Preparation and Hydrolytic Degradation of Hydroxyapatite-Filled PLGA Composite Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PLGA
2.3. Formation of Microspheres Based on PLGA
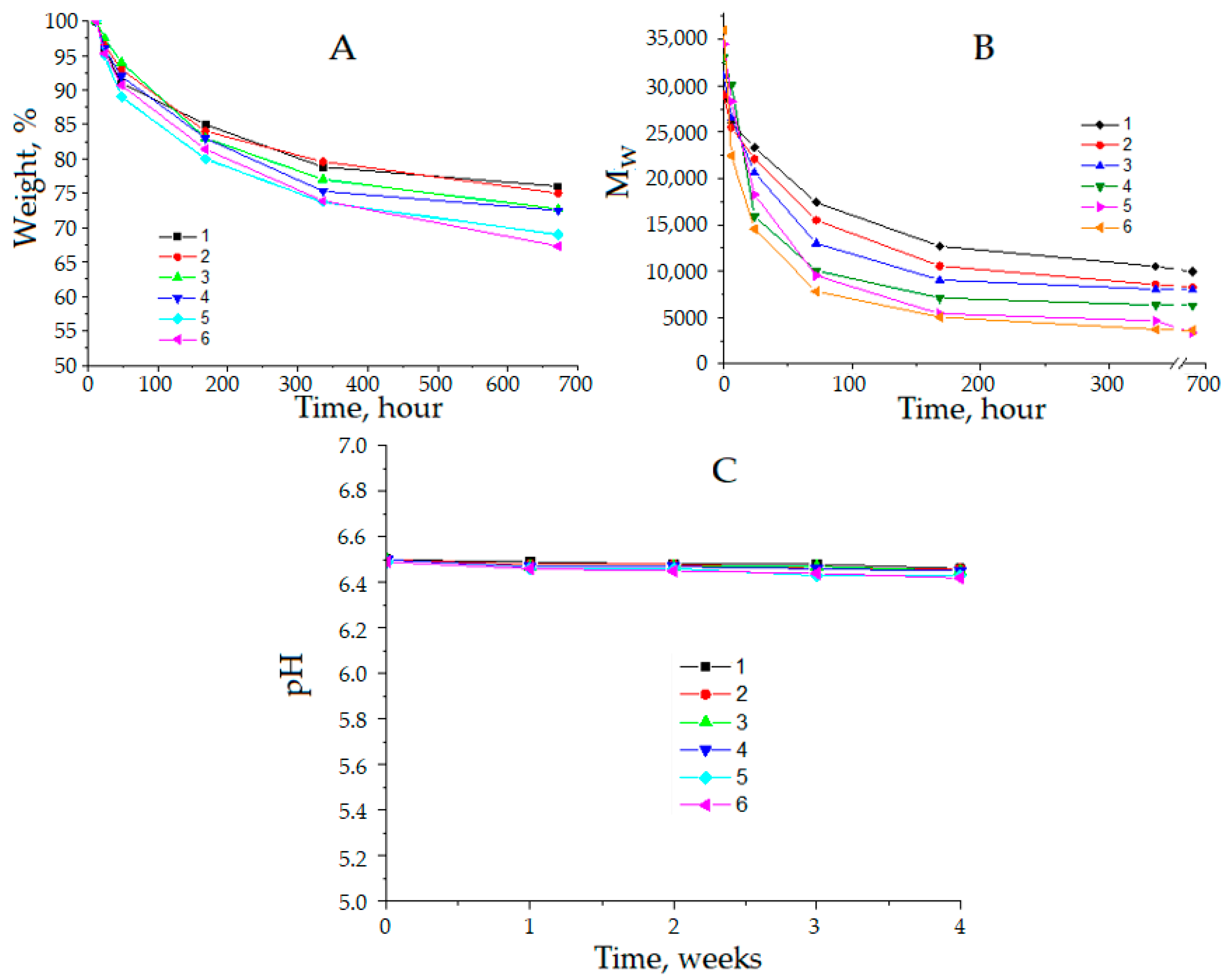
2.4. Study of Hydrolytic Degradation of PLGA
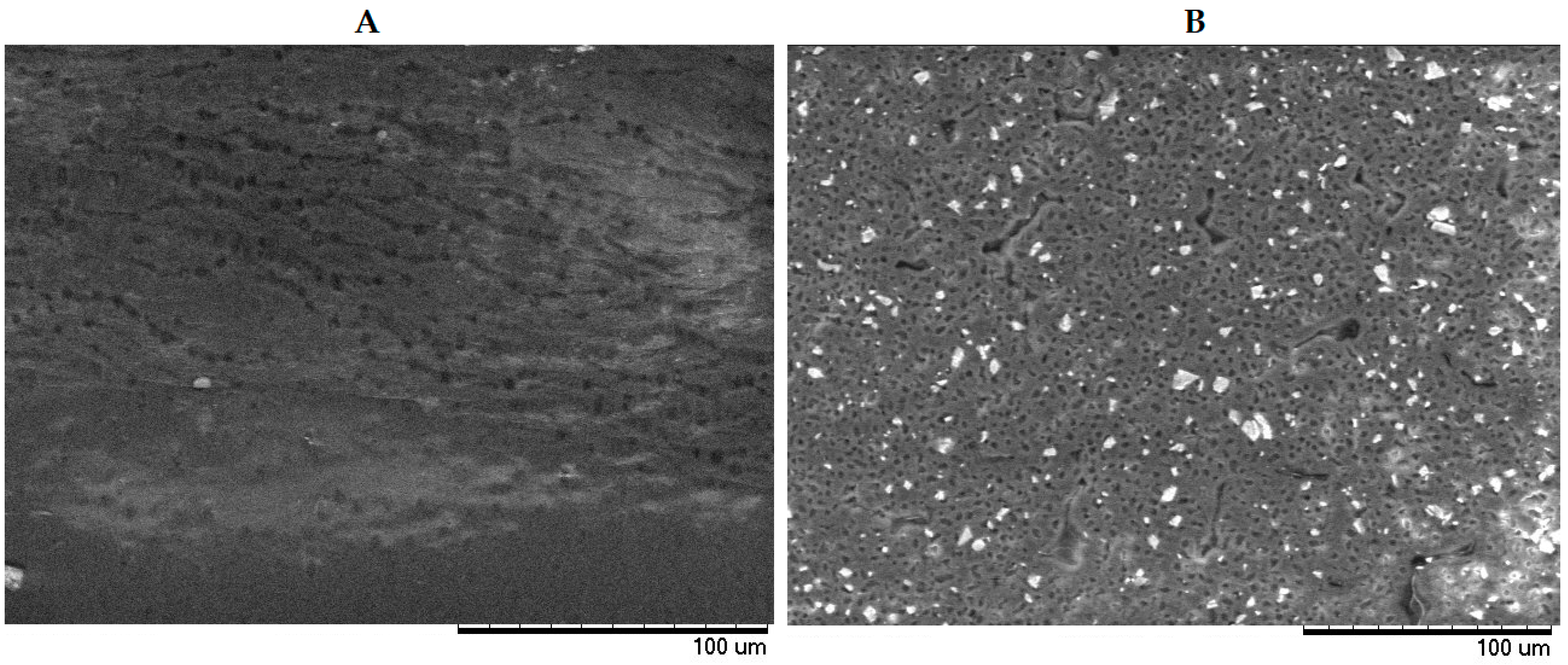
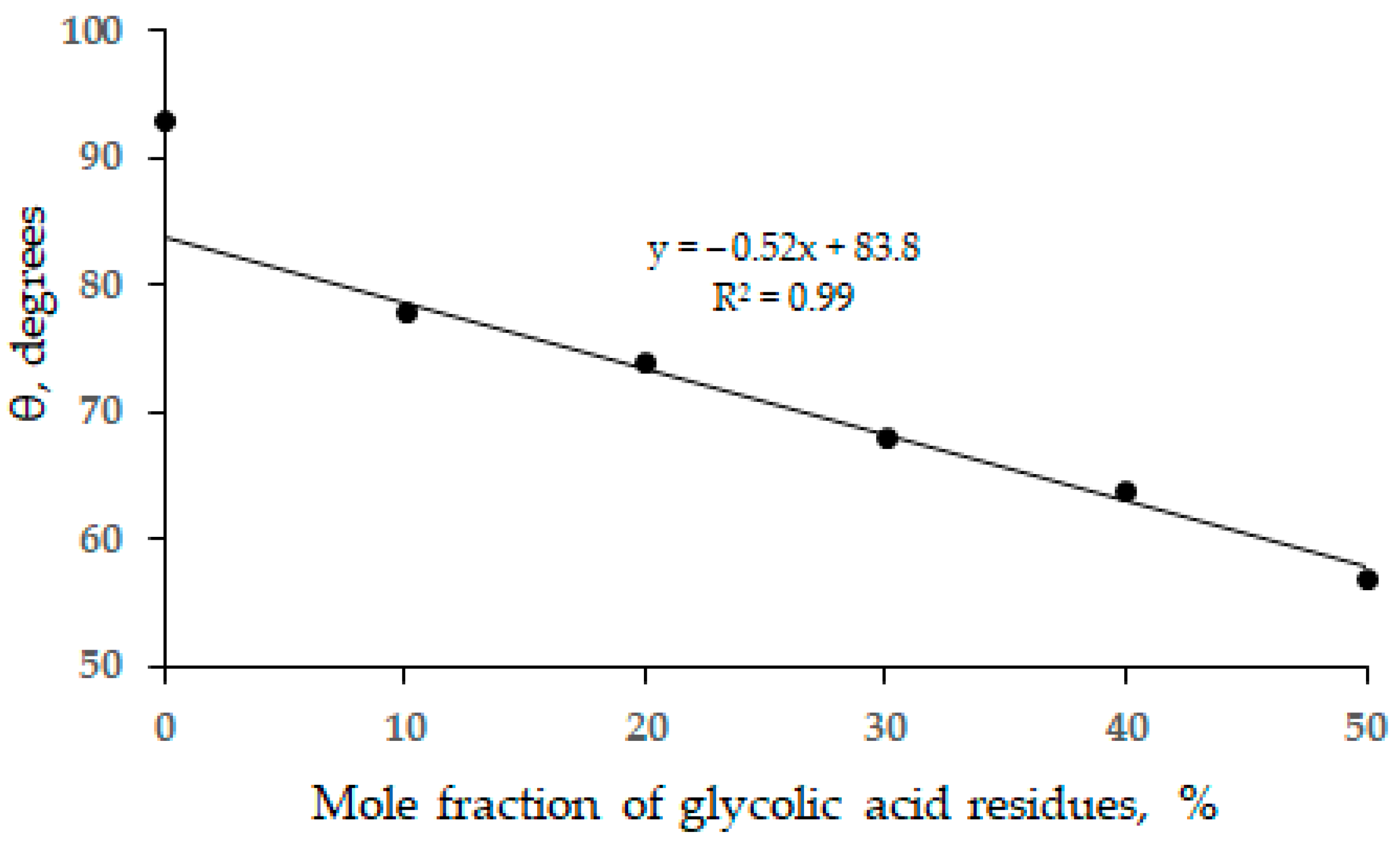
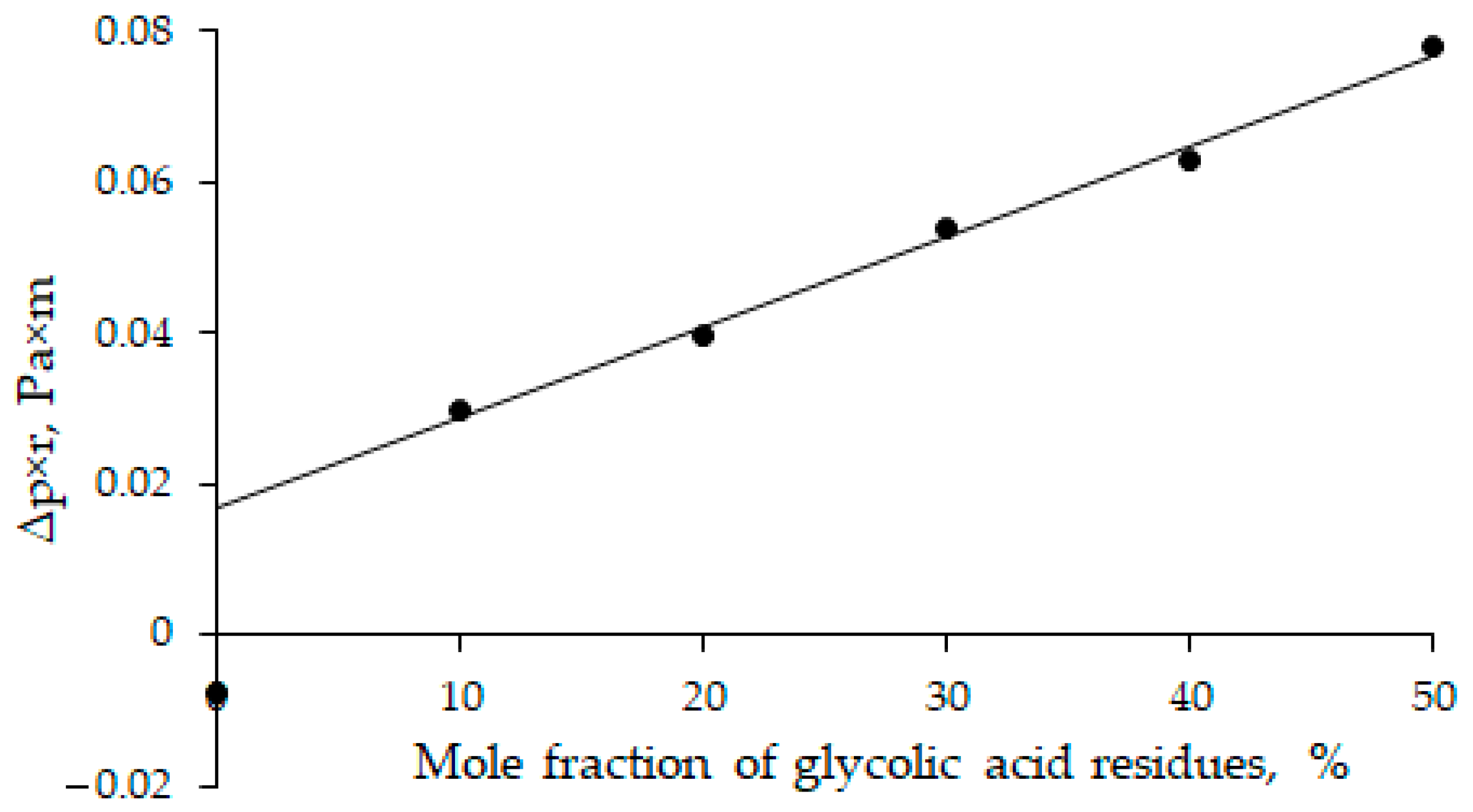
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar] [CrossRef]
- Kontogianni, G.-I.; Bonatti, A.F.; De Maria, C.; Naseem, R.; Melo, P.; Coelho, C.; Vozzi, G.; Dalgarno, K.; Quadros, P.; Vitale-Brovarone, C.; et al. Promotion of In Vitro Osteogenic Activity by Melt Extrusion-Based PLLA/PCL/PHBV Scaffolds Enriched with Nano-Hydroxyapatite and Strontium Substituted Nano-Hydroxyapatite. Polymers 2023, 15, 1052. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, E.; Cascone, M.G. Microfluidic Fabrication of Natural Polymer-Based Scaffolds for Tissue Engineering Applications: A Review. Biomimetics 2023, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, E.; Hassan, M.H.; Omar, A.M.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Blunn, G.; Koc, B.; Bartolo, P. Accelerated Degradation of Poly-ε-caprolactone Composite Scaffolds for Large Bone Defects. Polymers 2023, 15, 670. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Su, K.; Varshney, R.R.; Wang, Y.; Wang, D.-A. Sintered Microsphere Scaffolds for Controlled Release and Tissue Engineering. Pharm. Res. 2011, 28, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Kirby, G.T.S.; Cox, H.C.; Qodratnama, R.; Qutachi, O.; Rose, F.R.; Shakesheff, K.M. Accelerating Protein Release from Microparticles for Regenerative Medicine Applications. Mater. Sci. Eng. C 2013, 33, 2578–2583. [Google Scholar] [CrossRef]
- Rahman, C.V.; Ben-David, D.; Dhillon, A.; Kuhn, G.; Gould, T.W.A.; Müller, R.; Rose, F.R.; Shakesheff, K.M.; Livne, E. Controlled Release of BMP-2 from a Sintered Polymer Scaffold Enhances Bone Repair in a Mouse Calvarial Defect Model. J. Tissue Eng. Regen. Med. 2014, 8, 59–66. [Google Scholar] [CrossRef]
- Gupta, V.; Khan, Y.; Berkland, C.J.; Laurencin, C.T.; Detamore, M.S. Microsphere-Based Scaffolds in Regenerative Engineering. Annu. Rev. Biomed. Eng. 2017, 19, 135–161. [Google Scholar] [CrossRef]
- Belkas, J.S.; Shoichet, M.S.; Midha, R. Peripheral Nerve Regeneration through Guidance Tubes. Neurol. Res. 2004, 26, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Valmikinathan, C.M.; Tian, J.; Wang, J.; Yu, X. Novel Nanofibrous Spiral Scaffolds for Neural Tissue Engineering. J. Neural Eng. 2008, 5, 422–432. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Wolke, J.G.C.; Mikos, A.G.; Jansen, J.A. Injectable PLGA Microsphere/calcium Phosphate Cements: Physical Properties and Degradation Characteristics. J. Biomater. Sci. Polym. 2006, 17, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, L.; Bertoldi, S.; Cigada, A.; Tanzi, M.C.; Haugen, H.J.; Farè, S. Preparation and Characterization of Shape Memory Polymer Scaffolds via Solvent Casting/particulate Leaching. J. Appl. Biomater. Funct. Mater. 2012, 10, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.; Attawia, M.; Khan, Y.; El-Amin, S.F.; Laurencin, C.T. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J. Bone Jt. Surg. Br. 2004, 86, 1200. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, P.Q.; Hedberg-Dirk, E.L.; Padron, N.T.; Spauwen, P.H.; Jansen, J.A.; Mikos, A.G. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.; Attawia, M.; Laurencin, C.T. The Sintered Microsphere Matrix for Bone Tissue Engineering: In Vitro Osteoconductivity Studies. J. Biomed. Mater. 2002, 61, 421–429. [Google Scholar] [CrossRef]
- Chun, K.W.; Yoo, H.S.; Yoon, J.J.; Park, T.G. Biodegradable PLGA microcarriers for injectable delivery of chondrocytes: Effect of surface modification on cell attachment and function. Biotechnol. Prog. 2004, 20, 1797. [Google Scholar] [CrossRef]
- Holland, T.A.; Tabata, Y.; Mikos, A.G. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release 2005, 101, 111. [Google Scholar] [CrossRef]
- Singh, M.; Morris, C.P.; Ellis, R.J.; Detamore, M.S.; Berkland, C. Microsphere-Based Seamless Scaffolds Containing Macroscopic Gradients of Encapsulated Factors for Tissue Engineering. Tissue Eng. C Methods 2008, 14, 299–309. [Google Scholar] [CrossRef]
- Dormer, N.H.; Singh, M.; Wang, L.; Berkland, C.J.; Detamore, M.S. Osteochondral Interface Tissue Engineering Using Macroscopic Gradients of Bioactive Signals. Ann. Biomed. Eng. 2010, 38, 2167–2182. [Google Scholar] [CrossRef]
- Dormer, N.H.; Busaidy, K.; Berkland, C.J.; Detamore, M.S. Osteochondral Interface Regeneration of Rabbit Mandibular Condyle with Bioactive Signal Gradients. J. Oral. Maxill. Surg. 2011, 69, e50–e57. [Google Scholar] [CrossRef]
- Dormer, N.H.; Singh, M.; Zhao, L.; Mohan, N.; Berkland, C.J.; Detamore, M.S. Osteochondral Interface Regeneration of the Rabbit Knee with Macroscopic Gradients of Bioactive Signals. J. Biomed. Mater. Res. 2012, 100A, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Dormer, N.H.; Gupta, V.; Scurto, A.M.; Berkland, C.J.; Detamore, M.S. Effect of Different Sintering Methods on Bioactivity and Release of Proteins from PLGA Microspheres. Mater. Sci. Eng. C 2013, 33, 4343–4351. [Google Scholar] [CrossRef]
- Goraltchouk, A.; Scanga, V.; Morshead, C.M.; Shoichet, M.S. Incorporation of protein-eluting microspheres into biodegradable nerve guidance channels for controlled release. J. Control. Release 2006, 110, 400. [Google Scholar] [CrossRef]
- Rosner, B.I.; Siegel, R.A.; Grosberg, A.; Tranquillo, R.T. Rational design of contact guiding, neurotrophic matrices for peripheral nerve regeneration. Ann. Biomed. Eng. 2003, 31, 1383. [Google Scholar] [CrossRef]
- Beleño Acosta, B.; Advincula, R.C.; Grande-Tovar, C.D. Chitosan-Based Scaffolds for the Treatment of Myocardial Infarction: A Systematic Review. Molecules 2023, 28, 1920. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S.V. Gelatin based scaffolds for Tissue Engineering: A review. J. Polym. Res. 2015, 9, 15–32. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Remiszewska, E.; Sikorska, W.; Dulnik, J.; Chwojnowski, A. Scaffolds for Cartilage Tissue Engineering from a Blend of Polyethersulfone and Polyurethane Polymers. Molecules 2023, 28, 3195. [Google Scholar] [CrossRef]
- Chierchia, M.; Chirumbolo, S.; Valdenassi, L.; Franzini, M. Ozone-treated poly-ε-caprolactone scaffolds for bone regeneration. Chem. Biol. Interact. 2023, 381, 110509. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Bassand, C.; Freitag, J.; Benabed, L.; Verin, J.; Siepmann, F.; Siepmann, J. PLGA implants for controlled drug release: Impact of the diameter. Eur. J. Pharm. Biopharm. 2022, 177, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; La, W.G.; Kim, B.S. Open macroporous poly(lactic-co-glycolic Acid) microspheres as an injectable scaffold for cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2009, 20, 399–409. [Google Scholar] [CrossRef]
- Patel, M.; Jha, A.; Patel, R. Potential application of PLGA microsphere for tissue engineering. J. Polym. Res. 2021, 28, 214. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- van Apeldoorn, A.A.; van Manen, H.J.; Bezemer, J.M.; de Bruijn, J.D.; van Blitterswijk, C.A.; Otto, C. Raman imaging of PLGA microsphere degradation inside macrophages. J. Am. Chem. Soc. 2004, 126, 13226–13227. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Lazzari, S.; Perale, G.; Masi, M. Mathematical modeling of PLGA microparticles: From polymer degradation to drug release. Mol. Pharm. 2014, 11, 4036–4048. [Google Scholar] [CrossRef]
- Fu, K.; Pack, D.W.; Klibanov, A.M.; Langer, R. Visual Evidence of Acidic Environment within Degrading Poly(lactic-co-glycolic acid) (PLGA) Microspheres. Pharm. Res. 2000, 17, 100–106. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, J.U.; Back, J.H.; Jang, S.W.; Kim, H.J.; Kim, P.S.; Shin, S.; Kim, T. High strength PLGA/Hydroxyapatite composites with tunable surface structure using PLGA direct grafting method for orthopedic implants. Compos. Part B Eng. 2019, 178, 107449. [Google Scholar] [CrossRef]
- Stevanovic, M.; Selakovic, D.; Vasovic, M.; Ljujic, B.; Zivanovic, S.; Papic, M.; Zivanovic, M.; Milivojevic, N.; Mijovic, M.; Tabakovic, S.Z.; et al. Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study. Molecules 2022, 27, 1694. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Ahn, K.M.; Park, M.S.; Lee, J.H.; Choi, C.Y.; Kim, B.S. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J. Biomed. Mater. Res. A 2007, 80, 206–215. [Google Scholar] [CrossRef]
- Son, J.S.; Appleford, M.; Ong, J.L.; Wenke, J.C.; Kim, J.M.; Choi, S.H.; Oh, D.S. Porous hydroxyapatite scaffold with three-dimensional localized drug delivery system using biodegradable microspheres. J. Control. Release 2011, 153, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Radin, S.; Leboy, P.S.; Ducheyne, P. The effect of bioactive glass content on synthesis and bioactivity of composite poly (lactic-co-glycolic acid)/bioactive glass substrate for tissue engineering. Biomaterials 2005, 26, 1935. [Google Scholar] [CrossRef]
- Jaklenec, A.; Hinckfuss, A.; Bilgen, B.; Ciombor, D.M.; Aaron, R.; Mathiowitz, E. Sequential release of bioactive IGF-I and TGF-beta(1) from PLGA microsphere-based scaffolds. Biomaterials 2008, 29, 1518. [Google Scholar] [CrossRef]
- Jaklenec, A.; Wan, E.; Murray, M.E.; Mathiowitz, E. Novel scaffolds fabricated from protein-loaded microspheres for tissue engineering. Biomaterials. 2008, 29, 185. [Google Scholar] [CrossRef]
- Brown, J.L.; Nair, L.S.; Laurencin, C.T. Solvent/non-solvent sintering: A novel route to create porous microsphere scaffolds for tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86B, 396. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules 2008, 9, 1818. [Google Scholar] [CrossRef]
- Wu, L.; Ding, J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2004, 25, 5821. [Google Scholar] [CrossRef]
- Tracy, M.A.; Ward, K.L.; Firouzabadian, L.; Wang, Y.; Dong, N.; Qian, R.; Zhang, Y. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials 1999, 20, 1057. [Google Scholar] [CrossRef] [PubMed]
- Erbetta, C.; Viegas, C.C.B.; Freitas, R.; Sousa, R.G. Synthesis Thermal and Chemical Characterization of the Poly(D,L-lactide-co-glycolide) Copolymer. Polímeros 2010, 21, 376–382. [Google Scholar] [CrossRef][Green Version]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polym. Int. 2004, 54, 36–46. [Google Scholar] [CrossRef]
- Wu, X.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef]
- Schliecker, G.; Schmidt, C.; Fuchs, S.; Wombacher, R.; Kissel, T. Hydrolytic degradation of poly(lactide-co-glycolide) films: Effect of oligomers on degradation rate and crystallinity. Int. J. Pharm. 2003, 266, 39–49. [Google Scholar] [CrossRef]
- Cam, D.; Hyon, S.H.; Ikada, Y. Degradation of high molecular weight poly(L-lactide) in alkaline medium. Biomaterials 1995, 16, 833–843. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhu, R.; Wang, Y.; Li, B.; Ma, Y.; Yin, Y. Synthesis and characterization of serial random and block-copolymers based on lactide and glycolide. Polym. Sci. Ser. B 2016, 58, 720–729. [Google Scholar] [CrossRef]
- Istratov, V.; Gomzyak, V.; Vasnev, V.; Baranov, O.V.; Mezhuev, Y.; Gritskova, I. Branched Amphiphilic Polylactides as a Polymer Matrix Component for Biodegradable Implants. Polymers 2023, 15, 1315. [Google Scholar] [CrossRef]
- de Melo, L.P.; Salmoria, G.V.; Fancello, E.A.; Roesler, C.R.M. Effect of Injection Molding Melt Temperatures on PLGA Craniofacial Plate Properties during In Vitro Degradation. Int. J. Biomater. 2017, 2017, 1256537. [Google Scholar] [CrossRef] [PubMed]

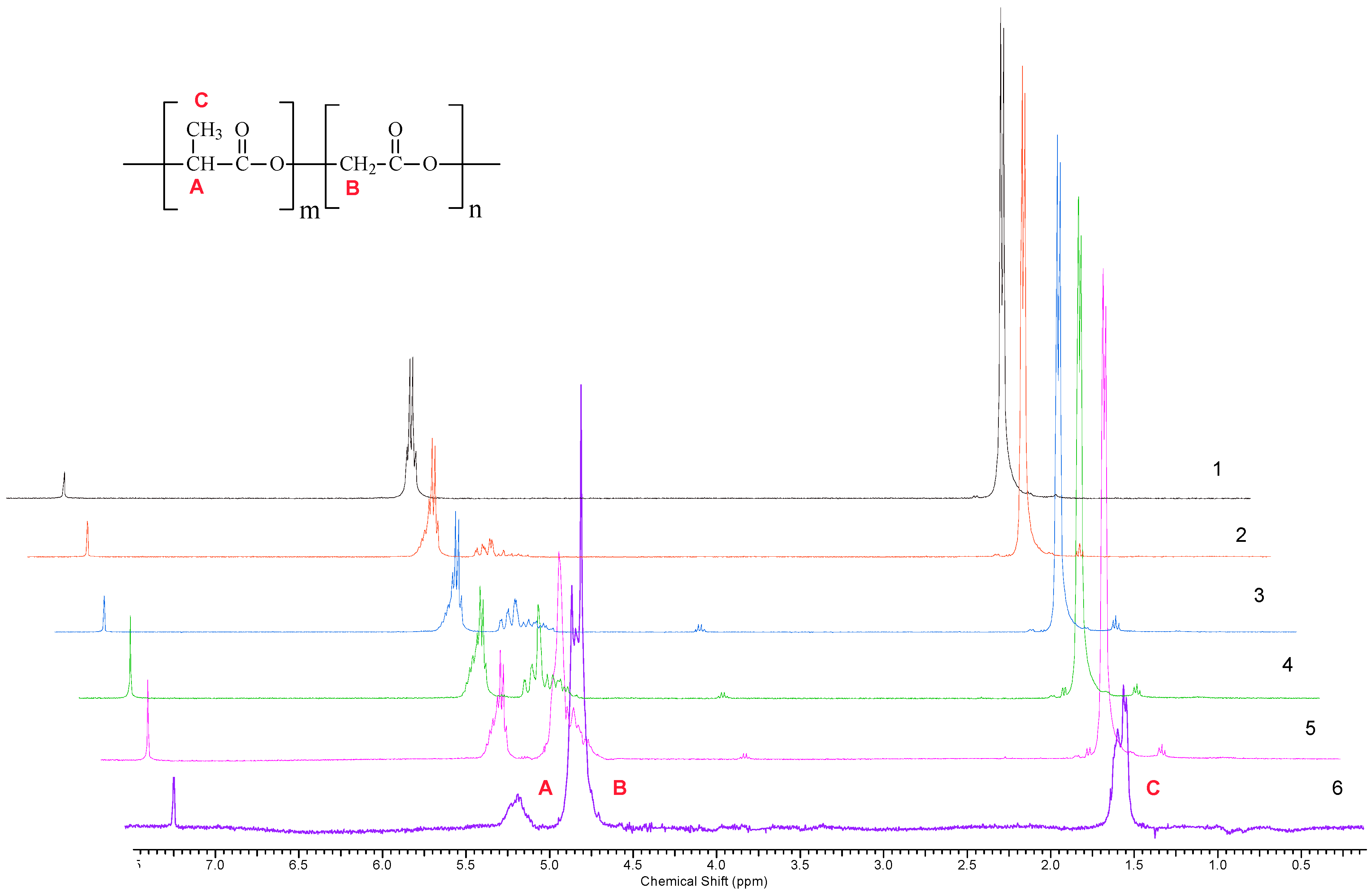


| Polymer | Yield, % | Ratio of Monomers x:y *, mol% | Mn ** | Mw/Mn ** | |
|---|---|---|---|---|---|
| Calculated | Determined | ||||
| 1 | 86 | 100:0 | 100:0 | 28,500 | 1.7 |
| 2 | 88 | 90:10 | 88:12 | 29,000 | 1.7 |
| 3 | 90 | 75:25 | 73:24 | 31,000 | 1.8 |
| 4 | 85 | 70:30 | 68:32 | 33,000 | 1.9 |
| 5 | 89 | 50:50 | 42:58 | - | - |
| 6 | 91 | 25:75 | 23:77 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istratov, V.; Gomzyak, V.; Baranov, O.; Markova, G.; Mezhuev, Y.; Vasnev, V. Preparation and Hydrolytic Degradation of Hydroxyapatite-Filled PLGA Composite Microspheres. J. Compos. Sci. 2023, 7, 346. https://doi.org/10.3390/jcs7090346
Istratov V, Gomzyak V, Baranov O, Markova G, Mezhuev Y, Vasnev V. Preparation and Hydrolytic Degradation of Hydroxyapatite-Filled PLGA Composite Microspheres. Journal of Composites Science. 2023; 7(9):346. https://doi.org/10.3390/jcs7090346
Chicago/Turabian StyleIstratov, Vladislav, Vitaliy Gomzyak, Oleg Baranov, Galy Markova, Yaroslav Mezhuev, and Valerii Vasnev. 2023. "Preparation and Hydrolytic Degradation of Hydroxyapatite-Filled PLGA Composite Microspheres" Journal of Composites Science 7, no. 9: 346. https://doi.org/10.3390/jcs7090346
APA StyleIstratov, V., Gomzyak, V., Baranov, O., Markova, G., Mezhuev, Y., & Vasnev, V. (2023). Preparation and Hydrolytic Degradation of Hydroxyapatite-Filled PLGA Composite Microspheres. Journal of Composites Science, 7(9), 346. https://doi.org/10.3390/jcs7090346








