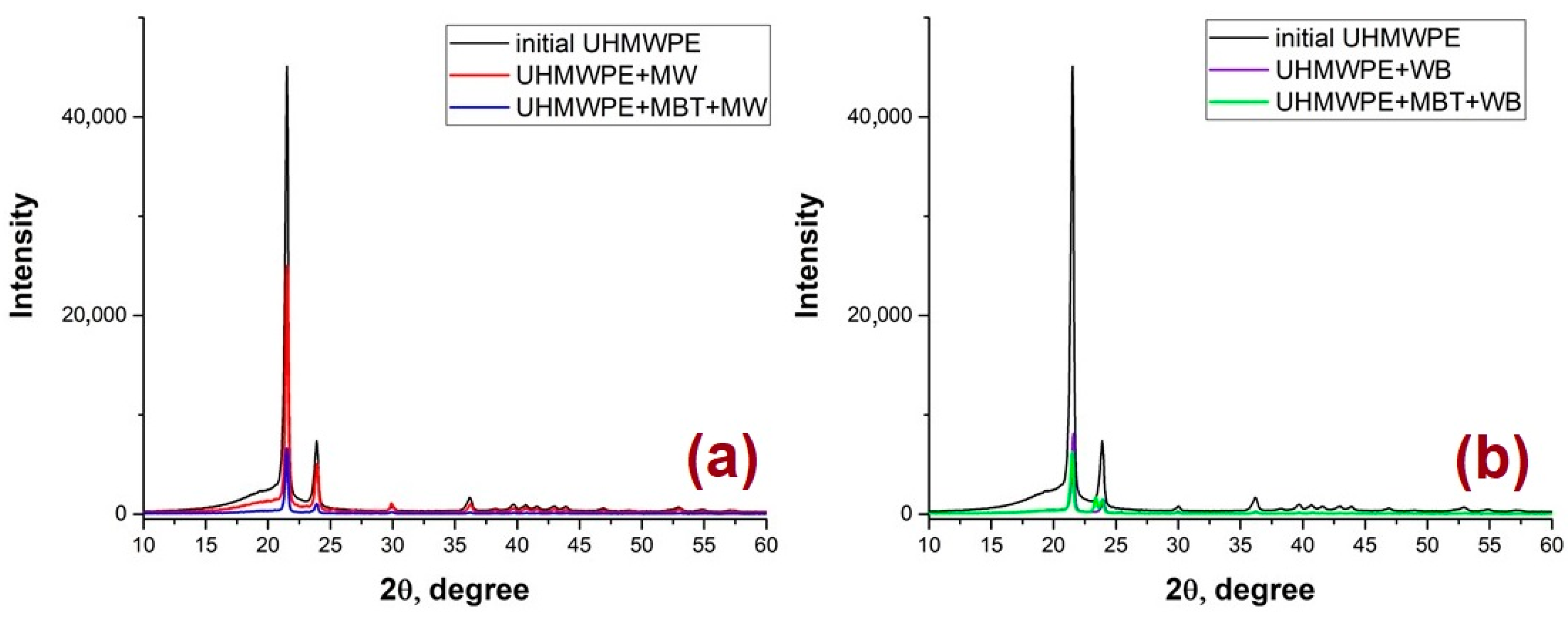
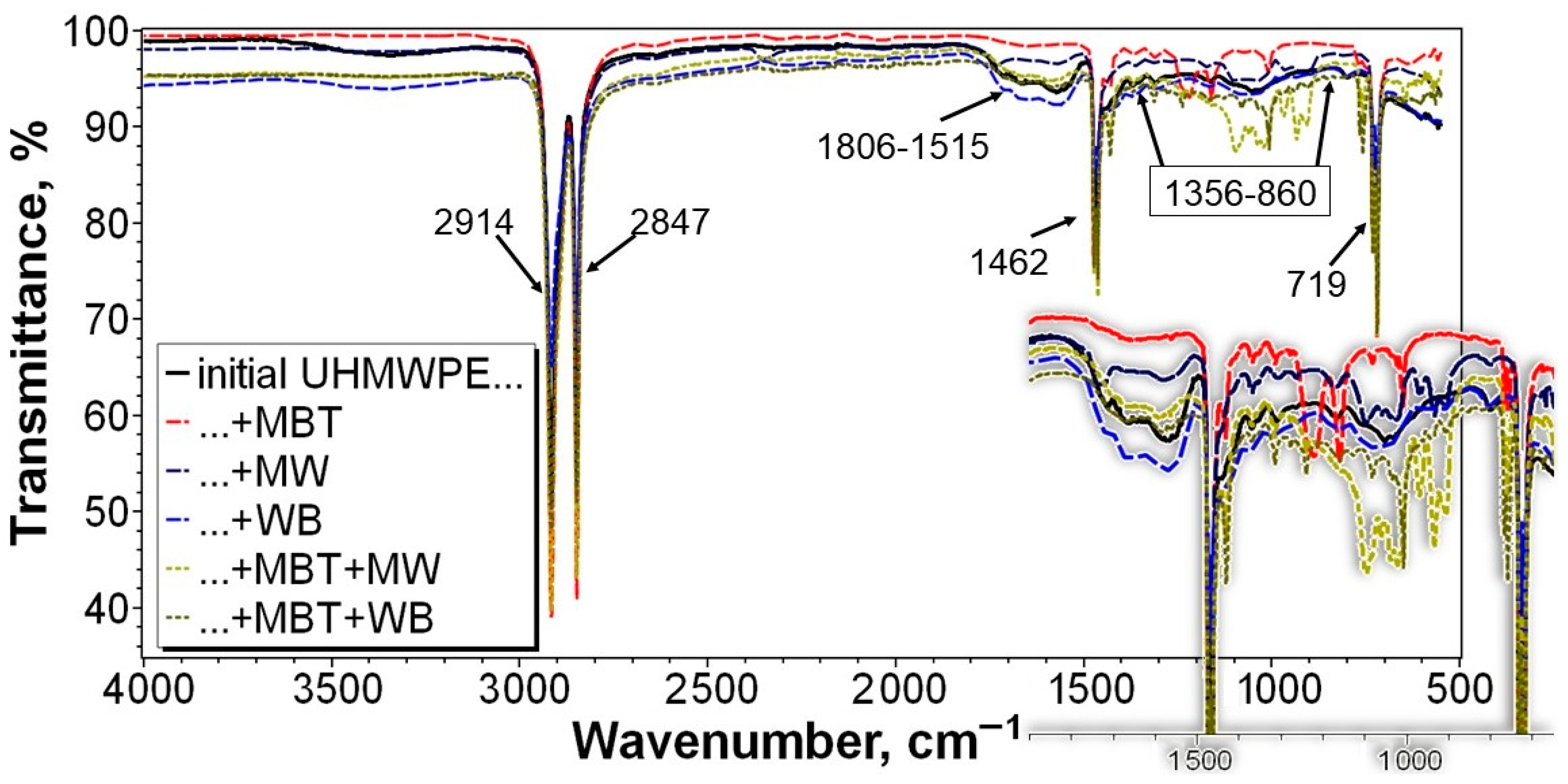
3.4. Study of the Structure of UHMWPE and the PCM
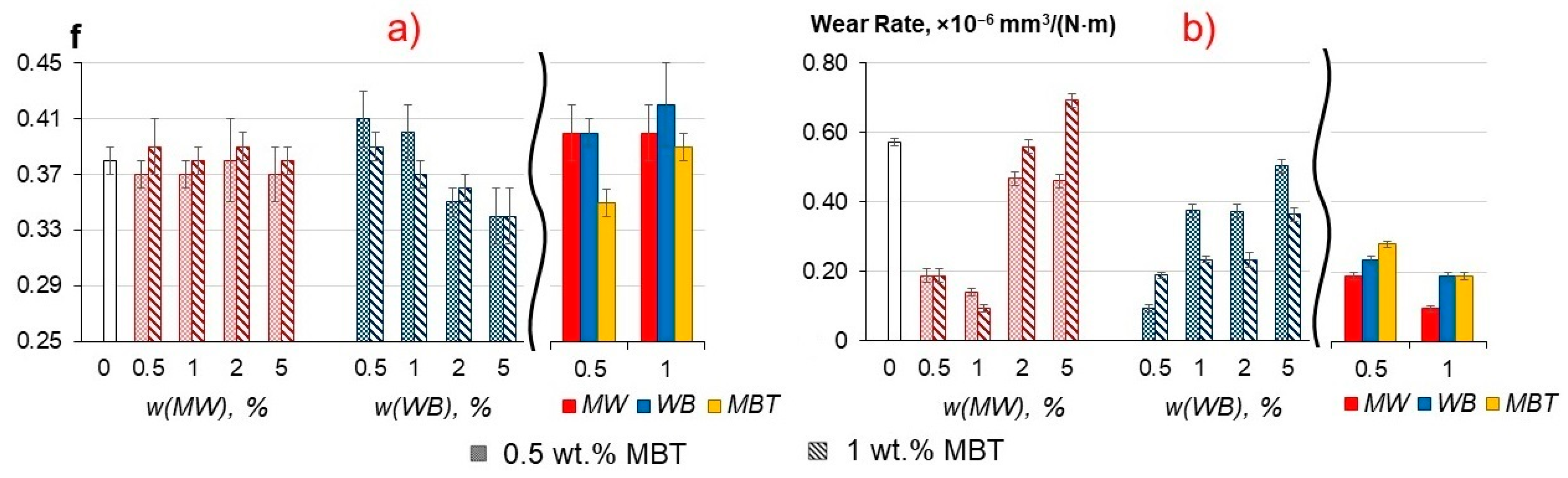
3.5. Tribological Properties of the Composites
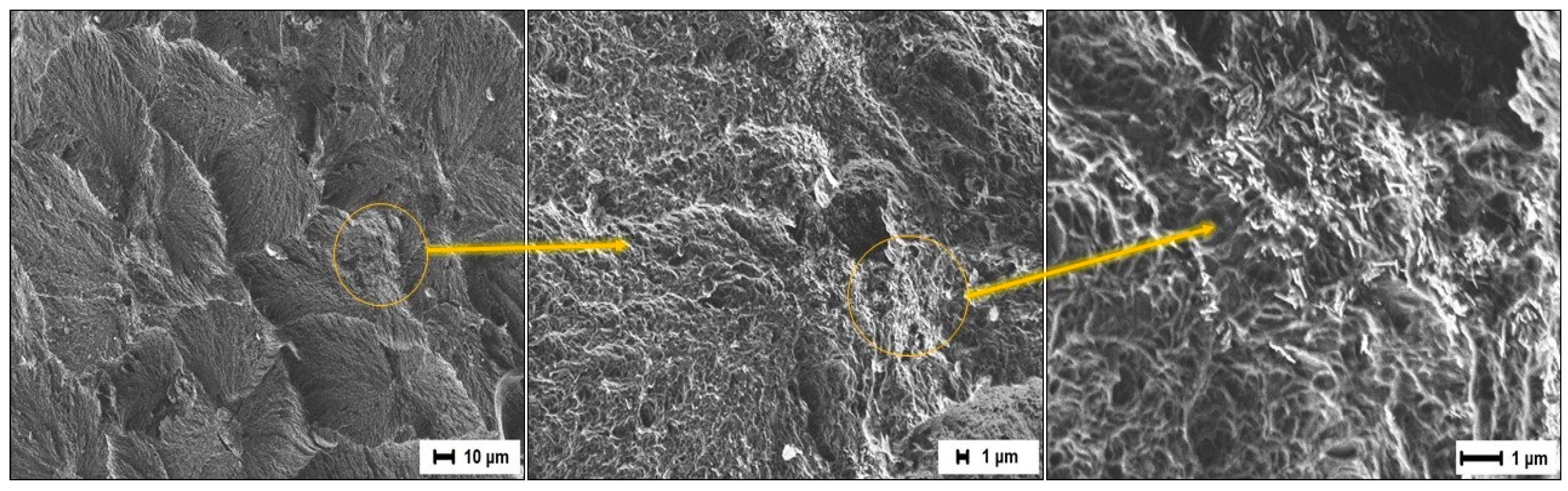
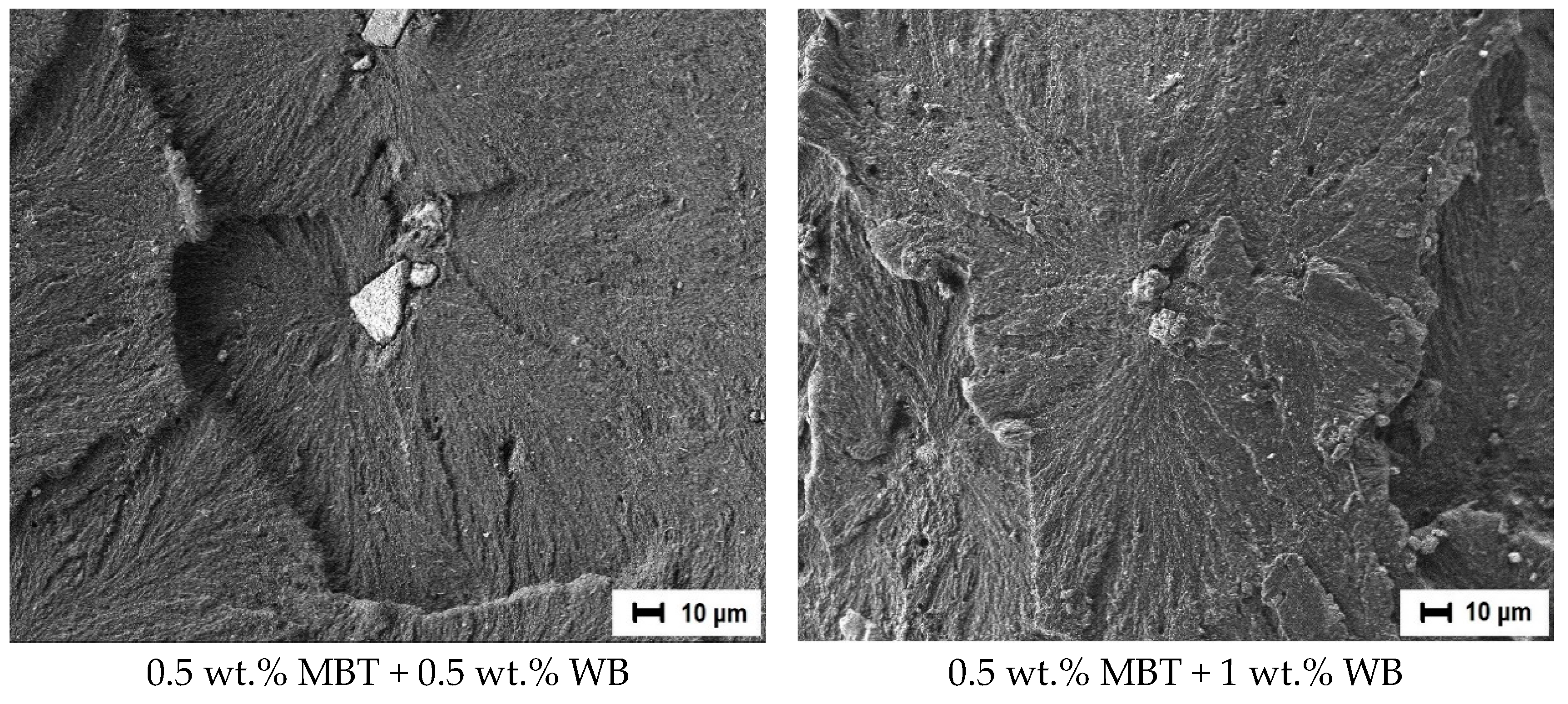
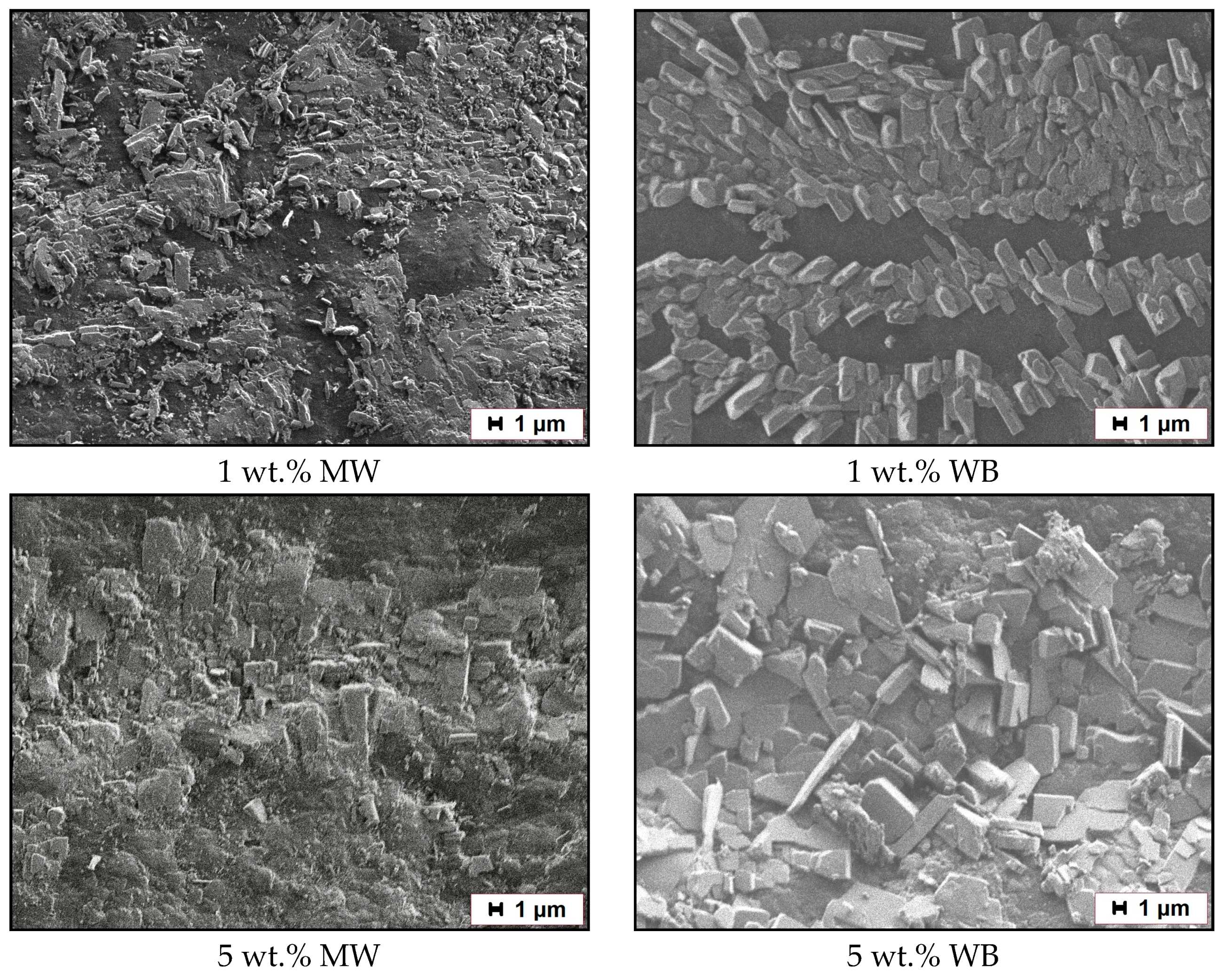
3.6. Study of the Friction Surface
- -
- In the first acts of friction, MBT (as a soft organic filler) can melt in the near-surface layer in the zone between the composites and the steel counterbody due to the increased temperature under mechanical stress. Consequently, MBT actively forms clusters on the friction surface of the composite and a transfer film on the surface of the steel counterbody [25,67]. In this case, MBT reduces the adhesion component of friction and facilitates the sliding processes of the composite relative to the surface of the steel counterbody, as if playing the role of a lubricant. The chemical aspect of MBT is described in more detail below.
- -
- The wollastonite particles (as solid particles) reinforce the polymer matrix, thereby preventing further molding of the surface layer. Solid particles can be localized in the friction zone, resulting in a gradual enrichment of the PCM surface with the more thermostable wollastonite particles. As a result, discrete areas are formed where the filler particles absorb the external load. The reduction of stresses at frictional contact is caused by the reduction of the actual contact area, increasing the loading capacity of the material and reducing the wear of the PCM, respectively.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolosov, A.E.; Sivetskii, V.I.; Kolosova, E.P.; Vanin, V.V.; Gondlyakh, A.V.; Sidorov, D.E.; Ivitskiy, I.I. Creation of Structural Polymer Composite Materials for Functional Application Using Physicochemical Modification. Adv. Polym. Technol. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Selyutin, G.E.; Gavrilov, Y.U.; Voskresenskaya, E.N.; Zakharov, V.A.; Nikitin, V.E.; Poluboyarov, V.A. Composite materials based on ultra-high molecular polyethylene: Properties, application prospects. Chem. Sustain. Dev. 2010, 18, 301–314. [Google Scholar]
- Kurtz, S.M. UHMWPE Biomaterials Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices, 2nd ed.; Elsevier: Amsterdam, The Netherland; Academic Press: Boston, MA, USA, 2009; ISBN 978-0-12-374721-1. [Google Scholar]
- Patel, K.; Chikkali, S.H.; Sivaram, S. Ultrahigh Molecular Weight Polyethylene: Catalysis, Structure, Properties, Processing and Applications. Prog. Polym. Sci. 2020, 109, 101290. [Google Scholar] [CrossRef]
- Sobieraj, M.C.; Rimnac, C.M. Ultra High Molecular Weight Polyethylene: Mechanics, Morphology, and Clinical Behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Hambir, S.; Jog, J.P. Sintering of Ultra High Molecular. Bull. Mater. Sci. 2000, 23, 221–226. [Google Scholar] [CrossRef]
- Grinev, V.G.; Krasheninnikov, V.G.; Zabolotnov, A.S.; Ladygina, T.A.; Brevnov, P.N.; Novokshonova, L.A.; Berlin, A.A. The Effect of Filler Type on the Mechanical Properties of Composite Materials Based on Ultra-High-Molecular-Weight Polyethylene. Polym. Sci. Ser. D 2018, 11, 202–208. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.; Xin, Z.; Ye, C.; Li, Z.; Xia, J.; Li, J. Mechanism of Size Effects of a Filler on the Wear Behavior of Ultrahigh Molecular Weight Polyethylene. Chin. J. Chem. Eng. 2020, 28, 1950–1963. [Google Scholar] [CrossRef]
- Liang, W.; Xiaochun, Y.; Guangjian, H.; Yanhong, F.; Jinping, Q. Ultrasound-Assisted Melt Mixing for the Preparation of UHMWPE/OMMT Nanocomposites. J. Thermoplast. Compos. Mater. 2018, 31, 784–802. [Google Scholar] [CrossRef]
- Yin, X.; Li, S.; He, G.; Feng, Y.; Wen, J. Preparation and Characterization of CNTs/UHMWPE Nanocomposites via a Novel Mixer under Synergy of Ultrasonic Wave and Extensional Deformation. Ultrason. Sonochem. 2018, 43, 15–22. [Google Scholar] [CrossRef]
- Bracco, P.; Brunella, V.; Luda, M.P.; Zanetti, M.; Costa, L. Radiation-Induced Crosslinking of UHMWPE in the Presence of Co-Agents: Chemical and Mechanical Characterisation. Polymer 2005, 46, 10648–10657. [Google Scholar] [CrossRef]
- Fu, J.; Ghali, B.W.; Lozynsky, A.J.; Oral, E.; Muratoglu, O.K. Wear Resistant UHMWPE with High Toughness by High Temperature Melting and Subsequent Radiation Cross-Linking. Polymer 2011, 52, 1155–1162. [Google Scholar] [CrossRef]
- Kiseleva, T.Y.; Devyatkina, E.T.; Grigoreva, T.F.; Yakuta, E.V.; Lazareva, E.V.; Vosmerikov, S.V.; Zholudev, S.I.; Ivanenko, I.P.; Markov, G.P.; Sangaa, D.; et al. Mechanosynthesized Composite Materials Based on Ultrahigh Molecular Weight Polyethylene Modified with Magnesium Ferrite Particles. Inorg. Mater. Appl. Res. 2021, 12, 740–749. [Google Scholar] [CrossRef]
- Panin, V.E.; Panin, S.V.; Kornienko, L.A.; Vannasri, S.; Ivanova, L.R.; Shil’ko, S.V. Effect of Mechanical Activation of Ultra-High-Molecular-Weight Polyethylene on Its Mechanical and Triboengineering Properties. J. Frict. Wear 2010, 31, 121–127. [Google Scholar] [CrossRef]
- Pak, S.Y.; Kim, H.M.; Kim, S.Y.; Youn, J.R. Synergistic Improvement of Thermal Conductivity of Thermoplastic Composites with Mixed Boron Nitride and Multi-Walled Carbon Nanotube Fillers. Carbon 2012, 50, 4830–4838. [Google Scholar] [CrossRef]
- Papynov, E.K.; Mayorov, V.Y.; Portnyagin, A.S.; Shichalin, O.O.; Kobylyakov, S.P.; Kaidalova, T.A.; Nepomnyashiy, A.V.; Sokol’nitskaya, T.A.; Zub, Y.L.; Avramenko, V.A. Application of Carbonaceous Template for Porous Structure Control of Ceramic Composites Based on Synthetic Wollastonite Obtained via Spark Plasma Sintering. Ceram. Int. 2015, 41, 1171–1176. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Apanasevich, V.I.; Portnyagin, A.S.; Yu, M.V.; Yu, B.I.; Merkulov, E.B.; Kaidalova, T.A.; Modin, E.B.; Afonin, I.S.; et al. Sol-Gel (Template) Synthesis of Osteoplastic CaSiO3/HAp Powder Biocomposite: “In Vitro” and “in Vivo” Biocompatibility Assessment. Powder Technol. 2020, 367, 762–773. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Apanasevich, V.I.; Afonin, I.S.; Evdokimov, I.O.; Mayorov, V.Y.; Portnyagin, A.S.; Agafonova, I.G.; Skurikhina, Y.E.; Medkov, M.A. Synthetic CaSiO3 Sol-Gel Powder and SPS Ceramic Derivatives: “In Vivo” Toxicity Assessment. Prog. Nat. Sci. Mater. Int. 2019, 29, 569–575. [Google Scholar] [CrossRef]
- Tong, J.; Ma, Y.; Jiang, M. Effects of the Wollastonite Fiber Modification on the Sliding Wear Behavior of the UHMWPE Composites. Wear 2003, 255, 734–741. [Google Scholar] [CrossRef]
- Panin, S.V.; Huang, Q.; Alexenko, V.O.; Buslovich, D.G.; Kornienko, L.А.; Berto, F.; Bochkareva, S.A.; Panov, I.L.; Ryabova, N.V. Design of Wear-Resistant UHMWPE-Based Composites Loaded with Wollastonite Microfibers Treated with Various Silane Coupling Agents. Appl. Sci. 2020, 10, 4511. [Google Scholar] [CrossRef]
- Danilova, S.N.; Yarusova, S.B.; Kulchin, Y.N.; Zhevtun, I.G.; Buravlev, I.Y.; Okhlopkova, A.A.; Gordienko, P.S.; Subbotin, E.P. UHMWPE/CaSiO3 Nanocomposite: Mechanical and Tribological Properties. Polymers 2021, 13, 570. [Google Scholar] [CrossRef]
- Danilova, S.N.; Yarusova, S.B.; Lazareva, N.N.; Buravlev, I.Y.; Shichalin, O.O.; Papynov, E.K.; Zhevtun, I.G.; Gordienko, P.S.; Okhlopkova, A.A. A Study of the Wear Mechanism of Composites Modified with Silicate Filler. Ceramics 2022, 5, 731–747. [Google Scholar] [CrossRef]
- Mahfoudh, A.; Cloutier, A.; Rodrigue, D. Characterization of UHMWPE/Wood Composites Produced via Dry-Blending and Compression Molding. Polym Compos 2013, 34, 510–516. [Google Scholar] [CrossRef]
- Duraccio, D.; Arrigo, R.; Strongone, V.; Capra, P.P.; Malucelli, G. Rheological, Mechanical, Thermal and Electrical Properties of UHMWPE/CNC Composites. Cellulose 2021, 28, 10953–10967. [Google Scholar] [CrossRef]
- Panin, S.V.; Kornienko, L.A.; Nguen Suan, T.; Ivanova, L.R.; Korchagin, M.A.; Shil’ko, S.V.; Pleskachevskii, Y.M. Wear Resistance of Composites Based on Hybrid UHMWPE–PTFE Matrix: Mechanical and Tribotechnical Properties of the Matrix. J. Frict. Wear 2015, 36, 249–256. [Google Scholar] [CrossRef]
- Massaccesi, L.; Ragone, V.; Papini, N.; Goi, G.; Corsi Romanelli, M.M.; Galliera, E. Effects of Vitamin E-Stabilized Ultra High Molecular Weight Polyethylene on Oxidative Stress Response and Osteoimmunological Response in Human Osteoblast. Front. Endocrinol. 2019, 10, 203. [Google Scholar] [CrossRef]
- GOST 739-74; 2-Mercaptobenzothiazole. Specifications, Standards Publishing House: Moscow, Russia, 2000; 11p.
- ASTM A. D3039/D3039M-14; Tensile Properties of Polymer Matrix Composite Materials, Annu. Book ASTM Stand. ASTM International: West Conshohocken, PA, USA, 2014; p. 13.
- Chesick, J.P.; Donohue, J. The Molecular and Crystal Structure of 2-Mercaptobenzothiazole. Acta Crystallogr. B Struct. Sci. 1971, 27, 1441–1444. [Google Scholar] [CrossRef]
- Rai, A.K.; Singh, R.; Singh, K.N.; Singh, V.B. FTIR, Raman Spectra and Ab Initio Calculations of 2-Mercaptobenzothiazole. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 483–490. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Sarkar, U.; Roy, D.R. Electrophilicity Index. Chem. Rev. 2006, 106, 2065–2091. [Google Scholar] [CrossRef]
- Obot, I.B.; Gasem, Z.M.; Umoren, S.A. Understanding the mechanism of 2-mercaptobenzimidazole adsorption on Fe (110), Cu (111) and Al (111) surfaces: DFT and molecular dynamics simulations approaches. Int. J. Electrochem. Sci. 2014, 9, 2367–2378. [Google Scholar] [CrossRef]
- Vernack, E.; Costa, D.; Tingaut, P.; Marcus, P. DFT Studies of 2-Mercaptobenzothiazole and 2-Mercaptobenzimidazole as Corrosion Inhibitors for Copper. Corros. Sci. 2020, 174, 108840. [Google Scholar] [CrossRef]
- Huang, W.; Dong, J.; Li, F.; Chen, B. The Performance and Antiwear Mechanism of (2-Sulfurone-Benzothiazole)-3-Methyl Esters as Additives in Synthetic Lubricant. Tribol. Int. 2000, 33, 553–557. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Zhang, H.; Li, P.; Wu, L.; Guo, W. Characterization of Iron Surface Modified by 2-Mercaptobenzothiazole Self-Assembled Monolayers. Appl. Surf. Sci. 2006, 253, 2812–2819. [Google Scholar] [CrossRef]
- Kress, K.E. Spectrophotometric Analysis of Accelerator-Rubber Mixtures. Anal. Chem. 1951, 23, 313–322. [Google Scholar] [CrossRef]
- Alam, M.N.; Debnath, S.C.; Boondamnoen, O.; Kumar, K.N.; Kim, J.H.; Choi, J. Synergistic Combination of 2-Mercaptobenzothiazole (MBT) and Nitrosoamine-Safe Thiuram Disulfide as Advanced Rubber Vulcanizing Accelerators. J. Elastomers Plast. 2022, 54, 1061–1077. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Guo, X.; Yi, M.; Wan, L.; Liao, S.; Wang, Z.; Fang, L. Xanthate-Modified NanoTiO2 as a Novel Vulcanization Accelerator Enhancing Mechanical and Antibacterial Properties of Natural Rubber. Nanotechnol. Rev. 2021, 10, 478–487. [Google Scholar] [CrossRef]
- Ilichev, V.A.; Silantyeva, L.I.; Yablonskiy, A.N.; Andreev, B.A.; Rumyantcev, R.V.; Fukin, G.K.; Bochkarev, M.N. Synthesis, Structure and Long-Lived NIR Luminescence of Lanthanide Ate Complexes with Perfluorinated 2-Mercaptobenzothiazole. Dalton Trans. 2019, 48, 1060–1066. [Google Scholar] [CrossRef]
- Form, G.R.; Raper, E.S.; Downie, T.C. The Crystal and Molecular Structure of 2-Mercaptobenzimidazole. Acta Crystallogr. B Struct. Sci. 1976, 32, 345–348. [Google Scholar] [CrossRef]
- Rogozhin, A.F.; Ilichev, V.A.; Fagin, A.A.; Rumyantcev, R.V.; Fukin, G.K.; Yablonskiy, A.N.; Andreev, B.A.; Bochkarev, M.N. Novel Ditopic 2-Mercaptothiazoles and Their Sodium Salts: Synthesis, Structural Diversity and Luminescence. New J. Chem. 2022, 46, 13987–13995. [Google Scholar] [CrossRef]
- Flakus, H.T.; Miros, A.; Jones, P.G. Polarization IR Spectra of Model Crystals Containing Cyclic N–H…S Bonded Dimers: 2-Mercaptothiazoline and 2-Mercapto-1-Methylimidazole. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 225–237. [Google Scholar] [CrossRef]
- Silva, A.L.R.; Ribeiro da Silva, M.D.M.C. Energetic, Structural and Tautomeric Analysis of 2-Mercaptobenzimidazole: An Experimental and Computational Approach. J. Therm. Anal. Calorim. 2017, 129, 1679–1688. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Mustafa, A.M.; Zoghaib, W.M.; Afifi, M.S.; Farag, R.S.; Badr, Y. Reinvestigation of Benzothiazoline-2-Thione and 2-Mercaptobenzothiazole Tautomers: Conformational Stability, Barriers to Internal Rotation and DFT Calculations. J. Mol. Struct. THEOCHEM 2008, 868, 27–36. [Google Scholar] [CrossRef]
- Radha, A. Crystal and Molecular Structure of 2-Mercaptobenzothiozole—A Redetermination. Z. Für Krist. Cryst. Mater. 1985, 171, 225–228. [Google Scholar] [CrossRef]
- Böhlig, H.; Ackermann, M.; Billes, F.; Kudra, M. Vibrational Analysis of Benzothiazoline-2-Thione. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1999, 55, 2635–2646. [Google Scholar] [CrossRef]
- Okhlopkova, T.A.; Borisova, R.V.; Nikiforov, L.A.; Spiridonov, A.M.; Sharin, P.P.; Okhlopkova, A.A. Technology of Liquid-Phase Compounding of Ultra-High-Molecular-Weight Polyethylene with Nanoparticles of Inorganic Compounds under the Action of Ultrasonic Vibrations. Russ. J. Appl. Chem. 2016, 89, 1469–1476. [Google Scholar] [CrossRef]
- Okhlopkova, T.A.; Borisova, R.V.; Nikiforov, L.A.; Spiridonov, A.M.; Okhlopkova, A.A.; Jeong, D.-Y.; Cho, J.-H. Supramolecular Structure and Mechanical Characteristics of Ultrahigh-Molecular-Weight Polyethylene-Inorganic Nanoparticle Nanocomposites: UHMWPE-Inorganic Nanoparticle Composites. Bull. Korean Chem. Soc. 2016, 37, 439–444. [Google Scholar] [CrossRef]
- Kapitonova, I.; Lazareva, N.; Tarasova, P.; Okhlopkova, A.; Laukkanen, S.; Mukhin, V. Morphology Analysis of Friction Surfaces of Composites Based on PTFE and Layered Silicates. Polymers 2022, 14, 4658. [Google Scholar] [CrossRef]
- Berladir, K.; Zhyhylii, D.; Gaponova, O.; Krmela, J.; Krmelová, V.; Artyukhov, A. Modeling of Polymer Composite Materials Chaotically Reinforced with Spherical and Cylindrical Inclusions. Polymers 2022, 14, 2087. [Google Scholar] [CrossRef]
- Mostovoy, A.; Bekeshev, A.; Tastanova, L.; Akhmetova, M.; Bredihin, P.; Kadykova, Y. The Effect of Dispersed Filler on Mechanical and Physicochemical Properties of Polymer Composites. Polym. Polym. Compos. 2021, 29, 583–590. [Google Scholar] [CrossRef]
- Sarokin, V.; Avdeychik, S.; Struk, V.; Antonov, A. Activation of components functional polymer materials according to energy technologies. In Proceedings of the 21st International Scientific Conference, Kaunas, Lithuania, 12–13 May 2016; pp. 241–244. [Google Scholar]
- Costa, P.; Maceiras, A.; San Sebastián, M.; García-Astrain, C.; Vilas, J.L.; Lanceros-Mendez, S. On the Use of Surfactants for Improving Nanofiller Dispersion and Piezoresistive Response in Stretchable Polymer Composites. J. Mater. Chem. C 2018, 6, 10580–10588. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, W.-H.; Teng, T.-P.; Yang, C.-R. Evaluation of Surfactants on Graphene Dispersion and Thermal Performance for Heat Dissipation Coating. Polymers 2022, 14, 952. [Google Scholar] [CrossRef]
- Atif, R.; Inam, F. Reasons and Remedies for the Agglomeration of Multilayered Graphene and Carbon Nanotubes in Polymers. Beilstein J. Nanotechnol. 2016, 7, 1174–1196. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Visco, A.; Galtieri, G.; Nocita, D.; Espro, C. Wear Behaviour of UHMWPE Reinforced by Carbon Nanofiller and Paraffin Oil for Joint Replacement. Mater. Sci. Eng. C 2017, 73, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Visco, A.; Yousef, S.; Scolaro, C.; Espro, C.; Cristani, M. Tribological Behavior of Nanocomposites Based on UHMWPE Aged in Simulated Synovial Fluid. Polymers 2018, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Scolaro, C.; Dal Poggetto, G.; Pacifico, S.; Visco, A. Wear Resistant Nanocomposites Based on Biomedical Grade UHMWPE Paraffin Oil and Carbon Nano-Filler: Preliminary Biocompatibility and Antibacterial Activity Investigation. Polymers 2020, 12, 978. [Google Scholar] [CrossRef] [PubMed]
- Parpaite, T.; Otazaghine, B.; Caro, A.S.; Taguet, A.; Sonnier, R.; Lopez-Cuesta, J.M. Janus Hybrid Silica/Polymer Nanoparticles as Effective Compatibilizing Agents for Polystyrene/Polyamide-6 Melted Blends. Polymer 2016, 90, 34–44. [Google Scholar] [CrossRef]
- Jiang, J.; Cao, J.; Wang, W.; Shen, H. Preparation of a Synergistically Stabilized Oil-in-Water Paraffin Pickering Emulsion for Potential Application in Wood Treatment. Holzforschung 2018, 72, 489–497. [Google Scholar] [CrossRef]
- Jiang, J.; Mei, C.; Pan, M.; Cao, J. How Does Pickering Emulsion Pre-Treatment Influence the Properties of Wood Flour and Its Composites with High-Density Polyethylene? Polymers 2019, 11, 1115. [Google Scholar] [CrossRef]
- Vignati, E.; Piazza, R.; Lockhart, T.P. Pickering Emulsions: Interfacial Tension, Colloidal Layer Morphology, and Trapped-Particle Motion. Langmuir 2003, 19, 6650–6656. [Google Scholar] [CrossRef]
- Kurkin, T.S.; Ozerin, A.N.; Kechek’yan, A.S.; Gritsenko, O.T.; Ozerina, L.A.; Alkhanishvili, G.G.; Sushchev, V.G.; Dolmatov, V.Y. The Structure and Properties of Polymer Composite Fibers Based on Poly(Vinyl Alcohol) and Nanodiamond of Detonation Synthesis. Nanotechnol. Russ. 2010, 5, 340–351. [Google Scholar] [CrossRef]
- Taguet, A.; Cassagnau, P.; Lopez-Cuesta, J.-M. Structuration, Selective Dispersion and Compatibilizing Effect of (Nano)Fillers in Polymer Blends. Prog. Polym. Sci. 2014, 39, 1526–1563. [Google Scholar] [CrossRef]
- Hu, X.-P.; Hsieh, Y.-L. Crystallite Sizes and Lattice Distortions of Gel-Spun Ultra-High Molecular Weight Polyethylene Fibers. Polym. J. 1998, 30, 771–774. [Google Scholar] [CrossRef]
- Danilova, S.N.; Dyakonov, A.A.; Vasilev, A.P.; Okhlopkova, A.A.; Tuisov, A.G.; Kychkin, A.K.; Kychkin, A.A. Effect of Borpolymer on Mechanical and Structural Parameters of Ultra-High Molecular Weight Polyethylene. Nanomaterials 2021, 11, 3398. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.K.; Bahadur, S. Micromechanism of Wear at Polymer-Metal Sliding Interface. Wear 1978, 46, 189–202. [Google Scholar] [CrossRef]
- Romani, S.; Moroder, L.; Bovermann, G.; Wünsch, E. On the Use of Five-Membered Heterocycles in Peptide Chemistry. Synthesis 1985, 1985, 738–742. [Google Scholar] [CrossRef]
- Yekeler, H.; Yekeler, M. Predicting the Efficiencies of 2-Mercaptobenzothiazole Collectors Used as Chelating Agents in Flotation Processes: A Density-Functional Study. J. Mol. Model. 2006, 12, 763–768. [Google Scholar] [CrossRef] [PubMed]
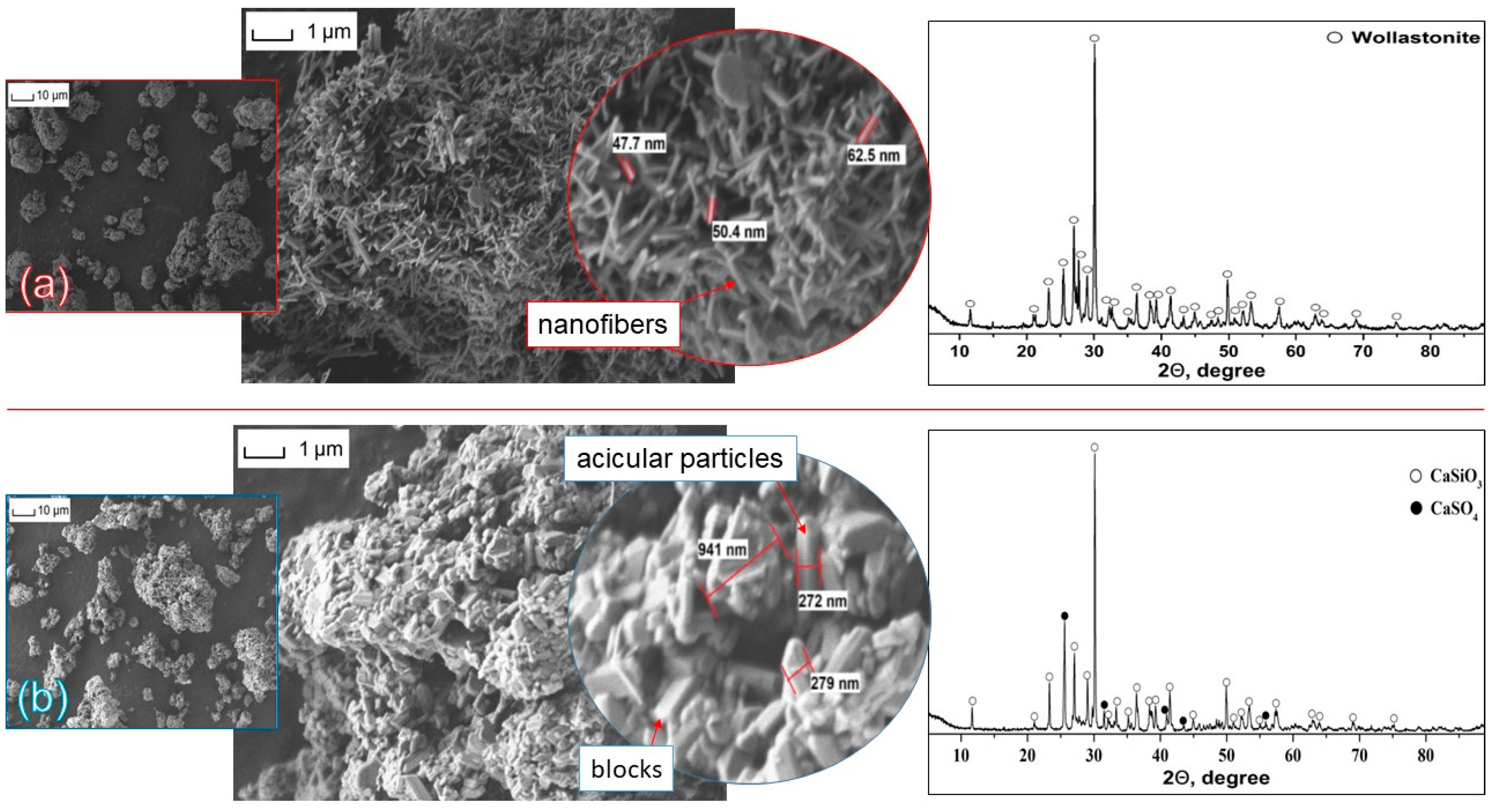

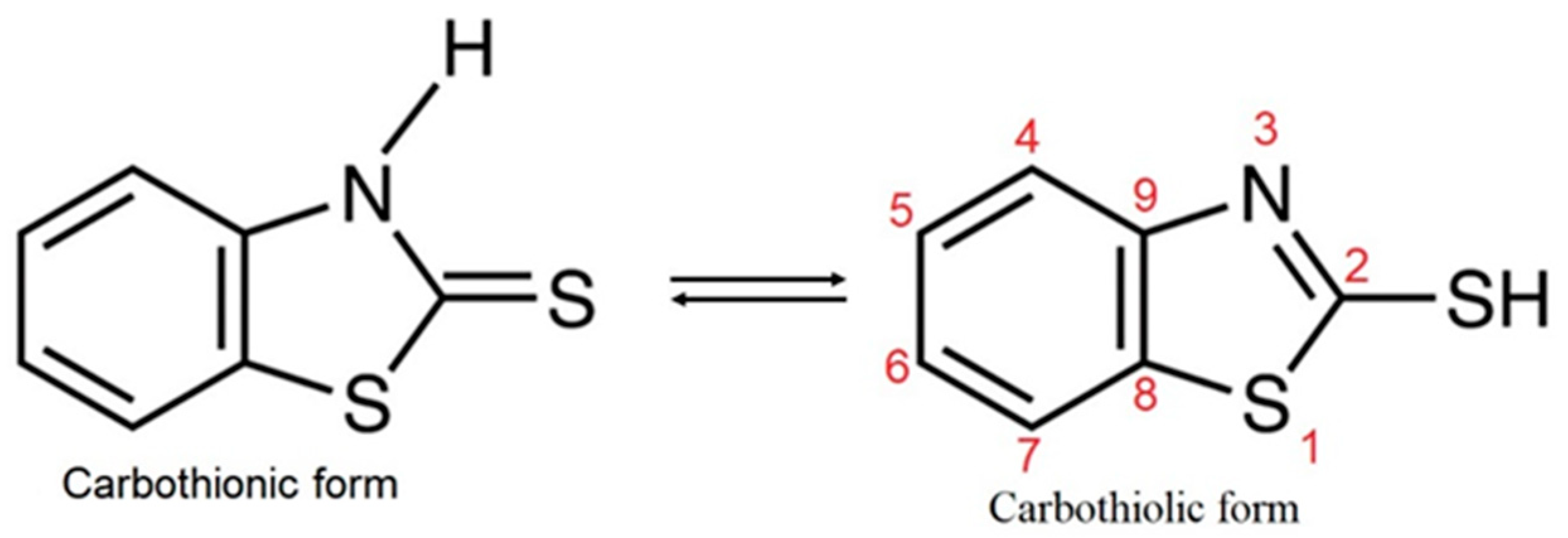


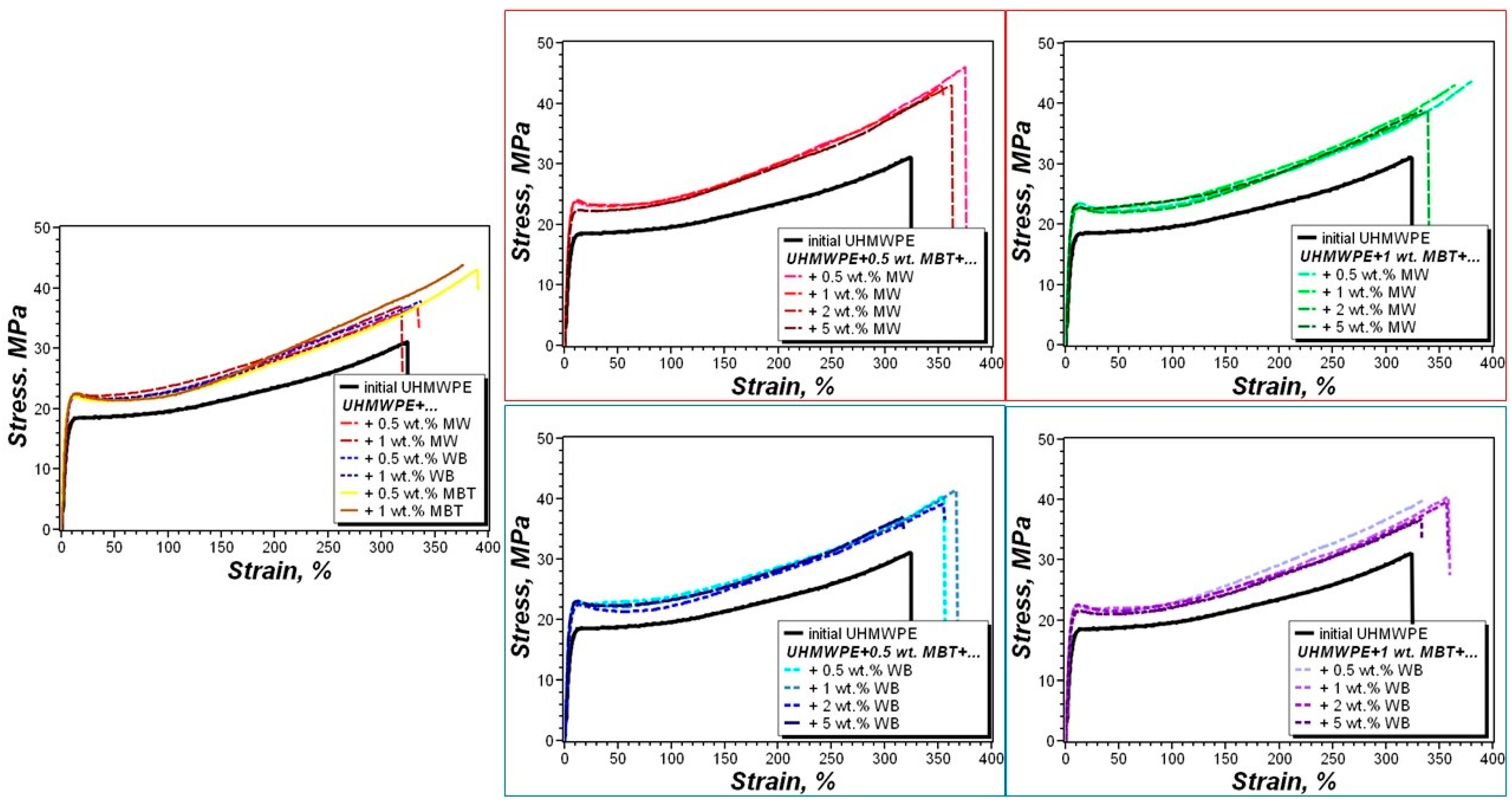

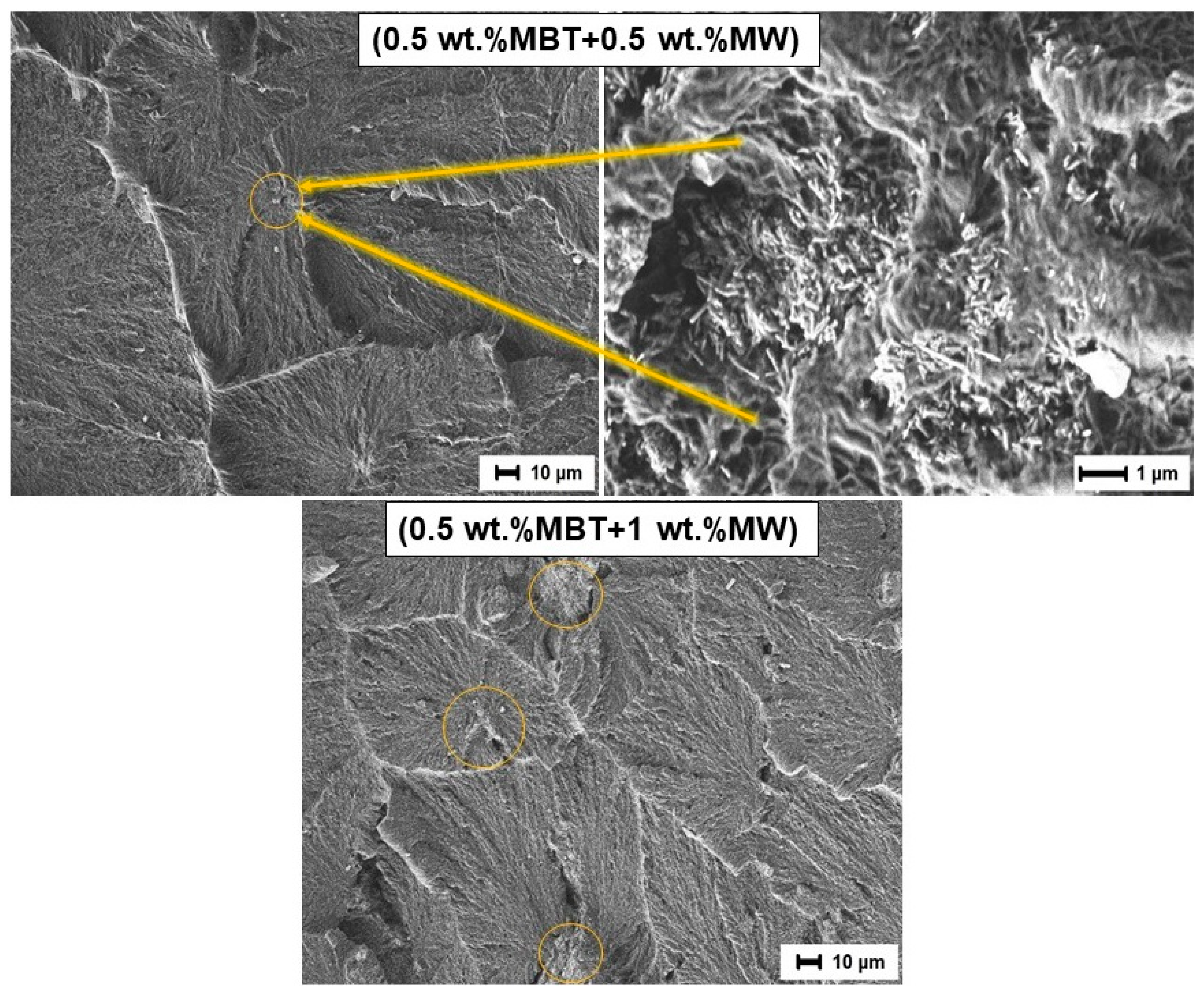




| Composite | UHMWPE | MBT | Wollastonite |
|---|---|---|---|
| 1 | 99 | 0.5 | 0.5 |
| 2 | 98.5 | 0.5 | 1 |
| 3 | 97.5 | 0.5 | 2 |
| 4 | 94.5 | 0.5 | 5 |
| 5 | 98.5 | 1 | 0.5 |
| 6 | 98 | 1 | 1 |
| 8 | 97 | 1 | 2 |
| 9 | 94 | 1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilova, S.N.; Okhlopkova, A.A.; Yarusova, S.B.; Dyakonov, A.A.; Gordienko, P.S.; Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Vasilev, A.P.; Zhevtun, I.G.; et al. Study on the Impact of a Combination of Synthetic Wollastonite and 2-Mercaptobenzothiazole-Based Fillers on UHMWPE Polymeric Matrix. J. Compos. Sci. 2023, 7, 431. https://doi.org/10.3390/jcs7100431
Danilova SN, Okhlopkova AA, Yarusova SB, Dyakonov AA, Gordienko PS, Papynov EK, Shichalin OO, Buravlev IY, Vasilev AP, Zhevtun IG, et al. Study on the Impact of a Combination of Synthetic Wollastonite and 2-Mercaptobenzothiazole-Based Fillers on UHMWPE Polymeric Matrix. Journal of Composites Science. 2023; 7(10):431. https://doi.org/10.3390/jcs7100431
Chicago/Turabian StyleDanilova, Sakhayana N., Aitalina A. Okhlopkova, Sofia B. Yarusova, Afanasy A. Dyakonov, Pavel S. Gordienko, Evgeniy K. Papynov, Oleg O. Shichalin, Igor Yu. Buravlev, Andrey P. Vasilev, Ivan G. Zhevtun, and et al. 2023. "Study on the Impact of a Combination of Synthetic Wollastonite and 2-Mercaptobenzothiazole-Based Fillers on UHMWPE Polymeric Matrix" Journal of Composites Science 7, no. 10: 431. https://doi.org/10.3390/jcs7100431
APA StyleDanilova, S. N., Okhlopkova, A. A., Yarusova, S. B., Dyakonov, A. A., Gordienko, P. S., Papynov, E. K., Shichalin, O. O., Buravlev, I. Y., Vasilev, A. P., Zhevtun, I. G., & Ivanenko, N. V. (2023). Study on the Impact of a Combination of Synthetic Wollastonite and 2-Mercaptobenzothiazole-Based Fillers on UHMWPE Polymeric Matrix. Journal of Composites Science, 7(10), 431. https://doi.org/10.3390/jcs7100431









