Curcumin-Loaded Nanofibrous Matrix Accelerates Fibroblast Cell Proliferation and Enhances Wound Healing via GSK3-β Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Docking
2.2. Cell Culture and Viability
2.3. Western Blot Analysis
2.4. Electrospinning
2.5. Morphological Analysis via Scanning Electron Microscopy
2.6. FTIR Instrumentation
2.7. Drug Release Study
2.8. Effect of PCLF–Cur on Cell Viability
2.9. FGF ELISA
2.10. Wound Healing Studies in Rodents
2.11. Statistical Analysis
3. Results
3.1. Molecular Interactions between Curcumin and GSK3-β
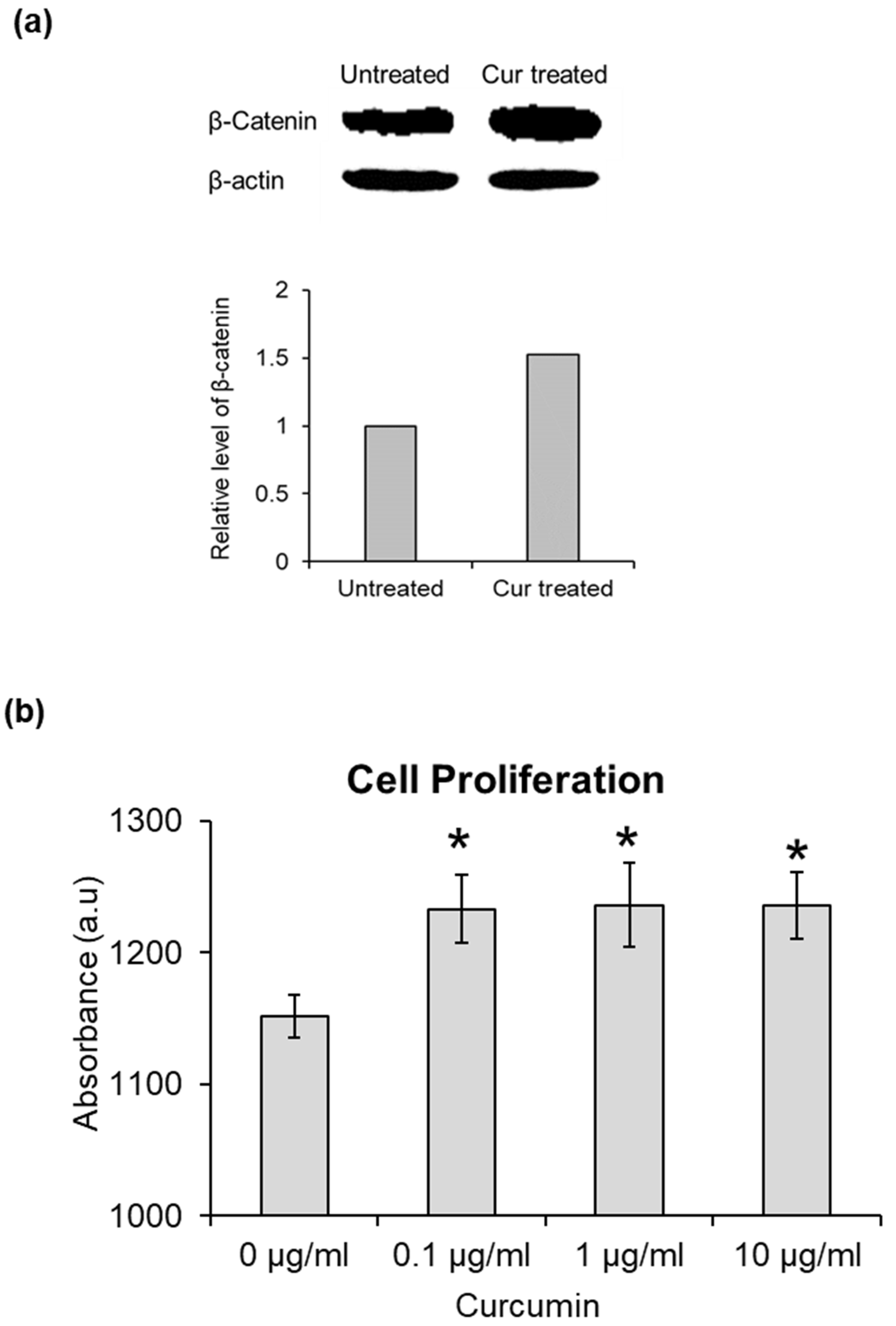
3.2. Stabilization of β-Catenin by Curcumin
3.3. Effect of Curcumin on Cell Proliferation
3.4. Morphological Characteristics of Nanofiber Scaffolds
3.5. Confirmation of Curcumin Incorporation in Nanofiber Scaffolds
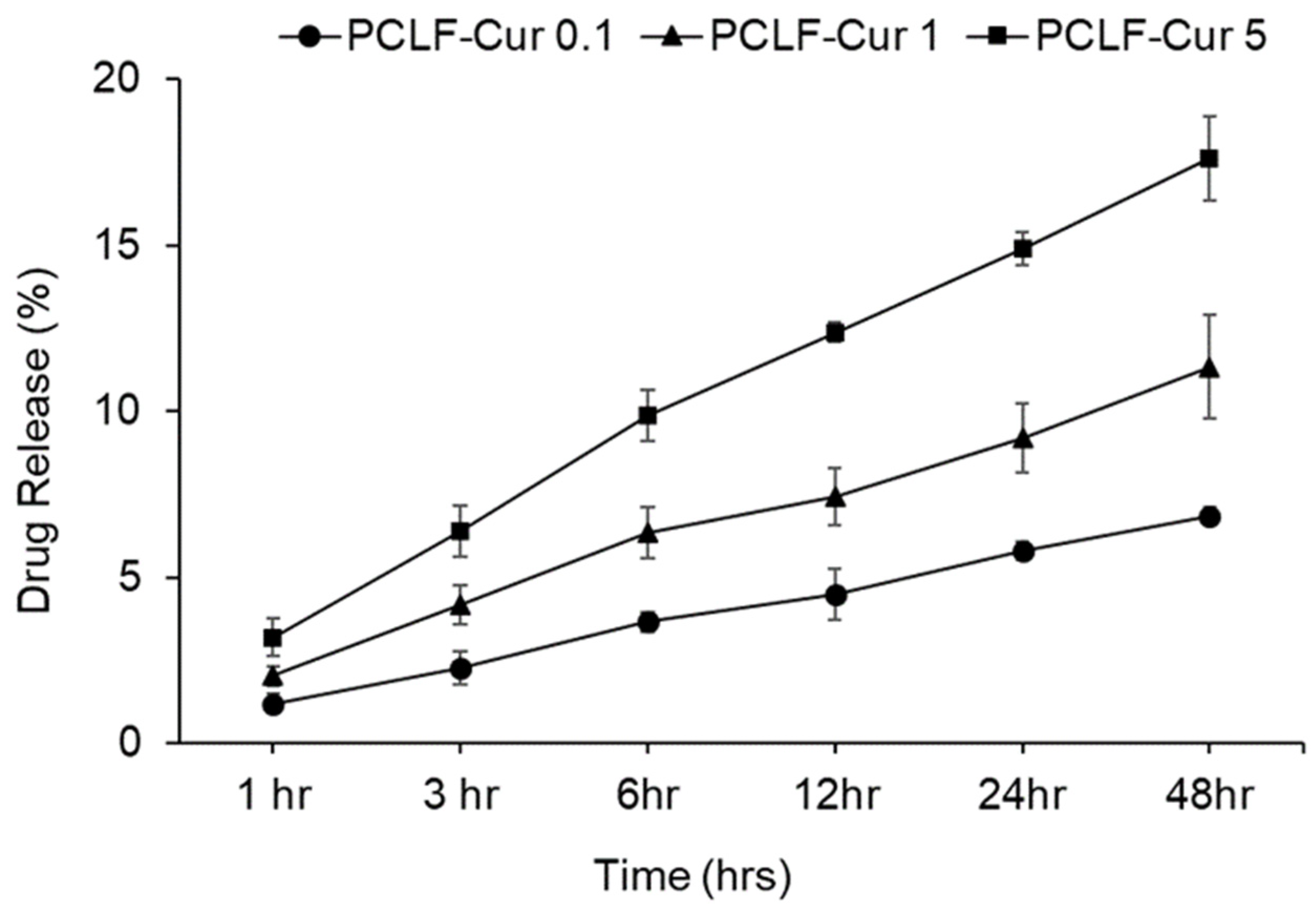
3.6. Controlled and Sustained Release of Curcumin from PCLF Scaffolds
3.7. Effect of Curcumin-Incorporated Nanofibers on Cell Proliferation
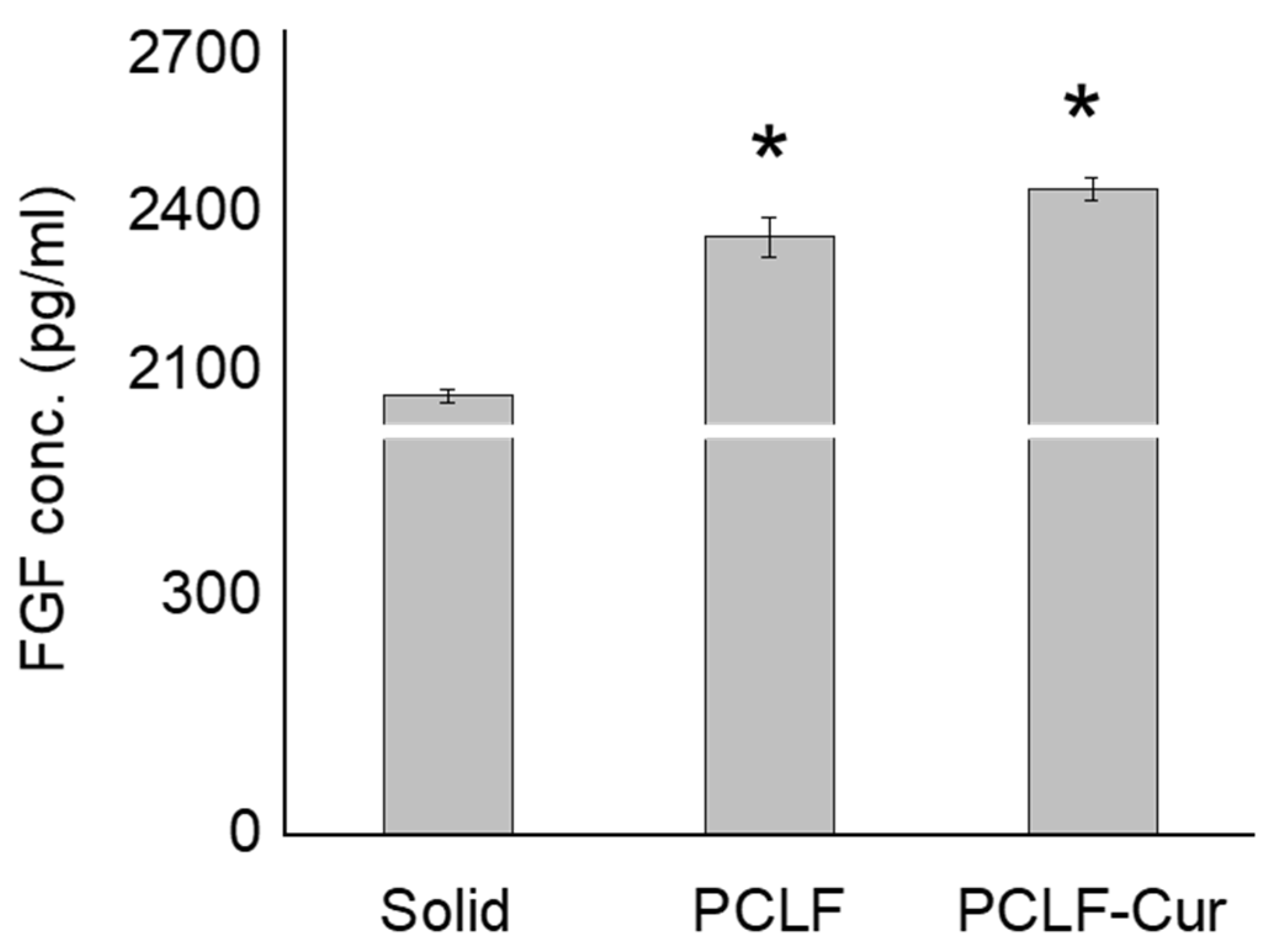
3.8. Biomimetic Nanofibrous Scaffolds on Fibroblast Growth Factor Expression
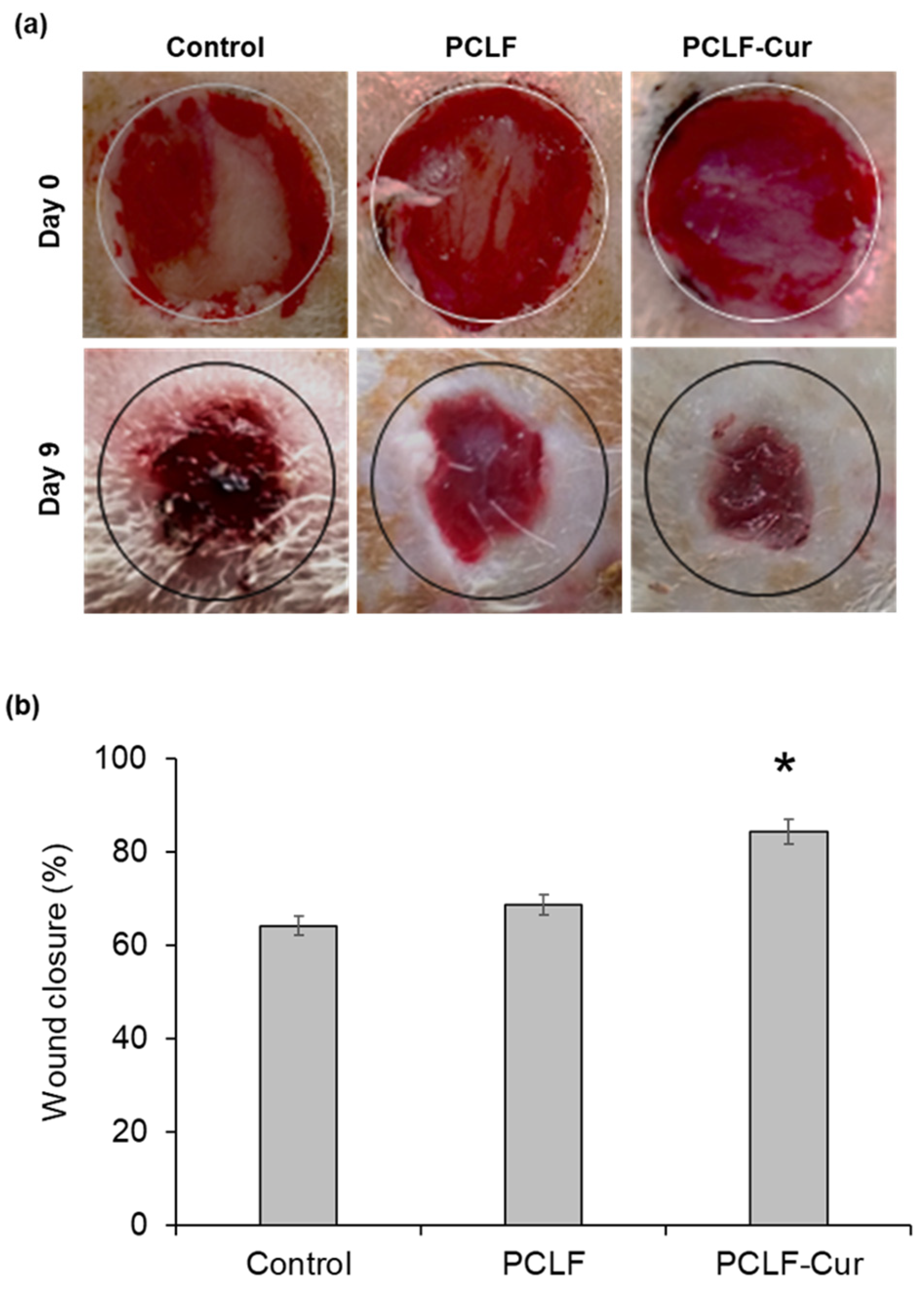
3.9. PCLF–Curcumin Scaffold for Wound Closure in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Chidambara Murthy, K.N. Challenges in Healing Wound: Role of Complementary and Alternative Medicine. Front. Nutr. 2022, 8, 791899. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef] [PubMed]
- Janoušková, O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Oh, J.-H.; Cho, Y.-D.; Chung, S.H.; Lee, G.; Baek, J.-H.; Ryoo, H.-M.; Woo, K.M. Fibrous topography-potentiated canonical Wnt signaling directs the odontoblastic differentiation of dental pulp-derived stem cells. ACS Appl. Mater. Interfaces 2018, 10, 17526–17541. [Google Scholar] [CrossRef]
- Rahman, S.U.; Kim, W.-J.; Chung, S.H.; Woo, K.M. Nanofibrous topography-driven altered responsiveness to Wnt5a mediates the three-dimensional polarization of odontoblasts. Mater. Today Bio 2022, 17, 100479. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Ponnusamy, S.; Nagrath, M.; Arany, P.R. Precision-engineered niche for directed differentiation of MSCs to lineage-restricted mineralized tissues. J. Tissue Eng. 2022, 13, 20417314211073934. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Duscher, D.; Rein, S.; Maan, Z.N.; Chelliah, M.P.; Cha, J.Y.; Weissenberg, K.; Siemers, F. Wnt Signaling During Cutaneous Wound Healing. In Regenerative Medicine and Plastic Surgery: Skin and Soft Tissue, Bone, Cartilage, Muscle, Tendon and Nerves; Duscher, D., Shiffman, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 147–155. [Google Scholar]
- Whyte, J.L.; Smith, A.A.; Helms, J.A. Wnt signaling and injury repair. Cold Spring Harb. Perspect. Biol. 2012, 4, a008078. [Google Scholar] [CrossRef]
- Subbukutti, V.; Sailatha, E.; Gunasekaran, S.; Manibalan, S.; Uma Devi, K.J.; Bhuvaneshwari, K.; Suvedha, R. Evaluation of wound healing active principles in the transdermal patch formulated with crude bio wastes and plant extracts against GSK-3 beta-an in silico study. J. Biomol. Struct. Dyn. 2023, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-loaded poly(epsilon-caprolactone) nanofibres: Diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2013, 39, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys. J. 2011, 101, 2525–2534. [Google Scholar] [CrossRef]
- Elumalai, M.; Muthaiah, R.; Alf, M.A. Identification of curcumin targets in neuroinflammatory pathways: Molecular docking scores with GSK-3β, p38 MAPK, COX, ICE and TACE enzymes. Acta Pol. Pharm. 2012, 69, 237–245. [Google Scholar]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. [Google Scholar] [CrossRef]
- Tabassum, N.; Khalid, S.; Ghafoor, S.; Woo, K.M.; Lee, E.H.; Samie, M.; Konain, K.; Ponnusamy, S.; Arany, P.; Rahman, S.U. Tideglusib-incorporated nanofibrous scaffolds potently induce odontogenic differentiation. J. Biomater. Appl. 2023, 38, 280–291. [Google Scholar] [CrossRef]
- Zhang, Q.; Oh, J.-H.; Park, C.H.; Baek, J.-H.; Ryoo, H.-M.; Woo, K.M. Effects of dimethyloxalylglycine-embedded poly (ε-caprolactone) fiber meshes on wound healing in diabetic rats. ACS Appl. Mater. Interfaces 2017, 9, 7950–7963. [Google Scholar] [CrossRef]
- Bustanji, Y.; Taha, M.O.; Almasri, I.M.; Al-Ghussein, M.A.; Mohammad, M.K.; Alkhatib, H.S. Inhibition of glycogen synthase kinase by curcumin: Investigation by simulated molecular docking and subsequent in vitro/in vivo evaluation. J. Enzyme Inhib. Med. Chem. 2009, 24, 771–778. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Cianfruglia, L.; Minnelli, C.; Laudadio, E.; Scirè, A.; Armeni, T. Side effects of curcumin: Epigenetic and antiproliferative implications for normal dermal fibroblast and breast cancer cells. Antioxidants 2019, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Ingavle, G.C.; Leach, J.K. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Maya Nandkumar, A.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA-Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Akhtar, M.; Woo, K.M.; Tahir, M.; Wu, W.; Elango, J.; Mirza, M.R.; Khan, M.; Shamim, S.; Arany, P.R.; Rahman, S.U. Enhancing osteoblast differentiation through small molecule-incorporated engineered nanofibrous scaffold. Clin. Oral Investig. 2022, 26, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Prudovsky, I. Cellular Mechanisms of FGF-Stimulated Tissue Repair. Cells 2021, 10, 1830. [Google Scholar] [CrossRef]
- Akita, S.; Akino, K.; Hirano, A. Basic Fibroblast Growth Factor in Scarless Wound Healing. Adv. Wound Care 2013, 2, 44–49. [Google Scholar] [CrossRef]
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: Getting back to the roots. Ann. N. Y. Acad. Sci. 2005, 1056, 206–217. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.; Mendonça, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Yozaki, M.; Utani, A.; Murota, H. Fibroblast growth factor 2 accelerates the epithelial–mesenchymal transition in keratinocytes during wound healing process. Sci. Rep. 2020, 10, 18545. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Sun, C.; Wang, T.; Shen, Y.; Cai, W.; Sun, J.; Chi, L.; Wang, H.; Song, N. Feedback activation of basic fibroblast growth factor signaling via the Wnt/β-catenin pathway in skin fibroblasts. Front. Pharmacol. 2017, 8, 32. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Schlie-Wolter, S.; Ngezahayo, A.; Chichkov, B.N. The selective role of ECM components on cell adhesion, morphology, proliferation and communication in vitro. Exp. Cell Res. 2013, 319, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Situm, M.; Kolić, M. Chronic wounds: Differential diagnosis. Acta Med. Croatica 2013, 67 (Suppl. S1), 11–20. [Google Scholar]
- Sidhu, G.S.; Singh, A.K.; Thaloor, D.; Banaudha, K.K.; Patnaik, G.K.; Srimal, R.C.; Maheshwari, R.K. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998, 6, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Sahoo, S.K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010, 31, 6597–6611. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konain, K.; Saddique, N.; Samie, M.; Rahman, Z.U.; Farid, S.; Hameed, S.; Mirza, M.R.; Wu, W.; Woo, K.M.; Arany, P.R.; et al. Curcumin-Loaded Nanofibrous Matrix Accelerates Fibroblast Cell Proliferation and Enhances Wound Healing via GSK3-β Inhibition. J. Compos. Sci. 2023, 7, 343. https://doi.org/10.3390/jcs7080343
Konain K, Saddique N, Samie M, Rahman ZU, Farid S, Hameed S, Mirza MR, Wu W, Woo KM, Arany PR, et al. Curcumin-Loaded Nanofibrous Matrix Accelerates Fibroblast Cell Proliferation and Enhances Wound Healing via GSK3-β Inhibition. Journal of Composites Science. 2023; 7(8):343. https://doi.org/10.3390/jcs7080343
Chicago/Turabian StyleKonain, Kiran, Nayyer Saddique, Muhammad Samie, Zia Ur Rahman, Sajida Farid, Shazia Hameed, Munazza R. Mirza, Wenhui Wu, Kyung Mi Woo, Praveen R. Arany, and et al. 2023. "Curcumin-Loaded Nanofibrous Matrix Accelerates Fibroblast Cell Proliferation and Enhances Wound Healing via GSK3-β Inhibition" Journal of Composites Science 7, no. 8: 343. https://doi.org/10.3390/jcs7080343






