Processing of 3-(Trimethoxysilyl)propyl Methacrylate (TMSPM) Functionalized Barium Titanate/Photopolymer Composites: Functionalization and Process Parameter Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Surface Functionalization of BTONP
2.2.1. Investigation of Silanization Temperature
2.2.2. Investigation of Silanization Time
2.2.3. Variation of TMSPM Concentration
2.2.4. Investigation of Acid-Catalyzed Silanization
2.3. UV Light Curable, BTONP Filled Photopolymer Resin Preparation
2.4. Composite Manufacturing
2.5. Characterization
3. Results and Discussion
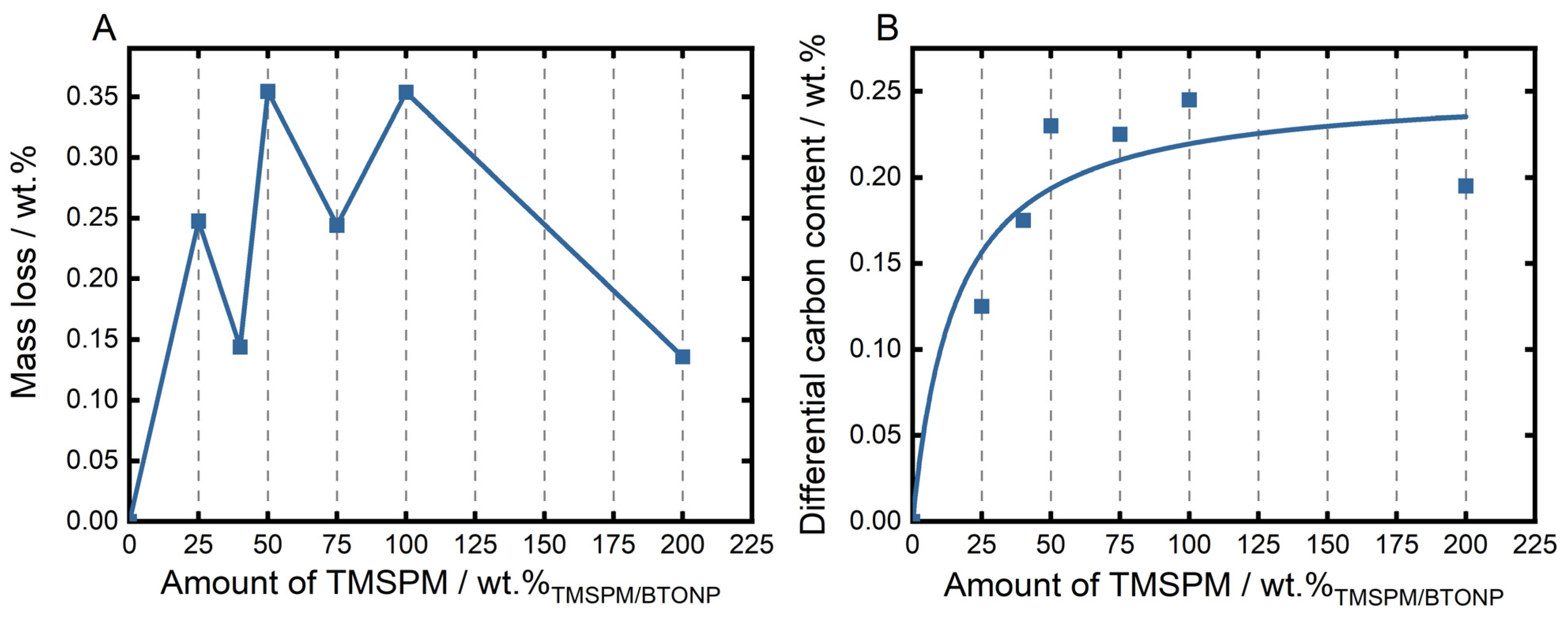
3.1. Particle Functionalization—Influence of TMSPM/BTONP Ratio
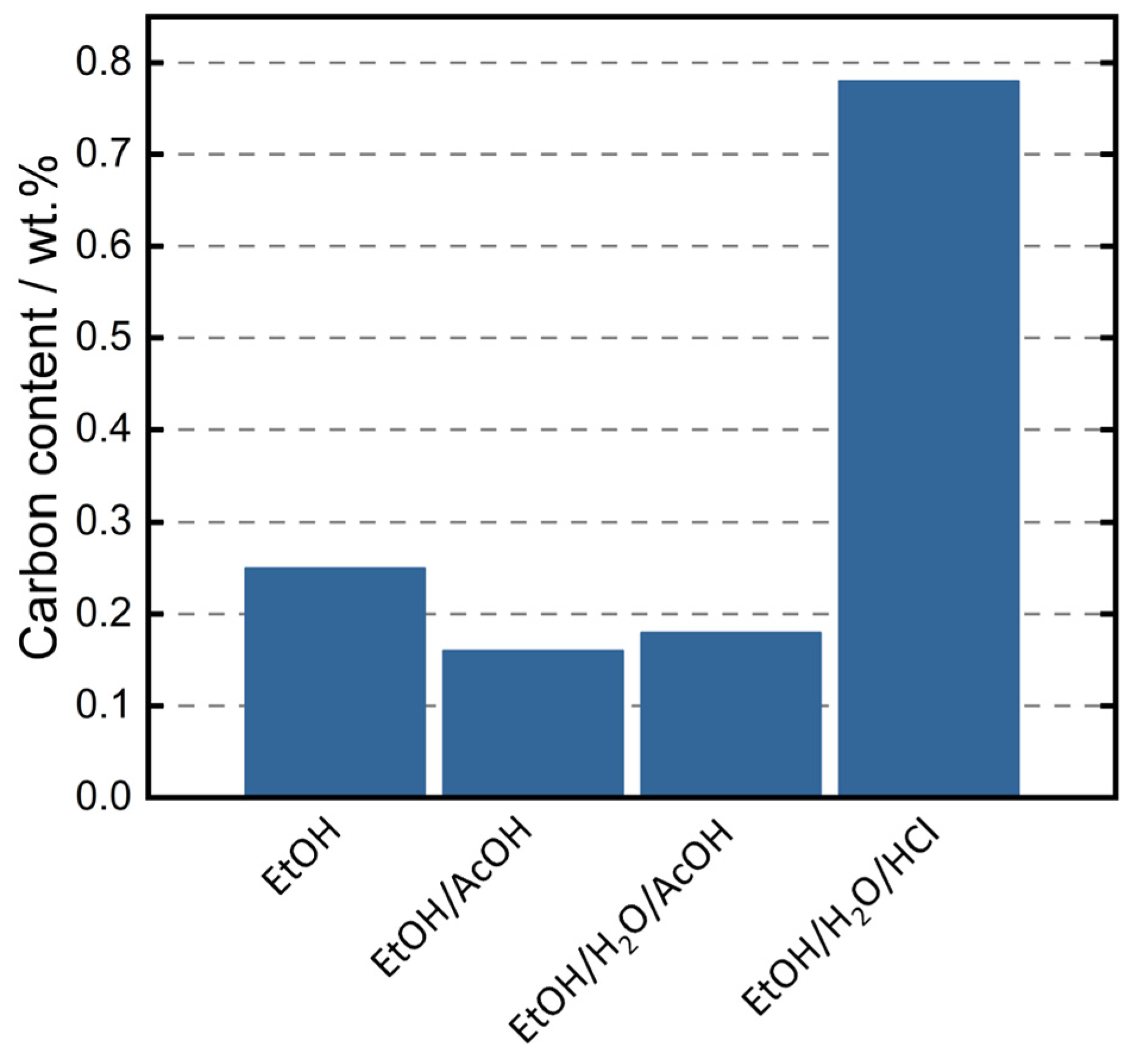
3.2. TMSPM Functionalization—Influence of Temperature, Time, and pH
3.3. Viscosity and Curing Depth
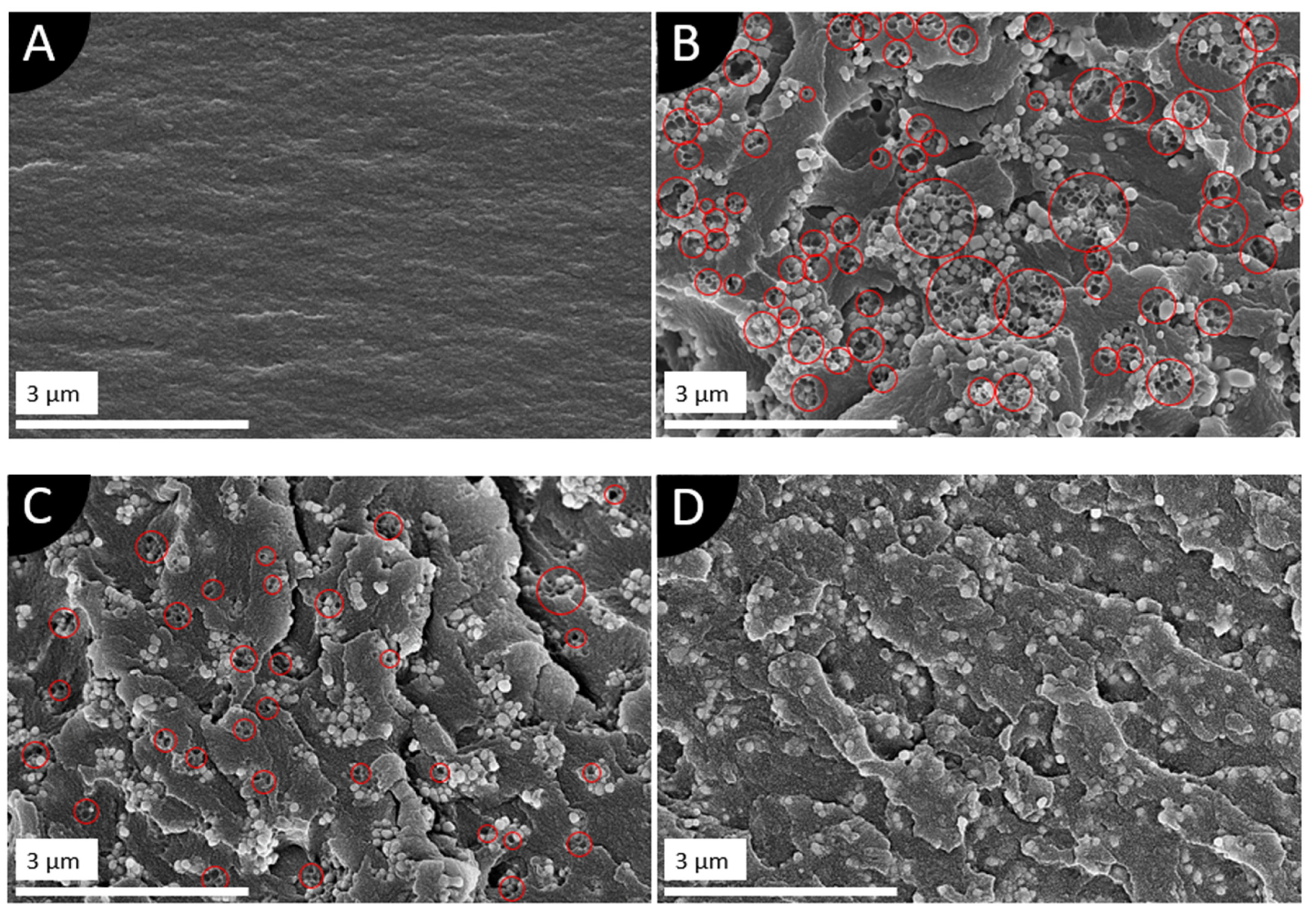
3.4. Microstructure of the Composites
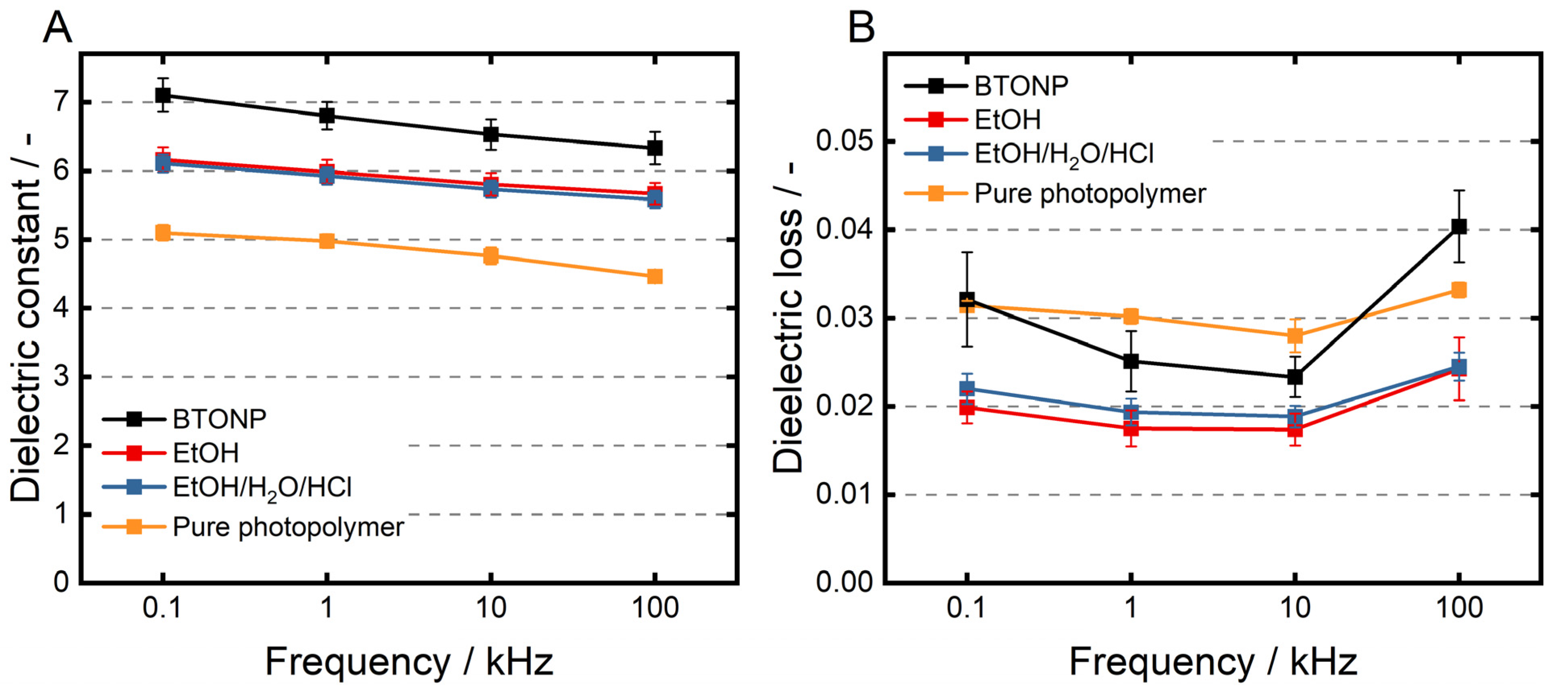
3.5. Dielectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, J.S. (Ed.) Sensor Technology Handbook; Newnes: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Wang, Z.; Narita, F. Corona Poling Conditions for Barium Titanate/Epoxy Composites and their Unsteady Wind Energy Harvesting Potential. Adv. Eng. Mater. 2019, 21, 1900169. [Google Scholar] [CrossRef]
- Wang, Z.; Abe, S.; Narita, F. On the Energy Harvesting Potential of Lead-Free Piezoelectric Composites from Air-Flow and Temperature Change. Res. Dev. Mater. Sci. 2018, 5, 000607. [Google Scholar] [CrossRef]
- Sinapius, J.M. Adaptronics—Smart Structures and Materials; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Giurgiutiu, V. Piezoelectric Wafer Active Sensors. In Structural Health Monitoring of Aerospace Composites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 177–248. [Google Scholar]
- Malhotra, J.; Patil, S.; Kaur, W.G.; Kumar, V. Energy Harvesting Applications using Piezoelectric Sensors. In Proceedings of the International Conference on Recent Advances in Computational Techniques (IC-RACT), Navi Mumbai, India, 27–28 March 2020. [Google Scholar] [CrossRef]
- Shrout, T.R.; Zhang, S.J. Lead-free piezoelectric ceramics: Alternatives for PZT? J. Electroceramics 2007, 19, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Mitkus, R.; Taleb Alashkar, A.; Sinapius, M. An Attempt to Topology Optimize 3D Printed Piezoelectric Composite Sensors for Highest D31 Output. In ASME 2021 Conference on Smart Materials, Adaptive Structures and Intelligent Systems; American Society of Mechanical Engineers: New York, NY, USA, 2021; p. 09142021. [Google Scholar]
- Roloff, T.; Mitkus, R.; Lion, J.N.; Sinapius, M. (Eds.) 3D Printable Piezoelectric Composite Sensors for Guided Ultrasonic Wave Detection; MDPI: Basel, Switzerland, 2021. [Google Scholar]
- Lin, J.; Chen, G.; Yang, W.; Li, H.; Lei, Q. New potassium sodium niobate/poly(vinylidene fluoride) functional composite films with high dielectric permittivity. J. Polym. Res. 2016, 23, 152. [Google Scholar] [CrossRef]
- Bodkhe, S.; Ermanni, P. Challenges in 3D printing of piezoelectric materials. Multifunct. Mater. 2019, 2, 22001. [Google Scholar] [CrossRef]
- Tiller, B.; Reid, A.; Zhu, B.; Guerreiro, J.; Domingo-Roca, R.; Jackson, J.C.; Windmill, J.F.C. Piezoelectric microphone via a digital light processing 3D printing process. Mater. Des. 2019, 165, 107593. [Google Scholar] [CrossRef]
- Kim, K.; Middlebrook, J.L.; Chen, J.E.; Zhu, W.; Chen, S.; Sirbuly, D.J. Tunable Surface and Matrix Chemistries in Optically Printed (0–3) Piezoelectric Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 33394–33398. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Song, X.; Zhu, B.; Hsiai, T.; Wu, P.-I.; Xiong, R.; Shi, J.; Chen, Y.; Zhou, Q.; et al. Three dimensional printing of high dielectric capacitor using projection based stereolithography method. Nano Energy 2016, 22, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Zhu, W.; Qu, X.; Aaronson, C.; McCall, W.R.; Chen, S.; Sirbuly, D.J. 3D optical printing of piezoelectric nanoparticle-polymer composite materials. ACS Nano 2014, 8, 9799–9806. [Google Scholar] [CrossRef]
- Popielarz, R.; Chiang, C.K.; Nozaki, R.; Obrzut, J. Dielectric Properties of Polymer/Ferroelectric Ceramic Composites from 100 Hz to 10 GHz. Macromolecules 2001, 34, 5910–5915. [Google Scholar] [CrossRef]
- Jang, J.H.; Wang, S.; Pilgrim, S.M.; Schulze, W.A. Preparation and Characterization of Barium Titanate Suspensions for Stereolithography. J. Am. Ceram. Soc. 2000, 83, 1804–1806. [Google Scholar] [CrossRef]
- Cui, H.; Hensleigh, R.; Yao, D.; Maurya, D.; Kumar, P.; Kang, M.G.; Priya, S.; Zheng, X. Three-dimensional printing of piezoelectric materials with designed anisotropy and directional response. Nat. Mater. 2019, 18, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Cui, H.; Hensleigh, R.; Smith, P.; Alford, S.; Bernero, D.; Bush, S.; Mann, K.; Wu, H.F.; Chin-Nieh, M.; et al. Achieving the Upper Bound of Piezoelectric Response in Tunable, Wearable 3D Printed Nanocomposites. Adv. Funct. Mater. 2019, 29, 1903866. [Google Scholar] [CrossRef]
- Mitkus, R.; Pierou, A.; Feder, J.; Sinapius, M. Investigation and Attempt to 3D Print Piezoelectric 0-3 Composites Made of Photopolymer Resins and PZT. In ASME 2020 Conference on Smart Materials, Adaptive Structures and Intelligent Systems; American Society of Mechanical Engineers: New York, NY, USA, 2020; p. 09152020. [Google Scholar]
- Schmidt, D. Modenselektive Übertragung von Lambwellen in Faserverbundstrukturen. Ph.D. Thesis, Deutsches Zentrum für Luft-und Raumfahrt eV, Braunschweig, Germany, 2014. [Google Scholar]
- Griffith, M.L.; Halloran, J.W. Freeform Fabrication of Ceramics via Stereolithography. J. Am. Ceram. Soc. 1996, 79, 2601–2608. [Google Scholar] [CrossRef] [Green Version]
- Badev, A.; Abouliatim, Y.; Chartier, T.; Lecamp, L.; Lebaudy, P.; Chaput, C.; Delage, C. Photopolymerization kinetics of a polyether acrylate in the presence of ceramic fillers used in stereolithography. J. Photochem. Photobiol. A Chem. 2011, 222, 117–122. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X. Experimental and numerical investigations on microstereolithography of ceramics. J. Appl. Phys. 2002, 92, 4796–4802. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, X. The influences of the material properties on ceramic micro-stereolithography. Sens. Actuators A Phys. 2002, 101, 364–370. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Sun, C. Micro-stereolithography of polymeric and ceramic microstructures. Sens. Actuators A Phys. 1999, 77, 149–156. [Google Scholar] [CrossRef]
- Liu, J.; Tian, G.; Qi, S.; Wu, Z.; Wu, D. Enhanced dielectric permittivity of a flexible three-phase polyimide–graphene–BaTiO3 composite material. Mater. Lett. 2014, 124, 117–119. [Google Scholar] [CrossRef]
- Luo, C.; Hu, S.; Xia, M.; Li, P.; Hu, J.; Li, G.; Jiang, H.; Zhang, W. A Flexible Lead-Free BaTiO3/PDMS/C Composite Nanogenerator as a Piezoelectric Energy Harvester. Energy Technol. 2018, 6, 922–927. [Google Scholar] [CrossRef]
- Zhang, Y. In Situ Fatigue Crack Detection using Piezoelectric Paint Sensor. J. Intell. Mater. Syst. Struct. 2016, 17, 843–852. [Google Scholar] [CrossRef]
- Escamilla-Díaz, T.; Serralta-Macías, J.J.; García-Zaldivar, O.; Cruz-Valeriano, E.; Ramírez-Bon, R.; Yanez Limon, J.M. Optical and dielectric studies of PMMA: Precursors of BNT hybrid films. Appl. Phys. A 2019, 125, 376. [Google Scholar] [CrossRef]
- Gupta, S.; Bhunia, R.; Fatma, B.; Maurya, D.; Singh, D.; Gupta, R.; Priya, S.; Gupta, R.K.; Gang, A. Multifunctional and Flexible Polymeric Nanocomposite Films with Improved Ferroelectric and Piezoelectric Properties for Energy Generation Devices. ACS Appl. Energy Mater. 2019, 2, 6364–6374. [Google Scholar] [CrossRef]
- Zarinwall, A.; Waniek, T.; Saadat, R.; Braun, U.; Sturm, H.; Garnweitner, G. Comprehensive Characterization of APTES Surface Modifications of Hydrous Boehmite Nanoparticles. Langmuir 2021, 37, 171–179. [Google Scholar] [CrossRef]
- Zarinwall, A.; Waniek, T.; Finke, B.; Saadat, R.; Sturm, H.; Garnweitner, G. Particle Surface Modification. In Acting Principles of Nano-Scaled Matrix Additives for Composite Structures; Sinapius, M., Ziegmann, G., Eds.; Springer: Cham, Switzerland, 2021; pp. 119–142. [Google Scholar]
- Mitkus, R.; Scharnofske, M.; Sinapius, M. Characterization 0.1 wt.% Nanomaterial/Photopolymer Composites with Poor Nanomaterial Dispersion: Viscosity, Cure Depth and Dielectric Properties. Polymers 2021, 13, 3948. [Google Scholar] [CrossRef]
- Mitkus, R.; Sinapius, M. Piezoelectric Ceramic/Photopolymer Composites Curable with UV Light: Viscosity, Curing Depth, and Dielectric Properties. J. Compos. Sci. 2022, 6, 212. [Google Scholar] [CrossRef]
- Phan TT, M.; Chu, N.C.; van Luu, B.; Nguyen Xuan, H.; Pham, D.T.; Martin, I.; Carriere, P. Enhancement of polarization property of silane-modified BaTiO3 nanoparticles and its effect in increasing dielectric property of epoxy/BaTiO3 nanocomposites. J. Sci. Adv. Mater. Devices 2016, 1, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Hasbullah, N.N.; Lee, O.J.; Chyi JL, Y.; Chen, S.K.; Talib, Z.A. Synthesis of BaTiO3 Nanoparticles via Hydrothermal Method. Solid State Phenom. 2017, 268, 172–176. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, X.-M.; He, T. Kinetics of (3-aminopropyl)triethoxylsilane (APTES) silanization of superparamagnetic iron oxide nanoparticles. Langmuir 2013, 29, 15275–15282. [Google Scholar] [CrossRef]
- Lee, T.-J.; Chau, L.-K.; Huang, C.-J. Controlled Silanization: High Molecular Regularity of Functional Thiol Groups on Siloxane Coatings. Langmuir 2020, 36, 5935–5943. [Google Scholar] [CrossRef]
- Rozlosnik, N.; Gerstenberg, M.C.; Larsen, N.B. Effect of Solvents and Concentration on the Formation of a Self-Assembled Monolayer of Octadecylsiloxane on Silicon (001). Langmuir 2003, 19, 1182–1188. [Google Scholar] [CrossRef]
- Zarinwall, A.; Asadian-Birjand, M.; Seleci, D.A.; Maurer, V.; Trautner, A.; Garnweitner, G.; Fuchs, H. Magnetic Nanoparticle-Based Dianthin Targeting for Controlled Drug Release Using the Endosomal Escape Enhancer SO1861. Nanomaterials 2021, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Zarinwall, A.; Maurer, V.; Pierick, J.; Oldhues, V.M.; Porsiel, J.C.; Finke, J.H.; Garnweitner, G. Amorphization and modified release of ibuprofen by post-synthetic and solvent-free loading into tailored silica aerogels. Drug Deliv. 2022, 29, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Bertini, F.; Scavia, G.; Bolognesi, A. A controlled approach to iron oxide nanoparticles functionalization for magnetic polymer brushes. J. Colloid Interface Sci. 2011, 360, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, K.C.; van der Voort, P.; Possemiers, K.; Vansant, E.F. Surface and Structural Properties of Silica Gel in the Modification with γ-Aminopropyltriethoxysilane. J. Colloid Interface Sci. 1995, 174, 86–91. [Google Scholar] [CrossRef]
- Čampelj, S.; Makovec, D.; Drofenik, M. Functionalization of magnetic nanoparticles with 3-aminopropyl silane. J. Magn. Magn. Mater. 2009, 321, 1346–1350. [Google Scholar] [CrossRef]
- Kaur, J.; Kotnala, R.K.; Verma, K.C. Surfactant free hydrothermal synthesis, electrical, optical and ferroelectric properties of BaTiO3 nanoparticles. J. Optoelectron. Adv. Mater. 2012, 14, 219–223. [Google Scholar]
- Singh, M.; Yadav, B.C.; Ranjan, A.; Kaur, M.; Gupta, S.K. Synthesis and characterization of perovskite barium titanate thin film and its application as LPG sensor. Sens. Actuators B Chem. 2017, 241, 1170–1178. [Google Scholar] [CrossRef]
- Ashiri, R. Detailed FT-IR spectroscopy characterization and thermal analysis of synthesis of barium titanate nanoscale particles through a newly developed process. Vib. Spectrosc. 2013, 66, 24–29. [Google Scholar] [CrossRef]
- Haroon, A.; Rai, P.; Uddin, I. Synthesis, Characterization and Dielectric Properties of BaTiO3 Nanoparticles. Int. J. Nanosci. 2020, 19, 1950001. [Google Scholar] [CrossRef]
- Bressy, C.; van Ngo, G.; Ziarelli, F.; Margaillan, A. New insights into the adsorption of 3-(trimethoxysilyl)propylmethacrylate on hydroxylated ZnO nanopowders. Langmuir 2012, 28, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Arantes, T.M.; Pinto, A.H.; Leite, E.R.; Longo, E.; Camargo, E.R. Synthesis and optimization of colloidal silica nanoparticles and their functionalization with methacrylic acid. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 209–217. [Google Scholar] [CrossRef]
- Nazir, T.; Afzal, A.; Siddiqi, H.M.; Saeed, S.; Dumon, M. The influence of temperature and interface strength on the microstructure and performance of sol–gel silica–epoxy nanocomposites. Polym. Bull. 2011, 67, 1539–1551. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Rivillon Amy, S.; Chabal, Y.J. Attachment of 3-(Aminopropyl)triethoxysilane on silicon oxide surfaces: Dependence on solution temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef]
- Etienne, M. Analytical investigation of the chemical reactivity and stability of aminopropyl-grafted silica in aqueous medium. Talanta 2003, 59, 1173–1188. [Google Scholar] [CrossRef]
- Vansant, E.F.; van der Voort, P.; Vrancken, K.C. Characterization and Chemical Modification of the Silica Surface; Elsevier: Amsterdam, The Netherlands, 1995; p. 556. [Google Scholar]
- Fadeev, A.Y.; Helmy, R.; Marcinko, S. Self-Assembled Monolayers of Organosilicon Hydrides Supported on Titanium, Zirconium, and Hafnium Dioxides. Langmuir 2002, 18, 7521–7529. [Google Scholar] [CrossRef]
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes: A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]
- Pantoja, M.; Velasco, F.; Broekema, D.; Abenojar, J.; Del Real, J.C. The Influence of pH on the Hydrolysis Process of γ-Methacryloxypropyltrimethoxysilane, Analyzed by FT-IR, and the Silanization of Electrogalvanized Steel. J. Adhes. Sci. Technol. 2010, 24, 1131–1143. [Google Scholar] [CrossRef]
- Serjeant, E.P.; Dempsey, B. Ionisation Constants of Organic Acids in Aqueous Solution; Pergamon Pr: Oxford, UK, 1979; p. 989. [Google Scholar]
- Riedel, E. Anorganische Chemie, 6th ed.; de Gruyter: Berlin, Germany, 2004; p. 935. [Google Scholar]
- Dang, Z.-M.; Yu, Y.-F.; Xu, H.-P.; Bai, J. Study on microstructure and dielectric property of the BaTiO3/epoxy resin composites. Compos. Sci. Technol. 2008, 68, 171–177. [Google Scholar] [CrossRef]
- Andrew, W. Ceramic Thick Films for MEMS and Microdevices; William Andrew: Oxford, UK, 2011. [Google Scholar]
- Ramajo, L.; Castro, M.S.; Reboredo, M.M. Effect of silane as coupling agent on the dielectric properties of BaTiO3-epoxy composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1852–1859. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Zhao, C.; Yang, W. High dielectric constant and low dielectric loss hybrid nanocomposites fabricated with ferroelectric polymer matrix and BaTiO3 nanofibers modified with perfluoroalkylsilane. Appl. Surf. Sci. 2014, 305, 531–538. [Google Scholar] [CrossRef]
- Bareiro Ferreira, O.; Sridaran Venkat, R.; Adam, J.; Boller, C. Development of the Fabrication Process and Characterization of Piezoelectric BaTiO3 /Epoxy Composite Used for Coated Ultrasonic Transducer Patterns in Structural Health Monitoring. In Proceedings of the 19th World Conference on Non-Destructive Testing, Munich, Germany, 13–17 June 2016. [Google Scholar]
- Robertson, J.; Varlow, B.R. Non-linear ferroelectric composite dielectric materials. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 779–790. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Ding, F.; Zhang, W. Effect of piezoelectric ceramic particles size gradation on piezoelectric properties of 0–3 cement-based piezoelectric composites. Smart Mater. Struct. 2018, 27, 85029. [Google Scholar] [CrossRef]
- Cho, S.-D.; Lee, J.-Y.; Paik, K.-W. Effects of particle size on dielectric constant and leakage current of epoxy/barium titanate (BaTiO3) composite films for embedded capacitors. In Advances in Electronic Materials and Packaging 2001 (Cat. No.01EX506); IEEE: New York, NY, USA, 2001; pp. 63–68. [Google Scholar]
- Tan, Q.; Cao, Y.; Irwin, P. DC Breakdown in Polyetherimide Composites and Implication for Structural Engineering. In Proceedings of the 2007 IEEE International Conference on Solid Dielectrics, Winchester, UK, 8–13 July 2007; IEEE: New York, NY, USA, 2007; pp. 411–414. [Google Scholar]
- Wang, W. Li, S. A transition of interface characteristics in LDPE/Al2O3 nanocomposites by permittivity simulation. In IEEE Transactions on Dielectrics and Electrical Insulation; IEEE: New York, NY, USA, 2018; Volume 25, pp. 2–12. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarinwall, A.; Mitkus, R.; Marth, A.; Maurer, V.; Sinapius, M.; Garnweitner, G. Processing of 3-(Trimethoxysilyl)propyl Methacrylate (TMSPM) Functionalized Barium Titanate/Photopolymer Composites: Functionalization and Process Parameter Investigation. J. Compos. Sci. 2023, 7, 47. https://doi.org/10.3390/jcs7020047
Zarinwall A, Mitkus R, Marth A, Maurer V, Sinapius M, Garnweitner G. Processing of 3-(Trimethoxysilyl)propyl Methacrylate (TMSPM) Functionalized Barium Titanate/Photopolymer Composites: Functionalization and Process Parameter Investigation. Journal of Composites Science. 2023; 7(2):47. https://doi.org/10.3390/jcs7020047
Chicago/Turabian StyleZarinwall, Ajmal, Rytis Mitkus, Axel Marth, Viktor Maurer, Michael Sinapius, and Georg Garnweitner. 2023. "Processing of 3-(Trimethoxysilyl)propyl Methacrylate (TMSPM) Functionalized Barium Titanate/Photopolymer Composites: Functionalization and Process Parameter Investigation" Journal of Composites Science 7, no. 2: 47. https://doi.org/10.3390/jcs7020047




