Design and Fabrication of Aerogel Composites for Oil Water Separation and Spilled Oil Cleaning
Abstract
:1. Introduction
2. Aerogel Composite Manufacturing Technologies
2.1. Hydrothermal Deposition on Porous Template
2.2. Hydrogen Bond Induced Self-Assembling
2.3. Gas Foaming
2.4. Vacuum-Assisted Resin Transfer Molding and Particle Leaching
2.5. In-situ Growth
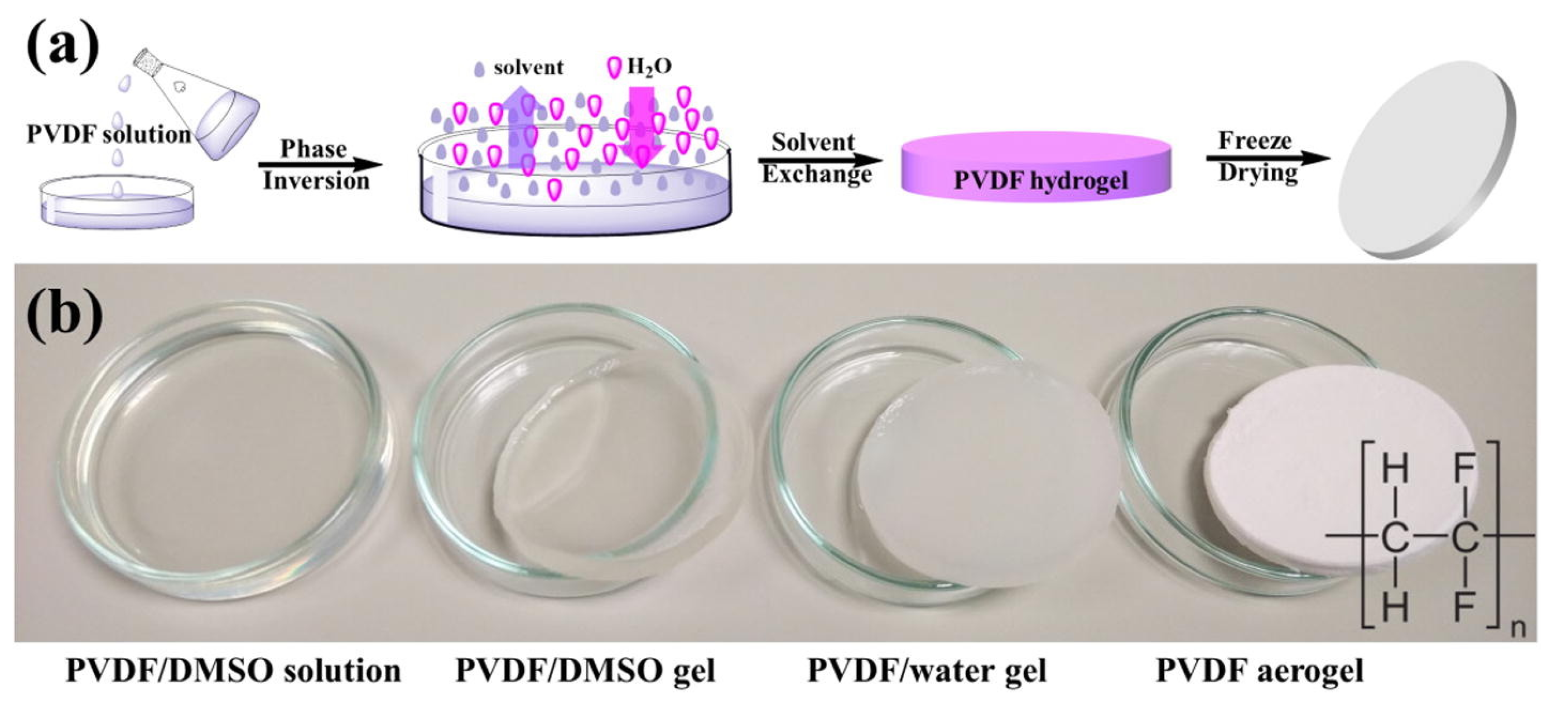
2.6. Vapor Induced Phase Inversion
3. Structure and Morphology Control of Aerogel Composites
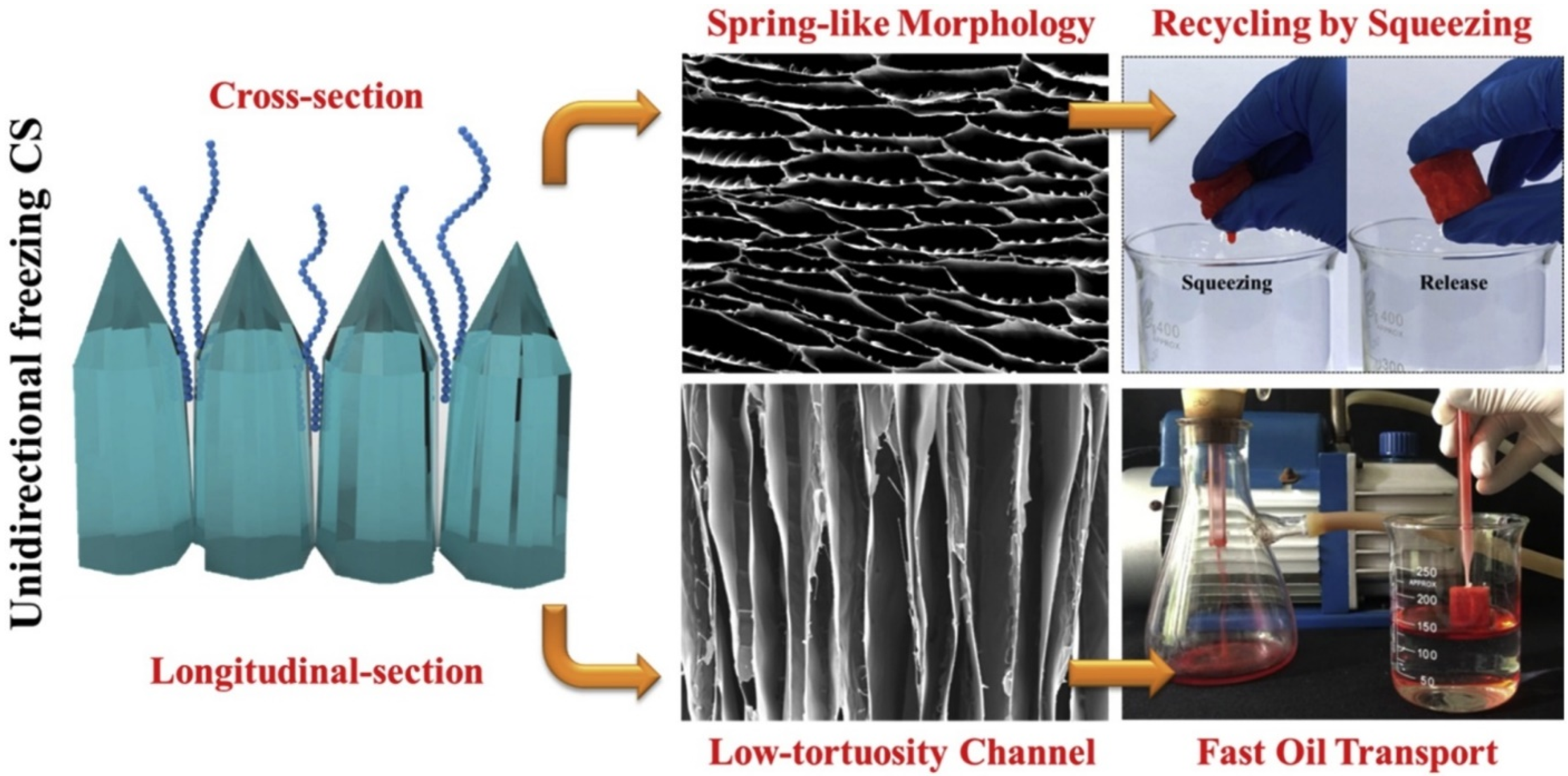
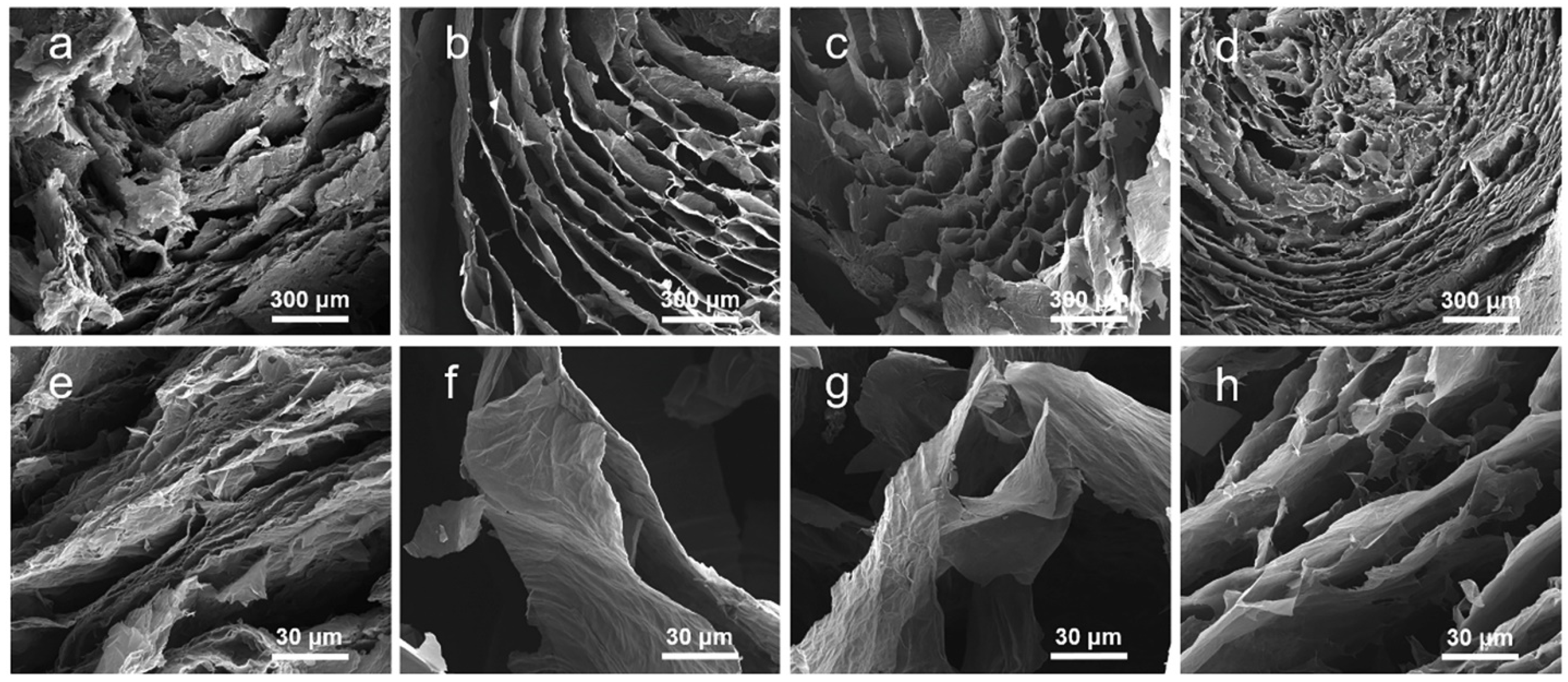
3.1. Directional Freeze Cast Oriented Structures
3.2. Stratified Structure
3.3. Fiber Network Structure
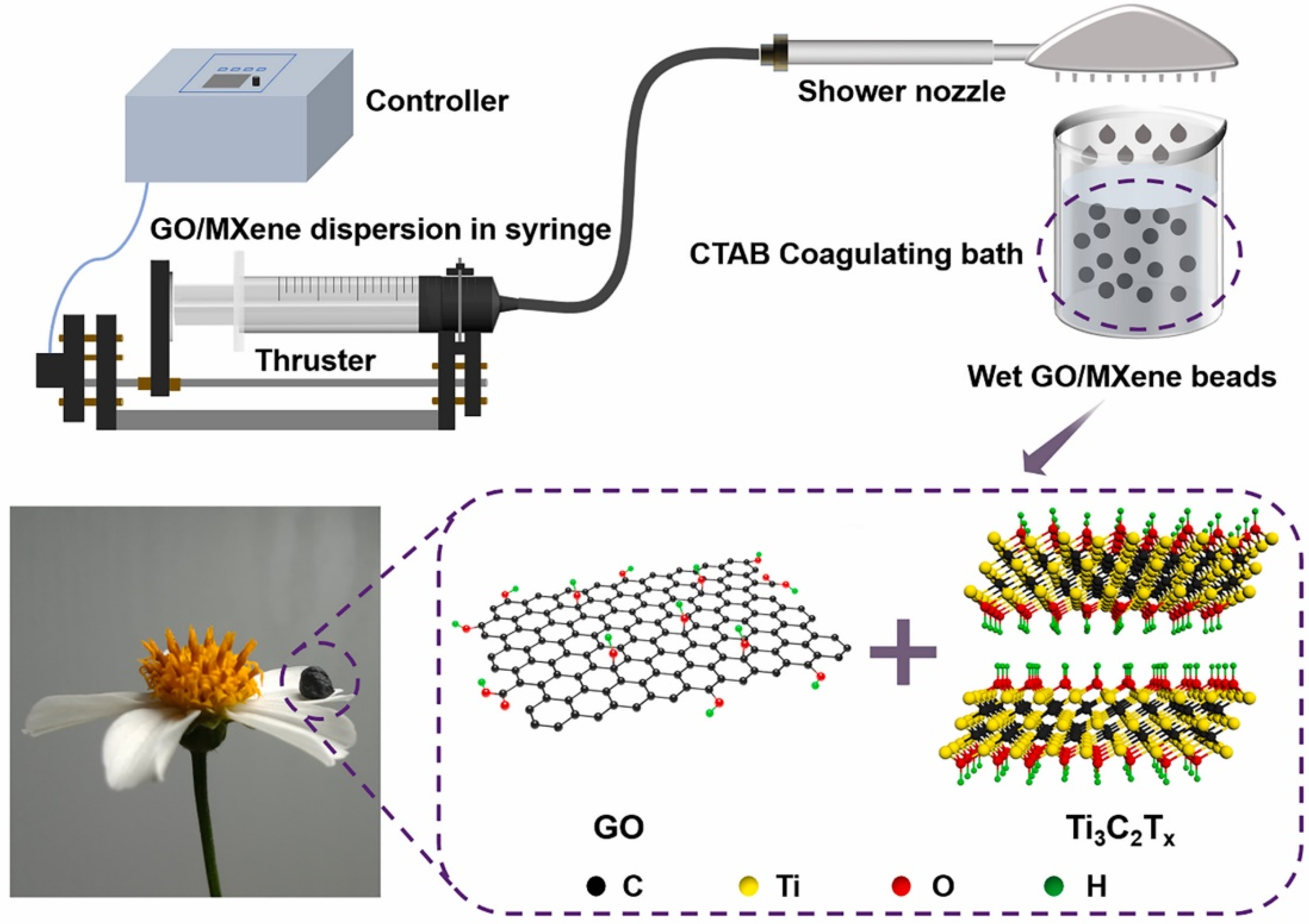
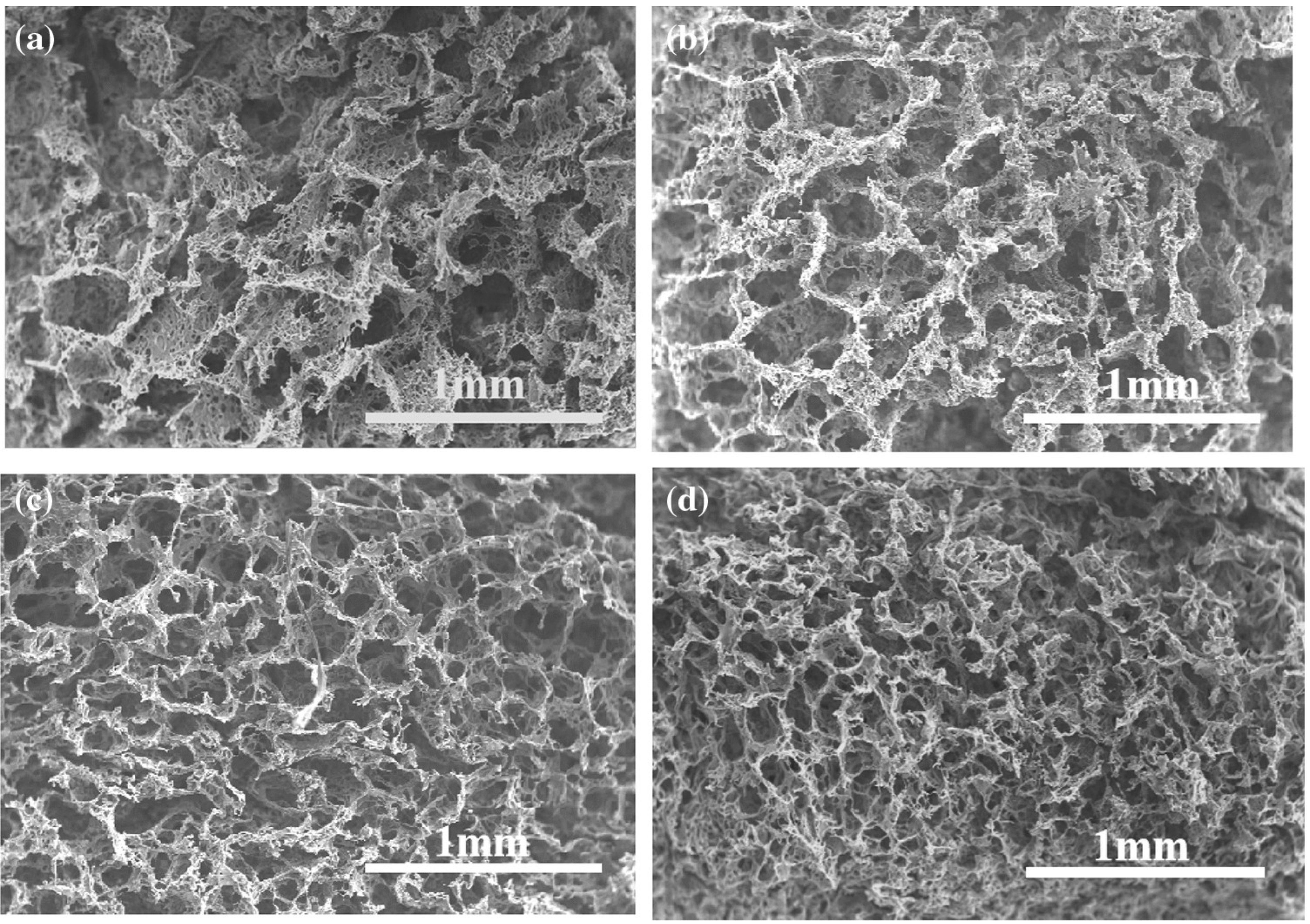
3.4. Beaded Structure
3.5. Biological Carbon Frame Structure
4. Oil/Water Separation Efficiency
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, W.J.; Jiang, Z.C.; Lu, T.; Xiong, R.H.; Huang, C.B. Lightweight, elastic and superhydrophobic multifunctional nanofibrous aerogel for self-cleaning, oil/water separation and pressure sensing. Chem. Eng. J. 2022, 430, 132989. [Google Scholar] [CrossRef]
- Karatum, O.; Bhuiya, M.M.H.; Carroll, M.K.; Anderson, A.M.; Plata, D.L. Life cycle assessment of aerogel manufacture on small and large scales: Weighing the use of advanced materials in oil spill remediation. J. Ind. Ecol. 2018, 22, 1365–1377. [Google Scholar] [CrossRef]
- Cui, Z.X.; Wu, J.H.; Chen, J.U.; Wang, X.F.; Si, J.H.; Wang, Q.T. Preparation of 3-D porous PVDF/TPU composite foam with superoleophilic/hydrophobicity for the efficient separation of oils and organics from water. J. Mater. Sci. 2021, 56, 12506–12523. [Google Scholar] [CrossRef]
- Liu, P.S.; Yu, H.H.; Hui, F.; Villena, M.A.; Li, X.H.; Lanza, M.; Zhang, Z.J. Fabrication of 3D silica with outstanding organic molecule separation and self-cleaning performance. Appl. Surf. Sci. 2020, 511, 145537. [Google Scholar] [CrossRef]
- Alghunaimi, F.I.; Alsaeed, D.J.; Harith, A.M.; Saleh, T.A. Synthesis of 9-octadecenoic acid grafted graphene modified with polystyrene for efficient light oil removal from water. J. Clean. Product. 2019, 233, 946–953. [Google Scholar] [CrossRef]
- Thakkar, S.V.; Pinna, A.; Carbonaro, C.M.; Malfatti, L.; Guardia, P.; Cabot, A.; Casula, M.F. Performance of oil sorbents based on reduced graphene oxide-silica composite aerogels. J. Environ. Chem. Eng. 2020, 8, 103632. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhou, Z.P.; Chang, Z.S.; Dai, X.H.; Li, C.; Dai, J.D. Superhydrophilic, underwater superoleophobic and self-cleaning nickel composite mesh via simultaneous acid etching and in-situ growth of Prussian blue analogue for oil-water separation. Colloids Surf. A Physiochem. Eng. Aspect. 2021, 627, 127140. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.H.; Altalhi, T.; Losic, D. Outstanding adsorption performance of graphene-carbon nanotube aerogels for continuous oil removal. Carbon 2014, 80, 523–533. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Khatri, O.P. h-BN and graphene-based ultralight hybrid aerogels: Highly efficient sorbent for recovery of hydrocarbon oils and organic solvents. J. Environ. Chem. Eng. 2021, 9, 106788. [Google Scholar] [CrossRef]
- Shi, Y.H.; Huang, J.H.; Zeng, G.M.; Cheng, W.J.; Hu, J.L.; Shi, L.X.; Yi, K.X. Evaluation of self-cleaning performance of the modified g-C3N4 and GO based PVDF membrane toward oil-in-water separation under visible-light. Chemosphere 2019, 230, 40–50. [Google Scholar] [CrossRef]
- Dai, J.G.; Tian, Q.F.; Sun, Q.Q.; Wei, W.K.; Zhuang, J.D.; Liu, M.Z.; Cao, Z.; Xie, W.Z.; Fan, M.Z. TiO2-alginate composite aerogels as novel oil/water separation and wastewater remediation filters. Compos. Part B Eng. 2019, 160, 480–487. [Google Scholar] [CrossRef]
- Zhuang, J.D.; Dai, J.G.; Ghaffar, S.H.; Yu, Y.Y.; Tian, Q.F.; Fan, M.Z. Development of highly efficient, renewable and durable alginate composite aerogels for oil/water separation. Surf. Coat. Technol. 2020, 388, 125551. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, J.D.; Ge, S.Z.; Jiang, C.W.; Guo, T.Y.; Peng, T.P.; Huang, T.; Xie, L. Biocompatible, hydrophobic and resilience graphene/chitosan composite aerogel for efficient oil-water separation. Surf. Coat. Technol. 2020, 385, 125361. [Google Scholar] [CrossRef]
- Duman, O.; Diker, C.O.; Ugurlu, H.; Tunc, S. Highly hydrophobic and superoleophilic agar/PVA aerogels for selective removal of oily substances from water. Carbohydr. Polym. 2022, 286, 119275. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Jiang, L.; Liu, J.; He, S.R.; Shao, W. Sustainable, highly efficient and superhydrophobic fluorinated silica functionalized chitosan aerogel for gravity-driven oil/water separation. Gels 2021, 7, 66. [Google Scholar] [CrossRef]
- Cao, M.; Li, S.L.; Cheng, J.B.; Zhang, A.N.; Wang, Y.Z.; Zhao, H.B. Fully bio-based, low fire-hazard and superelastic aerogel without hazardous cross-linkers for excellent thermal insulation and oil clean-up absorption. J. Hazard. Mater. 2021, 403, 123977. [Google Scholar] [CrossRef]
- Yi, L.F.; Yang, J.Y.; Fang, X.; Xia, Y.; Zhao, L.J.; Wu, H.; Guo, S.Y. Facile fabrication of wood-inspired aerogel from chitosan for efficient removal of oil from water. J. Hazard. Mater. 2020, 385, 121507. [Google Scholar] [CrossRef]
- Saharan, Y.; Singh, J.; Goyat, R.; Umar, A.; Algadi, H.; Ibrahim, A.A.; Kumar, R.; Baskoutas, S. Nanoporous and hydrophobic new chitosan-silica blend aerogels for enhanced oil adsorption capacity. J. Clean. Product. 2022, 351, 131247. [Google Scholar] [CrossRef]
- Pirzada, T.; Ashrafi, Z.; Xie, W.Y.; Khan, S.A. Cellulose silica hybrid nanofiber aerogels: From sol-gel electrospun nanofibers to multifunctional aerogels. Adv. Funct. Mater. 2020, 30, 1907359. [Google Scholar] [CrossRef]
- Paulauskiene, T.; Uebe, J.; Ziogas, M. Cellulose aerogel composites as oil sorbents and their regeneration. PeerJ 2021, 9, 11795. [Google Scholar] [CrossRef]
- Phat, L.N.; Thang, T.Q.; Nguyen, H.C.; Duyen, D.T.M.; Tien, D.X.; Khoa, B.D.D.; Khang, P.T.; Giang, N.T.H.; Nam, H.M.; Phong, M.T.; et al. Fabrication and modification of cellulose aerogels from Vietnamese water hyacinth for oil adsorption application. Korean J. Chem. Eng. 2021, 38, 2247–2255. [Google Scholar] [CrossRef]
- Shang, S.S.; Ye, X.; Jiang, X.; You, Q.; Zhong, Y.; Wu, X.D.; Cui, S. Preparation and characterization of cellulose/attapulgite composite aerogels with high strength and hydrophobicity. J. Non-Crystal. Solids 2021, 569, 120922. [Google Scholar] [CrossRef]
- Chhajed, M.; Yadav, C.; Agrawal, A.K.; Maji, P.K. Esterified superhydrophobic nanofibrillated cellulose based aerogel for oil spill treatment. Carbohydr. Polym. 2019, 226, 115286. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.M.; Wu, J.J.; Chen, S.Q.; Ji, X.X.; Wu, W.B. Superamphiphobic nanocellulose aerogels loaded with silica nanoparticles. Cellulose 2019, 26, 9661–9671. [Google Scholar] [CrossRef]
- Zhou, S.K.; Liu, P.P.; Wang, M.; Zhao, H.; Yang, J.; Xu, F. Sustainable, reusable, and superhydrophobic aerogels from microfibrillated cellulose for highly effective oil/water separation. ACS Sustain. Chem. Eng. 2016, 4, 6409–6416. [Google Scholar] [CrossRef]
- Yang, W.J.; Yuen, A.C.Y.; Li, A.; Lin, B.; Chen, T.B.Y.; Yang, W.; Lu, H.D.; Yeoh, G.H. Recent progress in bio-based aerogel absorbents for oil/water separation. Cellulose 2019, 26, 6449–6476. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, D.S.; Guo, Q.; Qiu, F.X.; Yang, D.Y.; Ou, Z.P. Preparation of a renewable biomass carbon aerogel reinforced with sisal for oil spillage clean-up: Inspired by green leaves to green Tofu. Food Bioprod. Process. 2019, 114, 154–162. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, T.L.; Yu, S.S.; Cheng, Y.; Lu, J.; Wang, H.S. A lignin-based carbon aerogel enhanced by graphene oxide and application in oil/water separation. Fuel 2020, 278, 118376. [Google Scholar] [CrossRef]
- Xu, B.; Ding, J.E.; Feng, L.; Ding, Y.Y.; Ge, Y.F.; Cai, Z.S. Self-cleaning cotton fabrics via combination of photocatalytic TiO2 and superhydrophobic SiO2. Surf. Coat. Technol. 2015, 262, 70–76. [Google Scholar] [CrossRef]
- Cao, C.Y.; Ge, M.Z.; Huang, J.Y.; Li, S.H.; Deng, S.; Zhang, S.N.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y.K. Robust fluorine-free superhydrophobic PDMS-ormosil@fabrics for highly effective self-cleaning and efficient oil-water separation. J. Mater. Chem. A 2016, 4, 12179–12187. [Google Scholar] [CrossRef]
- Dwivedi, P.; Vijayakumar, R.P.; Chaudhary, A.K. Synthesis of UMCNO-cotton fabric and its application in wastewater treatment. Cellulose 2019, 27, 969–980. [Google Scholar] [CrossRef]
- Rong, J.; Qiu, F.X.; Zhang, T.; Zhang, X.Y.; Zhu, Y.; Xu, J.C.; Yang, D.Y.; Dai, Y.T. A facile strategy toward 3D hydrophobic composite resin network decorated with biological ellipsoidal structure rapeseed flower carbon for enhanced oils and organic solvents selective absorption. Chem. Eng. J. 2017, 322, 397–407. [Google Scholar] [CrossRef]
- Nong, Y.L.; Ren, Y.W.; Wang, P.; Zhou, M.; Yu, Y.Y.; Yuan, J.G.; Xu, B.; Wang, Q. A facile strategy for the preparation of photothermal silk fibroin aerogels with antibacterial and oil-water separation abilities. J. Colloid Interface Sci. 2012, 603, 518–529. [Google Scholar] [CrossRef]
- Shome, A.; Moses, J.C.; Rather, A.M.; Mandal, B.B.; Manna, U. Unconventional and facile fabrication of chemically reactive silk fibroin sponges for environmental remediation. ACS Appl. Mater. Interfaces 2021, 13, 24258–24271. [Google Scholar] [CrossRef]
- Thai, Q.B.; Le-Cao, K.; Nguyen, P.T.T.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Fabrication and optimization of multifunctional nanoporous aerogels using recycled textile fibers from car tire wastes for oil-spill cleaning, heat-insulating and sound absorbing applications. Colloids Surf. A-Physiochem. Eng. Aspect. 2021, 628, 127363. [Google Scholar] [CrossRef]
- He, N.N.; Li, L.L.; Chen, J.Q.; Zhang, J.H.; Liang, C. Extraordinary superhydrophobic polycaprolactone-based composite membrane with an alternated micro-nano hierarchical structure as an eco-friendly oil/water separator. ACS Appl. Mater. Interfaces 2021, 13, 24117–24129. [Google Scholar] [CrossRef]
- Li, L.X.; Hu, T.; Li, A.; Zhang, J.P. Electrically conductive carbon aerogels with high salt-resistance for efficient solar-driven interfacial evaporation. ACS Appl. Mater. Interfaces 2020, 12, 32143–32153. [Google Scholar] [CrossRef]
- Wang, B.; Peng, S.; Wang, Y.C.; Li, X.X.; Zhang, K.M.; Liu, C.Y. A non- fluorine method for preparing multifunctional robust superhydrophobic coating with applications in photocatalysis, flame retardance, and oil- water separation. New J. Chem. 2019, 43, 7471–7481. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M.H. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.Q.; Sun, M.N.; Liu, C.A.; Fu, L.B.; Lv, Y.C.; Feng, Y.Z.; Huang, P.; Yang, F.Q.; Song, P.A.; Liu, M.H. Lightweight, amphipathic and fire-resistant prGO/MXene spherical beads for rapid elimination of hazardous chemicals. J. Hazard. Mater. 2022, 423, 127069. [Google Scholar] [CrossRef]
- Jin, X.; Al-Qatatsheh, A.; Subhani, K.; Salim, N.V. Biomimetic and flexible 3D carbon nanofiber networks with fire-resistant and high oil-sorption capabilities. Chem. Eng. J. 2021, 412, 128635. [Google Scholar] [CrossRef]
- Zeng, S.; Cui, Z.X.; Yang, Z.Q.; Si, J.H.; Wang, Q.T.; Wang, X.F.; Peng, K.P.; Chen, W.Z. Characterization of highly interconnected porous poly(lactic acid) and chitosan-coated poly(lactic acid) scaffold fabricated by vacuum-assisted resin transfer molding and particle leaching. J. Mater. Sci. 2016, 51, 9958–9970. [Google Scholar] [CrossRef]
- Yuan, D.S.; Zhang, T.; Guo, Q.; Qiu, F.X.; Yang, D.Y.; Ou, Z.P. A novel hierarchical hollow SiO2@MnO2 cubes reinforced elastic polyurethane foam for the highly efficient removal of oil from water. Chem. Eng. J. 2017, 327, 539–547. [Google Scholar] [CrossRef]
- Yuan, D.S.; Zhang, T.; Guo, Q.; Qiu, F.X.; Yang, D.Y.; Ou, Z.P. Recyclable biomass carbon@SiO2@MnO2 aerogel with hierarchical structures for fast and selective oil-water separation. Chem. Eng. J. 2018, 351, 622–630. [Google Scholar] [CrossRef]
- Chen, X.L.; Liang, Y.N.; Tang, X.Z.; Shen, W.M.; Hu, X. Additive-free poly (vinylidene fluoride) aerogel for oil/water separation and rapid oil absorption. Chem. Eng. J. 2017, 308, 18–26. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Wetting behavior of water and oil droplets in three-phase interfaces for hydrophobicity/philicity and oleophobicity/philicity. Langmuir 2009, 25, 14165–14173. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Xue, Q.Z.; Pan, X.L.; Jin, Y.K.; Lu, W.B.; Ding, D.G.; Guo, Q.K. Graphene oxide/polyacrylonitrile fiber hierarchical-structured membrane for ultra-fast microfiltration of oil-water emulsion. Chem. Eng. J. 2017, 307, 643–649. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Hui, L.; Liu, P.Z.; Liu, X.L.; Gan, S.P.; Xiao, C.; Lei, Z.; Wei, B.J.; Xue, Q.Z. Recyclable superhydrophilic meshes with scalable and robust coating for separating oily wastewater. Appl. Surf. Sci. 2022, 602, 154396. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.Q.; Zhu, L.; Liu, H.L.; Yu, S.F.; Xue, J.W.; Zhu, X.; Xue, Q.Z. Reusable membrane with multifunctional skin layer for effective removal of insoluble emulsified oils and soluble dyes. J. Hazard. Mater. 2021, 415, 125677. [Google Scholar] [CrossRef]








| Nanofiber | PAN | PP (PANi Coated PAN) | PPPP (PANi, PAA, and PEI on PAN) |
|---|---|---|---|
| FRR(%) | 7.9 | 56.2 | 95.9 |
| J1(L/m2/h/bar) | 953 | 2186 | 3071 |
| J2(L/m2/h/bar) | 665 | 1027 | 1827 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.X. Design and Fabrication of Aerogel Composites for Oil Water Separation and Spilled Oil Cleaning. J. Compos. Sci. 2023, 7, 95. https://doi.org/10.3390/jcs7030095
Gan YX. Design and Fabrication of Aerogel Composites for Oil Water Separation and Spilled Oil Cleaning. Journal of Composites Science. 2023; 7(3):95. https://doi.org/10.3390/jcs7030095
Chicago/Turabian StyleGan, Yong X. 2023. "Design and Fabrication of Aerogel Composites for Oil Water Separation and Spilled Oil Cleaning" Journal of Composites Science 7, no. 3: 95. https://doi.org/10.3390/jcs7030095






