Synthesis of Ni-Cu-CNF Composite Materials via Carbon Erosion of Ni-Cu Bulk Alloys Prepared by Mechanochemical Alloying
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ni-Cu via Mechanochemical Alloying
2.3. Studies on the CE Process
2.4. Synthesis of the Ni-Cu-CNF Composites
2.5. Characterization of the Ni-Cu Precursors and Ni-Cu-CNF Composites
3. Results and Discussion
3.1. Preparation of the Ni-Cu Precursors and Ni-Cu-CNF Composites
3.2. Characterization of the Ni-Cu-CNF Composites
- The diameter and morphology of the growing fibers can be changed over time due to the changeable dispersion of metal particles (primary and secondary disintegration).
- The surface of the already-formed CNF material can undergo restructuring due to its interaction with the gaseous hydrogen, which leads to the formation of CH4 and changes in the surface structure.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, S.; Kumaravel, S.; Jha, S.; Yen, M.; Kundu, S.; Liang, H. Impacts of Structure-Directing Agents on the Synthesis of Cu3Mo2O9 for Flexible Lignin-Based Supercapacitor Electrodes. J. Compos. Sci. 2023, 7, 155. [Google Scholar] [CrossRef]
- Morales, J.; Michell, R.M.; Sommer-Márquez, A.; Rodrigue, D. Effect of Biobased SiO2 on the Morphological, Thermal, Mechanical, Rheological, and Permeability Properties of PLLA/PEG/SiO2 Biocomposites. J. Compos. Sci. 2023, 7, 150. [Google Scholar] [CrossRef]
- Nachtane, M.; Tarfaoui, M.; Abichou, M.A.; Vetcher, A.; Rouway, M.; Aâmir, A.; Mouadili, H.; Laaouidi, H.; Naanani, H. An Overview of the Recent Advances in Composite Materials and Artificial Intelligence for Hydrogen Storage Vessels Design. J. Compos. Sci. 2023, 7, 119. [Google Scholar] [CrossRef]
- Cilento, F.; Bassano, A.; Sorrentino, L.; Martone, A.; Giordano, M.; Palmieri, B. PVB Nanocomposites as Energy Directors in Ultrasonic Welding of Epoxy Composites. J. Compos. Sci. 2023, 7, 160. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; Gao, X.; Zhao, D.; Wei, G.; Li, Z. Enhanced surface capacitive sodium storage by pores regulation in carbon/carbon composite nanofibers. Micropor. Mesopor. Mat. 2022, 332, 111706. [Google Scholar] [CrossRef]
- Song, M.; Tang, X.; Xu, J.; Yu, L.; Wei, Y. The formation of novel carbon/carbon composite by chemical vapor deposition: An efficient adsorbent for enhanced desulfurization performance. J. Analyt. Appl. Pyrol. 2016, 118, 34–41. [Google Scholar] [CrossRef]
- Rahaman, A.; Kar, K.K. Carbon nanomaterials grown on E-glass fibers and their application in composite. Compos. Sci. Technol. 2014, 101, 1–10. [Google Scholar] [CrossRef]
- Porto, L.S.; Silva, D.N.; de Oliveira, A.E.F.; Pereira, A.C.; Borges, K.B. Carbon nanomaterials: Synthesis and applications to development of electrochemical sensors in determination of drugs and compounds of clinical interest. Rev. Analyt. Chem. 2020, 38, 20190017. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Ngah Demon, S.Z.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Sudhakar, A.J.; Muthusubramanian, B. Development of Basalt Fiber Reinforced Fine-Grained Cementitious Composites for Textile Reinforcements. J. Compos. Sci. 2022, 6, 396. [Google Scholar] [CrossRef]
- Engül, M.; Ersoy, N. From Flat Plates to Sinusoidal Structures: Influence of Geometry on the Energy Absorption Capability of Carbon/Epoxy Composites. J. Compos. Sci. 2023, 7, 56. [Google Scholar] [CrossRef]
- Birgin, H.B.; D’Alessandro, A.; Meoni, A.; Ubertini, F. Self-Sensing Eco-Earth Composite with Carbon Microfibers for Sustainable Smart Buildings. J. Compos. Sci. 2023, 7, 63. [Google Scholar] [CrossRef]
- Guo, X.; Liu, G. Electromagnetic Shielding Enhancement of Butyl Rubber/Single-Walled Carbon Nanotube Composites via Water-Induced Modification. Polymers 2023, 15, 2101. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, Z.; Liu, J.; Jin, H.; Han, C.; Wang, X. Hierarchical Carbon Network Composites Derived from ZIF-8 for High-Efficiency Microwave Absorption. Materials 2023, 16, 3380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, T.; Zhang, Y.; Liu, H. Removal of Fe Impurity Ions from a Spent Vanadium Electrolyte Using Capacitive Deionization Based on Resin/Activated Carbon Composite Electrodes. Batteries 2023, 9, 240. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, G.; Chen, H.; Yue, H. Conductive Carbon-Wrapped Fluorinated Hard Carbon Composite as High-Performance Cathode for Primary Lithium Batteries. Coatings 2023, 13, 812. [Google Scholar] [CrossRef]
- Meng, T.; Shi, H.; Ao, F.; Wang, P.; Wang, L.; Wang, L.; Zhu, Y.; Lu, Y.; Zhao, Y. Study on Nitrogen-Doped Biomass Carbon-Based Composite Cobalt Selenide Heterojunction and Its Electrocatalytic Performance. Metals 2023, 13, 767. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress on carbon-based composite materials for microwave electromagnetic interference shielding. Carbon 2021, 177, 304–331. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsys. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Korneev, D.V.; Krasnikova, I.V.; Afonnikova, S.D.; Vedyagin, A.A.; Mishakov, I.V. Precise Characterization of CNF-Coated Microfibers Using Transmission Electron Microscopy. Coatings 2023, 13, 256. [Google Scholar] [CrossRef]
- Wang, X.; Dong, A.; Hu, Y.; Qian, J.; Huang, S. A review of recent work on using metal–organic frameworks to grow carbon nanotubes. Chem. Commun. 2020, 56, 10809–10823. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Yan, Y. Synthesis of CNTs on stainless steel microfibrous composite by CVD: Effect of synthesis condition on carbon nanotube growth and structure. Compos. Part B-Eng. 2019, 160, 369–383. [Google Scholar] [CrossRef]
- Hoyos-Palacio, L.M.; Cuesta Castro, D.P.; Ortiz-Trujillo, I.C.; Botero Palacio, L.E.; Galeano Upegui, B.J.; Escobar Mora, N.J.; Carlos Cornelio, J.A. Compounds of carbon nanotubes decorated with silver nanoparticles via in-situ by chemical vapor deposition (CVD). J. Mater. Res. Technol. 2019, 8, 5893–5898. [Google Scholar] [CrossRef]
- Jang, E.; Park, H.-K.; Choi, J.-H.; Lee, C.-S. Synthesis and Characterization of Carbon Nanofibers Grown on Ni and Mo Catalysts by Chemical Vapor Deposition. Bull. Korean Chem. Soc. 2015, 36, 1452–1459. [Google Scholar] [CrossRef]
- Shimamoto, D.; Muramatsu, H.; Fujisawa, K.; Hayashi, T.; Kim, Y.A.; Endo, M. Synthesis of catalytic chemical vapor grown carbon fibers: Carbon nanotube and carbon nanofiber. Carbon 2011, 49, 738. [Google Scholar] [CrossRef]
- Simon, A.; Seyring, M.; Kämnitz, S.; Richter, H.; Voigt, I.; Rettenmayr, M.; Ritter, U. Carbon nanotubes and carbon nanofibers fabricated on tubular porous Al2O3 substrates. Carbon 2015, 90, 25–33. [Google Scholar] [CrossRef]
- Shaikjee, A.; Coville, N.J. The role of the hydrocarbon source on the growth of carbon materials. Carbon 2012, 50, 3376–3398. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Z.H.; Lu, G.Q.; Yan, Z.F.; De Marco, R. Synthesis and characterization of turbostratic carbons prepared by catalytic chemical vapour decomposition of acetylene. Appl. Catal. A Gen. 2006, 309, 201–209. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, W.; Luo, S.; Ling, C.; Xiao, J.; Zhou, A.; Sun, Y.; Liao, K. 3D Ni-Co selenide nanorod array grown on carbon fiber paper: Towards high-performance flexible supercapacitor electrode with new energy storage mechanism. Electrochim. Acta 2017, 241, 41–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Z.; Wang, X.; Hu, H.; Wu, M. MXene/carbon composites for electrochemical energy storage and conversion. Mater. Today Sustain. 2023, 22, 100350. [Google Scholar] [CrossRef]
- Tokumitsu, N.; Shimamura, Y.; Fujii, T.; Inoue, Y. Effect of Annealing and Diameter on Tensile Property of Spinnable Carbon Nanotube and Unidirectional Carbon Nanotube Reinforced Epoxy Composite. J. Compos. Sci. 2022, 6, 389. [Google Scholar] [CrossRef]
- Gimenes Benega, M.A.; Silva, W.M.; Schnitzler, M.C.; Espanhol Andrade, R.J.; Ribeiro, H. Improvements in thermal and mechanical properties of composites based on epoxy-carbon nanomaterials—A brief landscape. Polymer. Test. 2021, 98, 107180. [Google Scholar] [CrossRef]
- Li, Q.; Guo, J.; Xu, D.; Guo, J.; Ou, X.; Hu, Y.; Qi, H.; Yan, F. Electrospun N-Doped Porous Carbon Nanofibers Incorporated with NiO Nanoparticles as Free-Standing Film Electrodes for High-Performance Supercapacitors and CO2 Capture. Small 2018, 14, 1704203. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, X.; Tan, S.; Wu, W.; Shi, J.; Zhou, H.; Chen, P. Preparation and characterisation of CNF/MWCNT carbon aerogel as efficient adsorbents. IET Nanobiotechnol. 2018, 12, 500–504. [Google Scholar] [CrossRef]
- Docekal, J. Hydrogen production from hydrocarbons. Int. J. Hydrog. Energy 1986, 11, 709–714. [Google Scholar] [CrossRef]
- Onsan, I. Catalytic Processes for Clean Hydrogen Production from Hydrocarbons. Turk. J. Chem. 2007, 31, 531–550. [Google Scholar]
- Murata, K.; Inaba, M.; Miki, M.; Yamaguchi, T. Formation of filamentous carbon and hydrogen by methane decomposition over Al2O3-supported Ni catalysts. Reac. Kinet. Catal. Lett. 2005, 85, 21–28. [Google Scholar] [CrossRef]
- Serrano, D.P.; Botas, J.A.; Fierro, J.L.G.; Guil-López, R.; Pizarro, P.; Gómez, G. Hydrogen production by methane decomposition: Origin of the catalytic activity of carbon materials. Fuel 2010, 89, 1241–1248. [Google Scholar] [CrossRef]
- Takenaka, S.; Shigeta, Y.; Tanabe, E.; Otsuka, K. Methane Decomposition into Hydrogen and Carbon Nanofibers over Supported Pd−Ni Catalysts: Characterization of the Catalysts during the Reaction. J. Phys. Chem. B 2004, 108, 7656–7664. [Google Scholar] [CrossRef]
- Tezel, E.; Figen, H.E.; Baykara, S.Z. Hydrogen production by methane decomposition using bimetallic Ni–Fe catalysts. Int. J. Hydrog. Energy 2019, 44, 9930–9940. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Al-Fatesh, A.S.; Abasaeed, A.E.; Khan, W.U. Methane decomposition over iron catalyst for hydrogen production. Int. J. Hydrog. Energy 2015, 40, 7593–7600. [Google Scholar] [CrossRef]
- Cazaña, F.; Afailal, Z.; González-Martín, M.; Sánchez, J.L.; Latorre, N.; Romeo, E.; Arauzo, J.; Monzón, A. Hydrogen and CNT Production by Methane Cracking Using Ni–Cu and Co–Cu Catalysts Supported on Argan-Derived Carbon. ChemEngineering 2022, 6, 47. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, H.; Huang, M.; Wan, C.; Li, D.; Jiang, L. Controlled preparation of Ni–Cu alloy catalyst via hydrotalcite-like precursor and its enhanced catalytic performance for methane decomposition. Fuel Proce. Technol. 2022, 233, 107271. [Google Scholar] [CrossRef]
- Sivamaran, V.; Balasubramanian, V.; Gopalakrishnan, M.; Viswabaskaran, V.; Rao, A.G. Identification of appropriate catalyst system for the growth of multi-walled carbon nanotubes via catalytic chemical vapor deposition process in a single step batch technique. Mater. Res. Express 2019, 6, 105620. [Google Scholar] [CrossRef]
- Schoemaker, S.E.; Welling, T.A.J.; Wezendonk, D.F.L.; Reesink, B.H.; van Bavel, A.P.; de Jongh, P.E. Carbon nanofiber growth from methane over carbon-supported NiCu catalysts: Two temperature regimes. Catal. Today 2023, 418, 114110. [Google Scholar] [CrossRef]
- Zhao, T.; Kvande, I.; Yu, Y.; Ronning, M.; Holmen, A.; Chen, D. Synthesis of Platelet Carbon Nanofiber/Carbon Felt Composite on in Situ Generated Ni−Cu Nanoparticles. J. Phys. Chem. C 2010, 115, 1123–1133. [Google Scholar] [CrossRef]
- Nam, K.-M.; Park, H.-K.; Lee, C.-S. Synthesis and Electrochemical Properties of Carbon Nanofibers and SiO2/Carbon Nanofiber Composite on Ni–Cu/C-Fiber Textiles. J. Nanosci. Nanotechnol. 2015, 15, 8989–8995. [Google Scholar] [CrossRef] [PubMed]
- Shelepova, E.V.; Maksimova, T.A.; Bauman, Y.I.; Mishakov, I.V.; Vedyagin, A.A. Experimental and Simulation Study on Coproduction of Hydrogen and Carbon Nanomaterials by Catalytic Decomposition of Methane-Hydrogen Mixtures. Hydrogen 2022, 3, 450–462. [Google Scholar] [CrossRef]
- Shen, Y.; Ge, M.; Lua, A.C. Deactivation of bimetallic nickel–copper alloy catalysts in thermocatalytic decomposition of methane. Catal. Sci. Technol. 2018, 8, 3853–3862. [Google Scholar] [CrossRef]
- Xu, M.; Lopez-Ruiz, J.A.; Riedel, N.W.; Weber, R.S.; Bowden, M.E.; Kovarik, L.; Jiang, C.; Hu, J.; Dagle, R.A. Promotional role of NiCu alloy in catalytic performance and carbon properties for CO2-free H2 production from thermocatalytic decomposition of methane. Catal. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Schlereth, C.; Hack, K.; Galetz, M.C. Parameters to estimate the metal dusting attack in different gases. Corros. Sci. 2022, 206, 110483. [Google Scholar] [CrossRef]
- Guo, X.; Vanhaecke, E.; Vullum, P.E.; Ma, J.; Gunawardana, P.V.D.S.; Walmsley, J.C.; Chen, D.; Venvik, H.J. Effects of metal dusting relevant exposures of alloy 601 surfaces on carbon formation and oxide development. Catal. Today 2021, 369, 48–61. [Google Scholar] [CrossRef]
- Bentria, E.T.; Akande, S.O.; Ramesh, A.; Laycock, N.; Hamer, W.; Normand, M.; Becquart, C.; Bouhali, O.; El-Mellouhi, F. Insights on the effect of water content in carburizing gas mixtures on the metal dusting corrosion of iron. Appl. Surf. Sci. 2022, 579, 152138. [Google Scholar] [CrossRef]
- Afonnikova, S.D.; Popov, A.A.; Bauman, Y.I.; Plyusnin, P.E.; Mishakov, I.V.; Trenikhin, M.V.; Shubin, Y.V.; Vedyagin, A.A.; Korenev, S.V. Porous Co-Pt Nanoalloys for Production of Carbon Nanofibers and Composites. Materials 2022, 15, 7456. [Google Scholar] [CrossRef]
- Popov, A.A.; Afonnikova, S.D.; Varygin, A.D.; Bauman, Y.I.; Trenikhin, M.V.; Plyusnin, P.E.; Shubin, Y.V.; Vedyagin, A.A.; Mishakov, I.V. Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER. Catalysts 2023, 13, 599. [Google Scholar] [CrossRef]
- Chun, C.; Bhargava, G.; Ramanarayanan, T. Metal Dusting Corrosion of Nickel-Based Alloys. ECS Trans. 2007, 3, 43–57. [Google Scholar] [CrossRef]
- Grabke, H.J.; Krajak, R.; Müller-Lorenz, E.M.; Strauß, S. Metal dusting of nickel-base alloys. Mater. Corros. 1996, 47, 495–504. [Google Scholar] [CrossRef]
- Jarrah, N.A.; Li, F.; van Ommen, J.G.; Lefferts, L. Immobilization of a layer of carbon nanofibres (CNFs) on Ni foam: A new structured catalyst support. J. Mater. Chem. 2005, 15, 1946. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Korneev, D.V.; Bauman, Y.I.; Vedyagin, A.A.; Nalivaiko, A.Y.; Shubin, Y.V.; Gromov, A.A. Interaction of chlorinated hydrocarbons with nichrome alloy: From surface transformations to complete dusting. Surf. Interf. 2022, 30, 101914. [Google Scholar] [CrossRef]
- Chang, J.-K.; Tsai, H.-Y.; Tsai, W.-T. Different Types of Nanosized Carbon Materials Produced by a Metal Dusting Process. J. Phys. Chem. C 2008, 112, 20143–20148. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chang, J.-K.; Tsai, W.-T. A feasibility study of preparing carbon nanotubes by using a metal dusting process. Diamond Rel. Mater. 2009, 18, 324–327. [Google Scholar] [CrossRef]
- Buyanov, R.A.; Chesnokov, V.V. On the Processes that Occur in the Metal Particles with Their Use in Catalytic Decomposition of Hydrocarbons through the Carbide Cycle Mechanism. Chem. Sustain. Develop. 2005, 13, 37–40. [Google Scholar]
- Mishakov, I.V.; Buyanov, R.A.; Zaikovskii, V.I.; Strel’tsov, I.A.; Vedyagin, A.A. Catalytic synthesis of nanosized feathery carbon structures via the carbide cycle mechanism. Kinet. Catal. 2008, 49, 868–872. [Google Scholar] [CrossRef]
- Rudneva, Y.V.; Shubin, Y.V.; Plyusnin, P.E.; Bauman, Y.I.; Mishakov, I.V.; Korenev, S.V.; Vedyagin, A.A. Preparation of highly dispersed Ni1-xPdx alloys for the decomposition of chlorinated hydrocarbons. J. Alloys Compnd. 2019, 782, 716–722. [Google Scholar] [CrossRef]
- Popov, A.A.; Shubin, Y.V.; Bauman, Y.I.; Plyusnin, P.E.; Mishakov, I.V.; Sharafutdinov, M.R.; Maksimovskiy, E.A.; Korenev, S.V.; Vedyagin, A.A. Preparation of porous Co-Pt alloys for catalytic synthesis of carbon nanofibers. Nanotechnology 2020, 31, 495604. [Google Scholar] [CrossRef]
- McCormick, P.G.; Froes, F.H. The fundamentals of mechanochemical processing. JOM 1998, 50, 61–65. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Boldyreva, E.V.; Belenguer, A.M.; Emmerling, F.; Boldyrev, V.V. Tribochemistry, Mechanical Alloying, Mechanochemistry: What is in a Name? Front. Chem. 2021, 9, 685789. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Afonnikova, S.D.; Bauman, Y.I.; Shubin, Y.V.; Trenikhin, M.V.; Serkova, A.N.; Vedyagin, A.A. Carbon Erosion of a Bulk Nickel–Copper Alloy as an Effective Tool to Synthesize Carbon Nanofibers from Hydrocarbons. Kinet. Catal. 2022, 63, 97–107. [Google Scholar] [CrossRef]
- Afonnikova, S.D.; Mishakov, I.V.; Bauman, Y.I.; Trenikhin, M.V.; Shubin, Y.V.; Serkova, A.N.; Vedyagin, A.A. Preparation of Ni–Cu Catalyst for Carbon Nanofiber Production by the Mechanochemical Route. Top. Catal. 2022, 66, 393–404. [Google Scholar] [CrossRef]
- Karimi, S.; Bibak, F.; Meshkani, F.; Rastegarpanah, A.; Deng, J.; Liu, Y.; Dai, H. Promotional roles of second metals in catalyzing methane decomposition over the Ni-based catalysts for hydrogen production: A critical review. Int. J. Hydrog. Energy 2021, 46, 20435–20480. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- ICDD. The Powder Diffraction File 2 (PDF2) 2009 Release; International Centre for Diffraction Data: Newtown Square, PA, USA, 2009. [Google Scholar]
- Kraus, W.; Nolze, G. PowderCell—A program to visualize crystal structures, calculate the corresponding powder patterns and refine experimental curves. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Robertson, J.H. Elements of X-ray diffractionby B. D. Cullity. Acta Crystallogr. Section A 1979, 35, 350. [Google Scholar] [CrossRef]
- Krumm, S. An Interactive Windows Program for Profile Fitting and Size/Strain Analysis. Mater. Sci. Forum 1996, 228–231, 183–190. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, & Porosity, 2nd ed.; Academic Press Inc.: London, UK, 1982. [Google Scholar]
- Ashok, J.; Reddy, P.S.; Raju, G.; Subrahmanyam, M.; Venugopal, A. Catalytic Decomposition of Methane to Hydrogen and Carbon Nanofibers over Ni−Cu−SiO2 Catalysts. Energ. Fuels 2008, 23, 5–13. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, T. Alloy formation and strength of Ni-Cu interaction in Ni-Cu/ZnO catalysts. Reac. Kinet. Catal. Lett. 2000, 70, 91–96. [Google Scholar] [CrossRef]
- Znak, L.; Zieliński, J. Interaction of Hydrogen with Unsupported and Supported Nickel. Langmuir 2006, 22, 8758–8763. [Google Scholar] [CrossRef]
- Znak, L.; Zieliński, J. Effects of support on hydrogen adsorption/desorption on nickel. Appl. Catal. A Gen. 2008, 334, 268–276. [Google Scholar] [CrossRef]
- Angelo, J.E.; Moody, N.R.; Baskes, M.I. Trapping of hydrogen to lattice defects in nickel. Modell. Simul. Mater. Sci. Eng. 1995, 3, 289–307. [Google Scholar] [CrossRef]
- Connétable, D.; Wang, Y.; Tanguy, D. Segregation of hydrogen to defects in nickel using first-principles calculations: The case of self-interstitials and cavities. J. Alloys Compnd. 2014, 614, 211–220. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Mishakov, I.V.; Vedyagin, A.A.; Ramakrishna, S. Synthesis of bimodal carbon structures via metal dusting of Ni-based alloys. Mater. Lett. 2017, 201, 70–73. [Google Scholar] [CrossRef]
- Potylitsyna, A.R.; Rudneva, Y.V.; Bauman, Y.I.; Plyusnin, P.E.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A.; Shubin, Y.V.; Mishakov, I.V. Efficient Production of Segmented Carbon Nanofibers via Catalytic Decomposition of Trichloroethylene over Ni-W Catalyst. Materials 2023, 16, 845. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.; Baker, R.T.K. Influence of Chlorine on the Decomposition of Ethylene over Iron and Cobalt Particles. J. Phys. Chem. B 1997, 101, 1621–1630. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Maksimova, T.A.; Mishakov, I.V.; Bauman, Y.I.; Ayupov, A.B.; Mel’gunov, M.S.; Dmitrachkov, A.M.; Nartova, A.V.; Stoyanovskii, V.O.; Vedyagin, A.A. Effect of Pretreatment with Acids on the N-Functionalization of Carbon Nanofibers Using Melamine. Materials 2022, 15, 8239. [Google Scholar] [CrossRef]
- Fenelonov, V.B.; Derevyankin, A.Y.; Okkel, L.G.; Avdeeva, L.B.; Zaikovskii, V.I.; Moroz, E.M.; Salanov, A.N.; Rudina, N.A.; Likholobov, V.A.; Shaikhutdinov, S.K. Structure and texture of filamentous carbons produced by methane decomposition on Ni and Ni-Cu catalysts. Carbon 1997, 35, 1129–1140. [Google Scholar] [CrossRef]
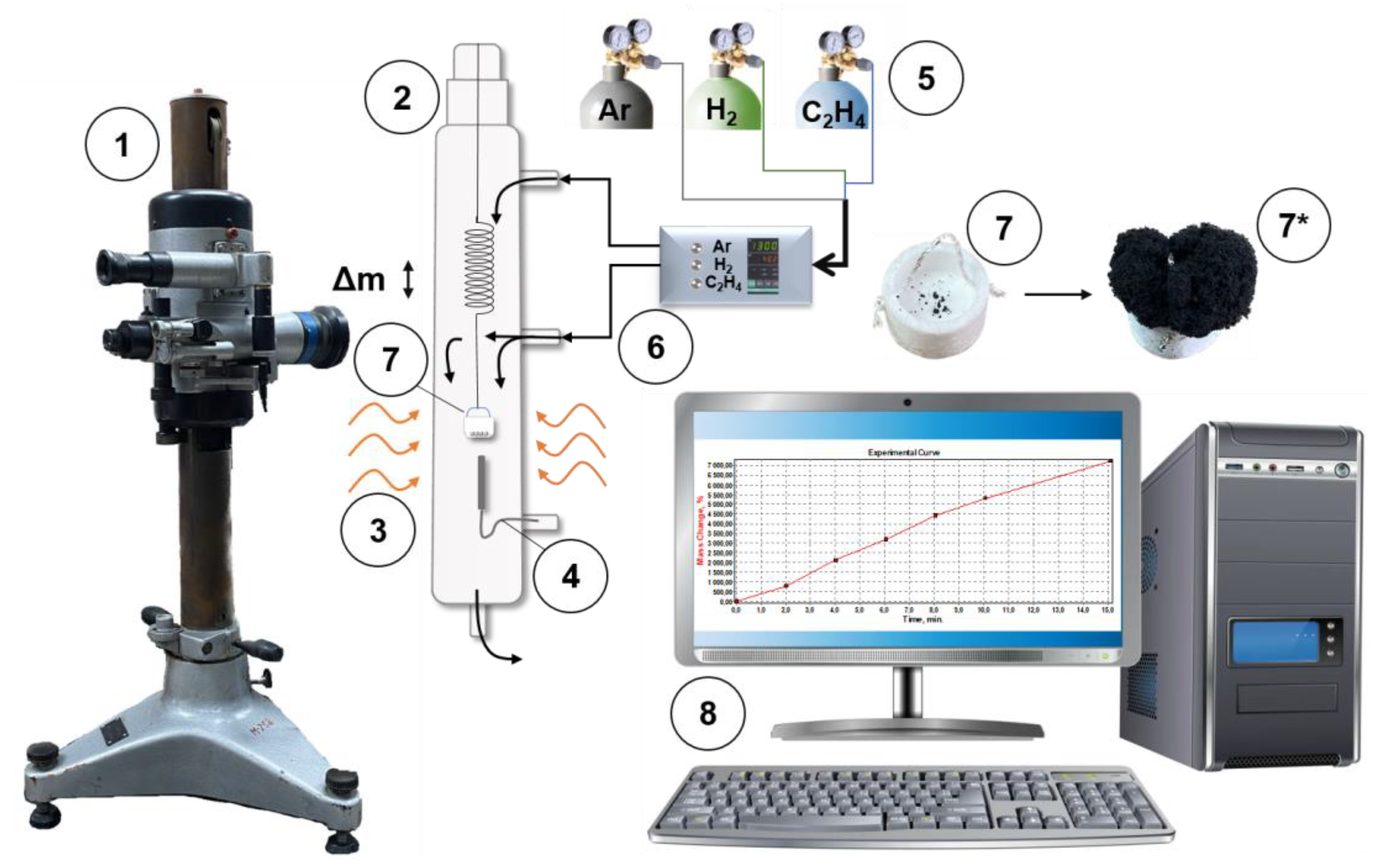



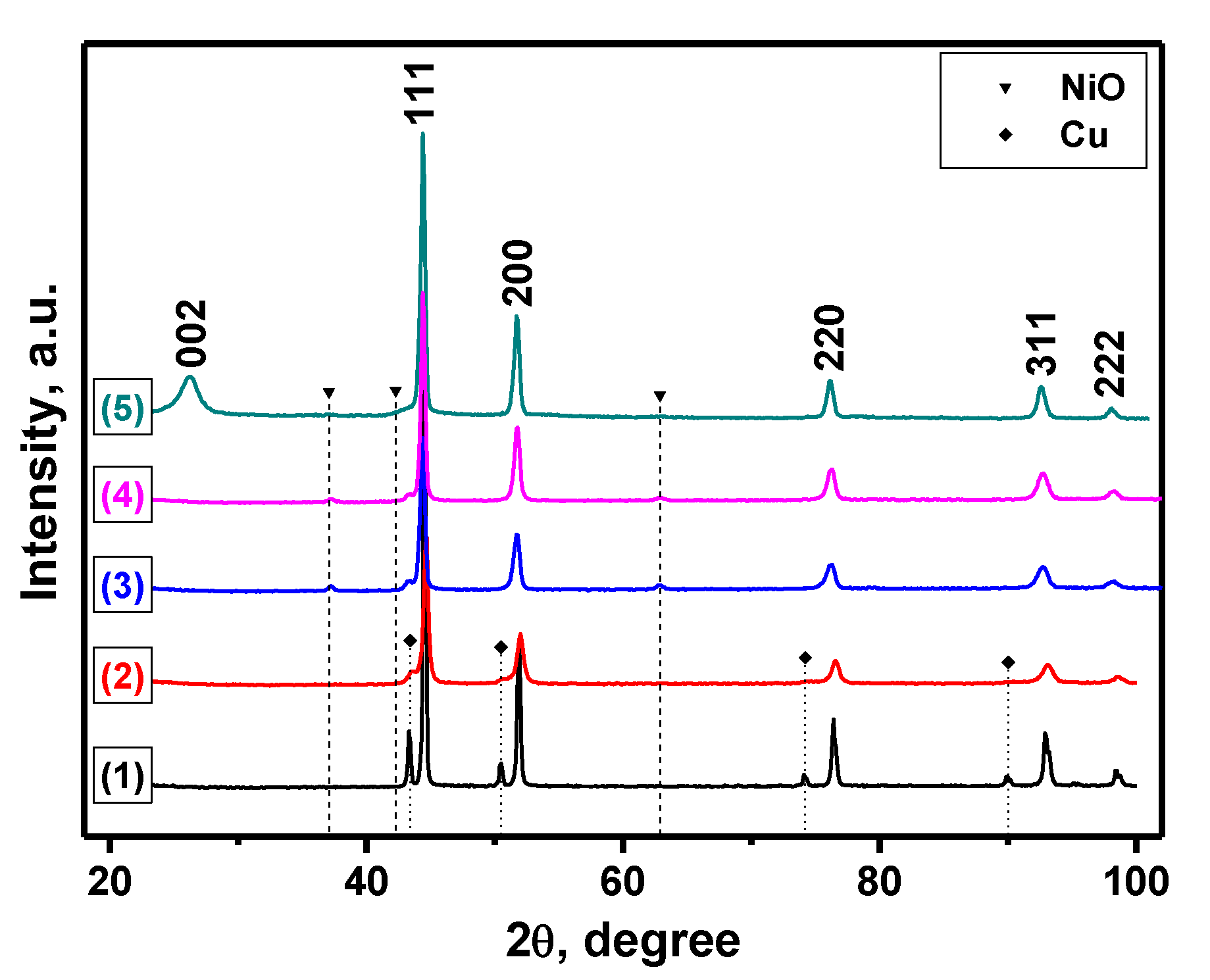
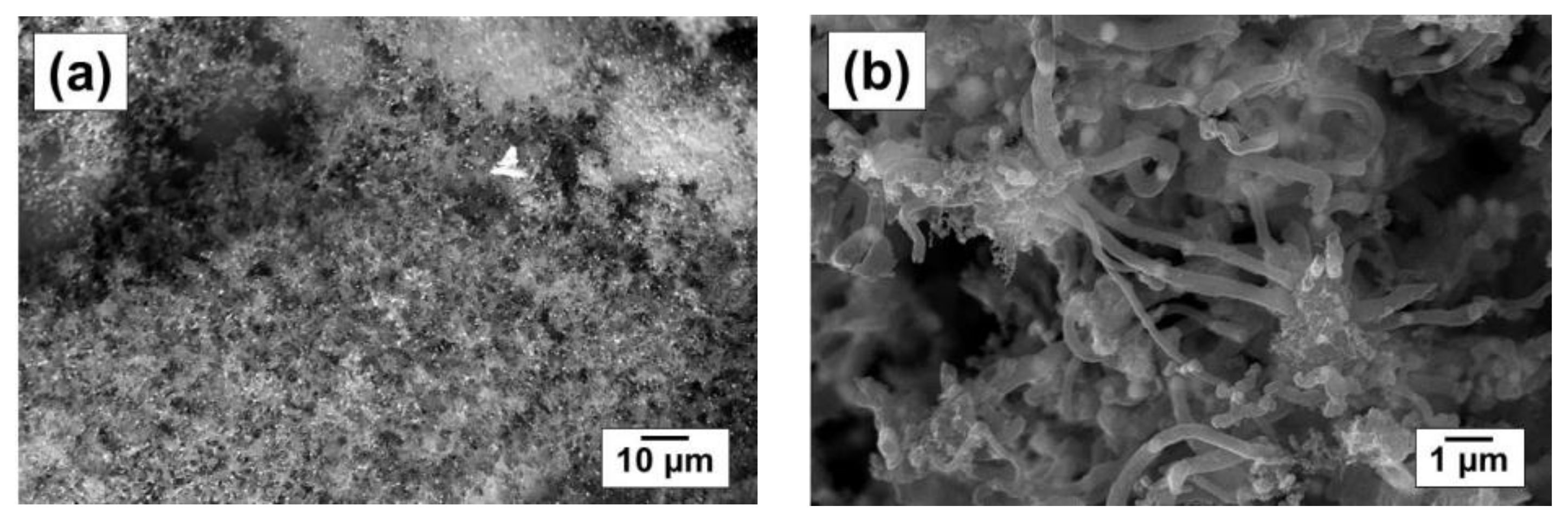
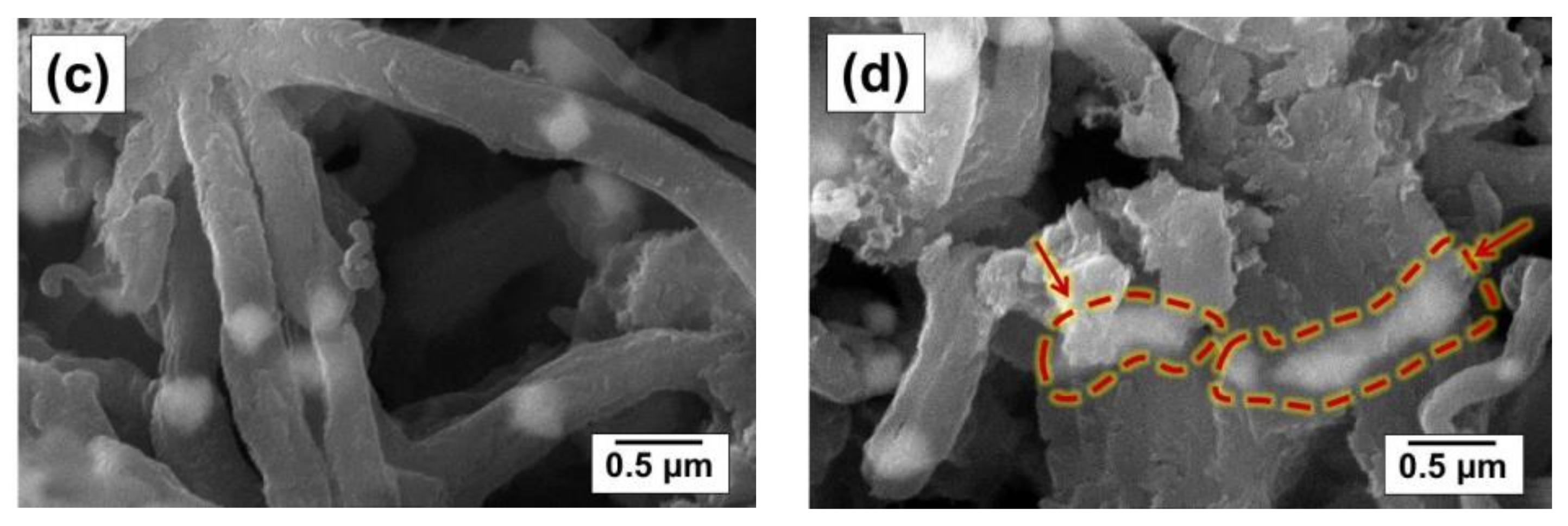

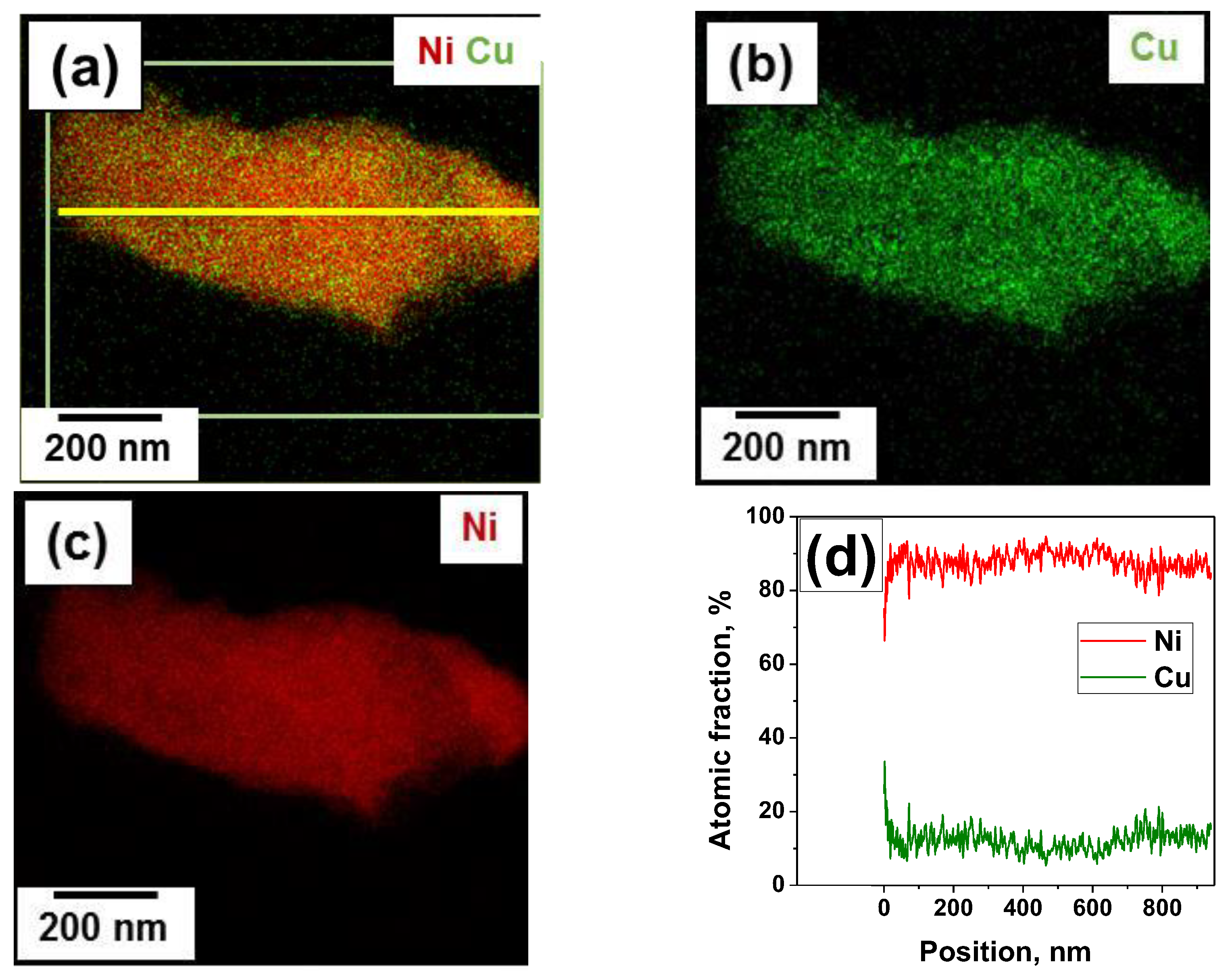


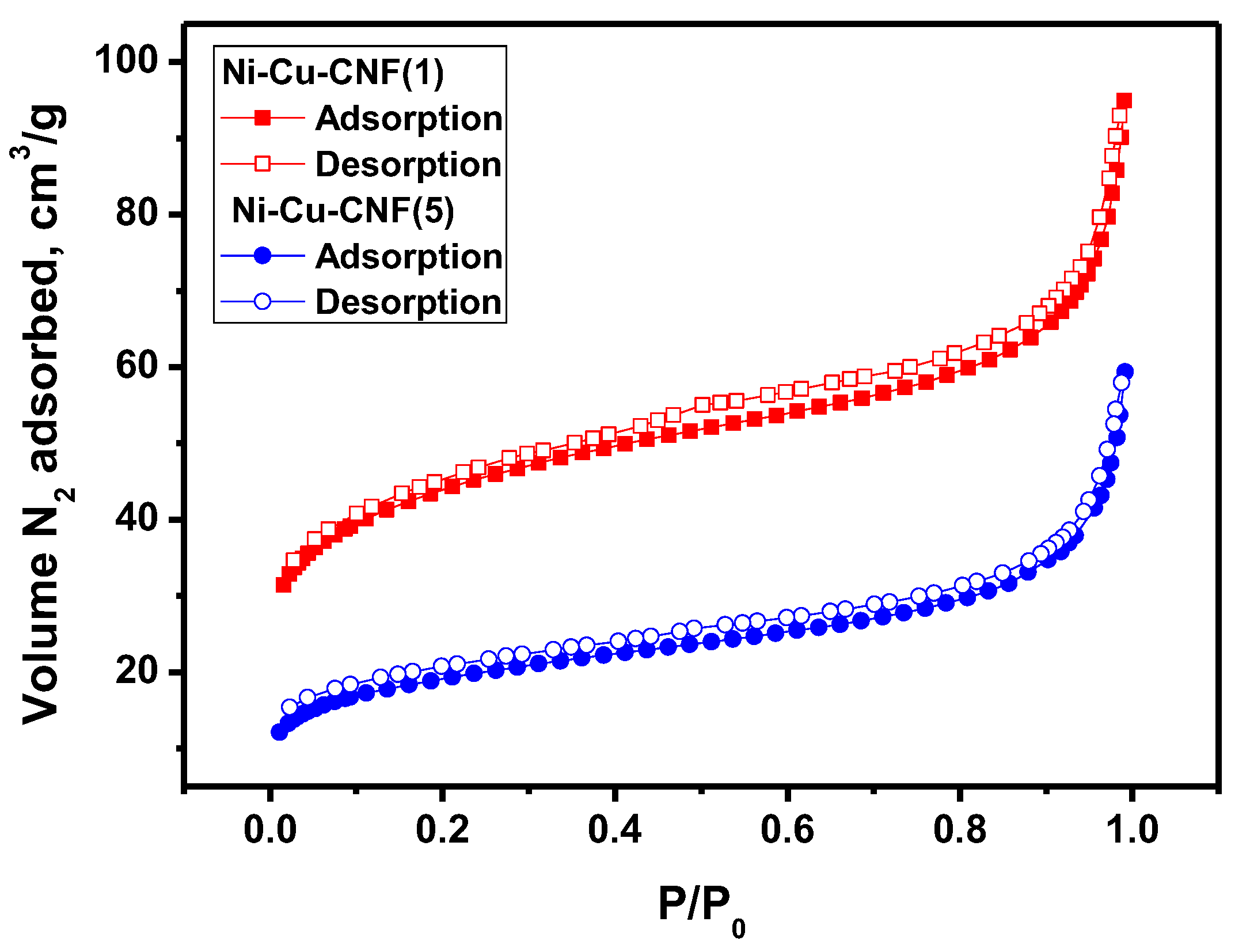
| Sample | Activation Time (τ, min) | Carbon Yield, g/gcat | Lattice Parameter, Å | Crystallite Size, nm | Hydrogen Uptake, mmol/g | SSA, m2/g | |
|---|---|---|---|---|---|---|---|
| Peak A | Peak B | ||||||
| Premix | - | 22 ± 2 | 3.525 ± 0.001 | 50 ± 10 | 0.17 ± 0.01 | - | 0.5 ± 0.3 |
| Ni(7) | 7 | 51 ± 5 | 3.525 ± 0.001 | 11 ± 3 | 0.77 ± 0.02 | −0.12 ± 0.02 | 1.7 ± 0.4 |
| NiCu(3) | 3 | 165 ± 17 | 3.523 ± 0.002 | 17 ± 5 | 0.18 ± 0.01 | −0.14 ± 0.02 | 0.4 ± 0.1 |
| NiCu(7) | 7 | 127 ± 13 | 3.532 ± 0.003 | 9 ± 3 | 0.76 ± 0.01 | −0.24 ± 0.02 | 1.7 ± 0.4 |
| NiCu(11) | 11 | 102 ± 10 | 3.535 ± 0.003 | 8 ± 3 | 1.20 ± 0.01 | −0.21 ± 0.02 | 1.9 ± 0.5 |
| Sample | Reaction Conditions | Lattice Parameter, Å | Crystallite Size, nm | Phase Composition |
|---|---|---|---|---|
| Premix | - | 3.525 ± 0.001 | 52 ± 11 | A mixture of Ni and Cu phases |
| NiCu(3) | MCA for 3 min | 3.523 ± 0.002 | 17 ± 5 | A mixture of Ni and Cu phases |
| NiCu(3)550 | MCA for 3 min; heating in Ar to 550 °C | 3.535 ± 0.002 | 16 ± 5 | Ni-Cu alloy |
| NiCu(3)550/1′ | MCA for 3 min; heating in Ar to 550 °C; reduction with H2 for 1 min at 550 °C | 3.532 ± 0.001 | 18 ± 5 | Ni-Cu alloy |
| NiCu(3)550/1″ * | MCA for 3 min; heating in Ar to 550 °C; exposure to the reaction mixture for 1 min at 550 °C | 3.535 ± 0.001 | 21 ± 6 | Ni-Cu alloy |
| Sample | tr, min | YC, g/gcat | CMe, wt.% | ρ, g/L | SSA, m2/g | Vp, cm3/g | Vμ, cm3/g | Davg (4Vp/SSA), nm |
|---|---|---|---|---|---|---|---|---|
| Ni-Cu-CNF_20 | 20 | 32 ± 3 | 3.1 ± 0.3 | 25 ± 3 | 159 ± 8 | 0.15 ± 0.01 | 0.040 ± 0.002 | 3.7 ± 0.2 |
| Ni-Cu-CNF_40 | 40 | 62 ± 6 | 1.6 ± 0.2 | 25 ± 3 | 158 ± 8 | 0.15 ± 0.01 | 0.042 ± 0.001 | 3.8 ± 0.2 |
| Ni-Cu-CNF_60 | 60 | 90 ± 9 | 1.1 ± 0.1 | 25 ± 3 | 158 ± 8 | 0.16 ± 0.01 | 0.041 ± 0.001 | 3.9 ± 0.2 |
| Ni-Cu-CNF_90 | 90 | 120 ± 12 | 0.8 ± 0.1 | 25 ± 3 | 134 ± 7 | 0.15 ± 0.01 | 0.035 ± 0.002 | 4.4 ± 0.2 |
| Ni-Cu-CNF_120 | 120 | 148 ± 15 | 0.7 ± 0.1 | 30 ± 3 | 69 ± 4 | 0.09 ± 0.01 | 0.014 ± 0.001 | 5.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonnikova, S.D.; Veselov, G.B.; Bauman, Y.I.; Gerasimov, E.Y.; Shubin, Y.V.; Mishakov, I.V.; Vedyagin, A.A. Synthesis of Ni-Cu-CNF Composite Materials via Carbon Erosion of Ni-Cu Bulk Alloys Prepared by Mechanochemical Alloying. J. Compos. Sci. 2023, 7, 238. https://doi.org/10.3390/jcs7060238
Afonnikova SD, Veselov GB, Bauman YI, Gerasimov EY, Shubin YV, Mishakov IV, Vedyagin AA. Synthesis of Ni-Cu-CNF Composite Materials via Carbon Erosion of Ni-Cu Bulk Alloys Prepared by Mechanochemical Alloying. Journal of Composites Science. 2023; 7(6):238. https://doi.org/10.3390/jcs7060238
Chicago/Turabian StyleAfonnikova, Sofya D., Grigory B. Veselov, Yury I. Bauman, Evgeny Y. Gerasimov, Yury V. Shubin, Ilya V. Mishakov, and Aleksey A. Vedyagin. 2023. "Synthesis of Ni-Cu-CNF Composite Materials via Carbon Erosion of Ni-Cu Bulk Alloys Prepared by Mechanochemical Alloying" Journal of Composites Science 7, no. 6: 238. https://doi.org/10.3390/jcs7060238
APA StyleAfonnikova, S. D., Veselov, G. B., Bauman, Y. I., Gerasimov, E. Y., Shubin, Y. V., Mishakov, I. V., & Vedyagin, A. A. (2023). Synthesis of Ni-Cu-CNF Composite Materials via Carbon Erosion of Ni-Cu Bulk Alloys Prepared by Mechanochemical Alloying. Journal of Composites Science, 7(6), 238. https://doi.org/10.3390/jcs7060238









