Solid Lipid Nanoparticles from Platonia insignis Seeds, a Brazilian Amazon Fruit: Characterization, In Vitro and In Vivo Toxicological and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Precipitation of Lipids from Bacuri Seed Butter (BBI)
2.3. Obtaining Methyl Derivatives of the Triacylglycerols from the Bacuri Seed Butter (BBI) Precipitate
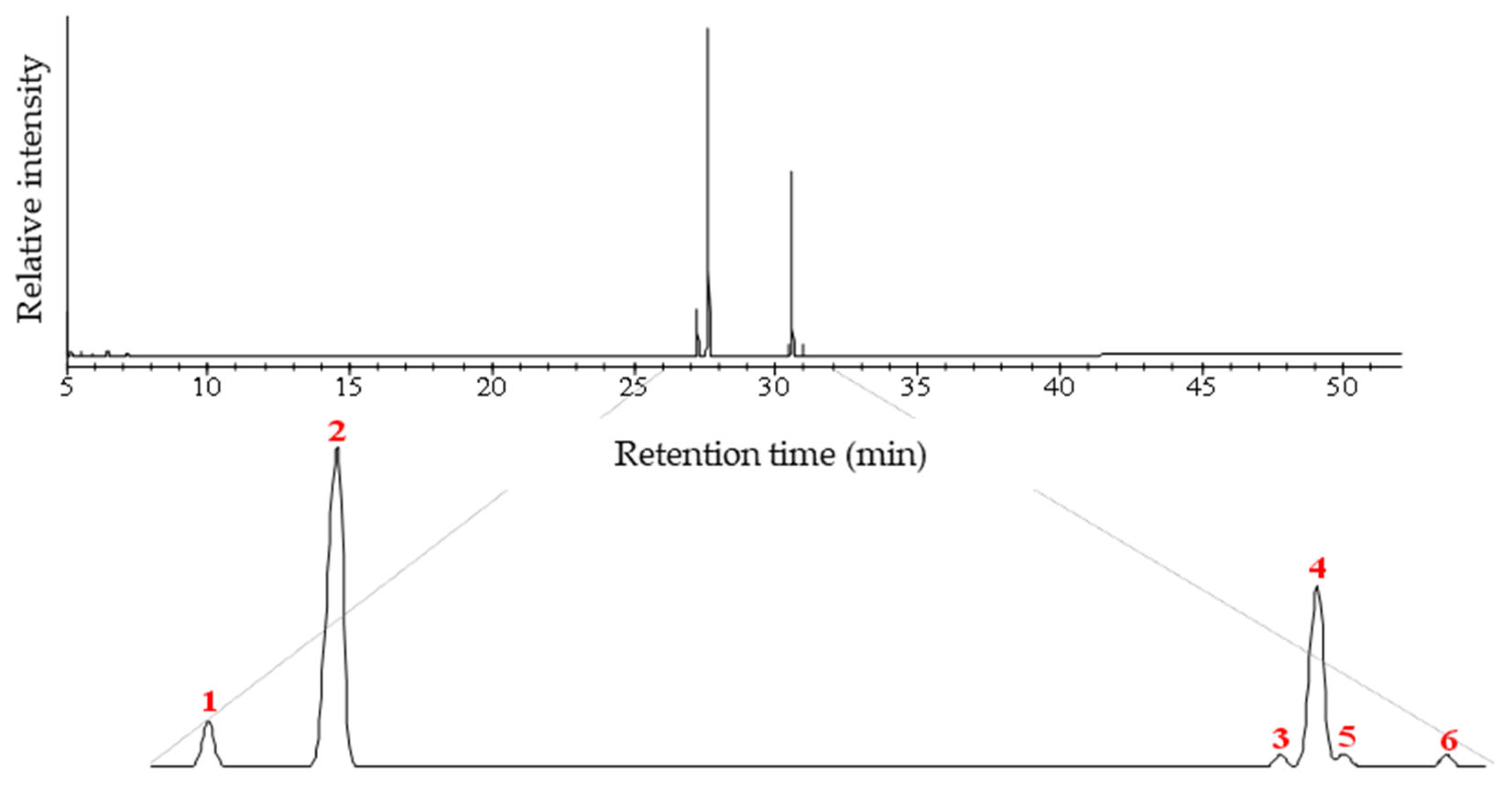
2.4. Analysis of the Methyl Derivatives by Gas Chromatography Coupled with Mass Spectrometer (GC-MS)
2.5. The Production and Characterization of Solid Lipid Nanoparticles (SLNs)
2.6. Storage Stability Study of SLNs
2.7. Raman Spectroscopy
2.8. In vitro Antioxidant Evaluation by Electron Paramagnetic Resonance (EPR) Spectroscopy
2.9. Morphological Analysis of the SLN by Atomic Force Microscopy
2.10. The Cellular Viability of Macrophages by the MTT Test
2.11. The Evaluation of the In Vivo Toxicity and Antioxidant Potential of SLN/TW-1.5 Formulations on Zophobas Morio Larvae
2.11.1. Reduced Non-Protein Sulfhydryl Groups—Reduced Glutathione (GSH)
2.11.2. Nitrite by Griess Method
2.11.3. Superoxide Dismutase (SOD) Enzyme Activity
2.11.4. Myeloperoxidase (MPO) Enzyme Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. The Chemical Composition of the Fatty Acids in Bacuri Seed Butter (BBI)
3.2. The Production and Physicochemical Characterization of Solid Lipid Nanoparticles (SLNs)
3.2.1. Organoleptic Characteristics
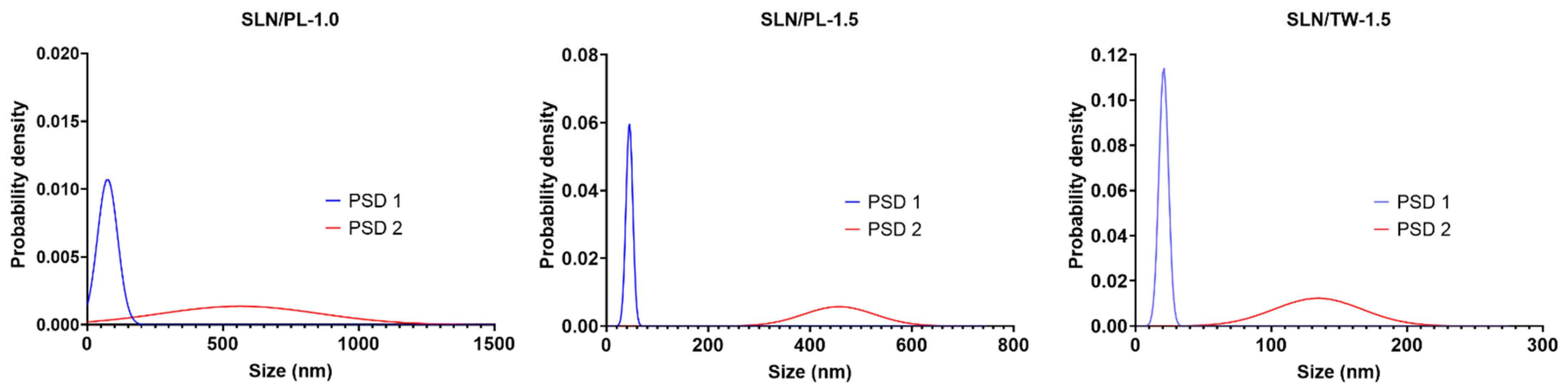
3.2.2. Particle Size, Polydispersity Index and Zeta Potential of SLN
3.3. Stability Study of SLNs
3.3.1. Organoleptic Characteristics
3.3.2. pH Evaluation
3.3.3. Particle Size
3.3.4. Polydispersion Index (PdI)
3.3.5. Zeta Potential (ZP)
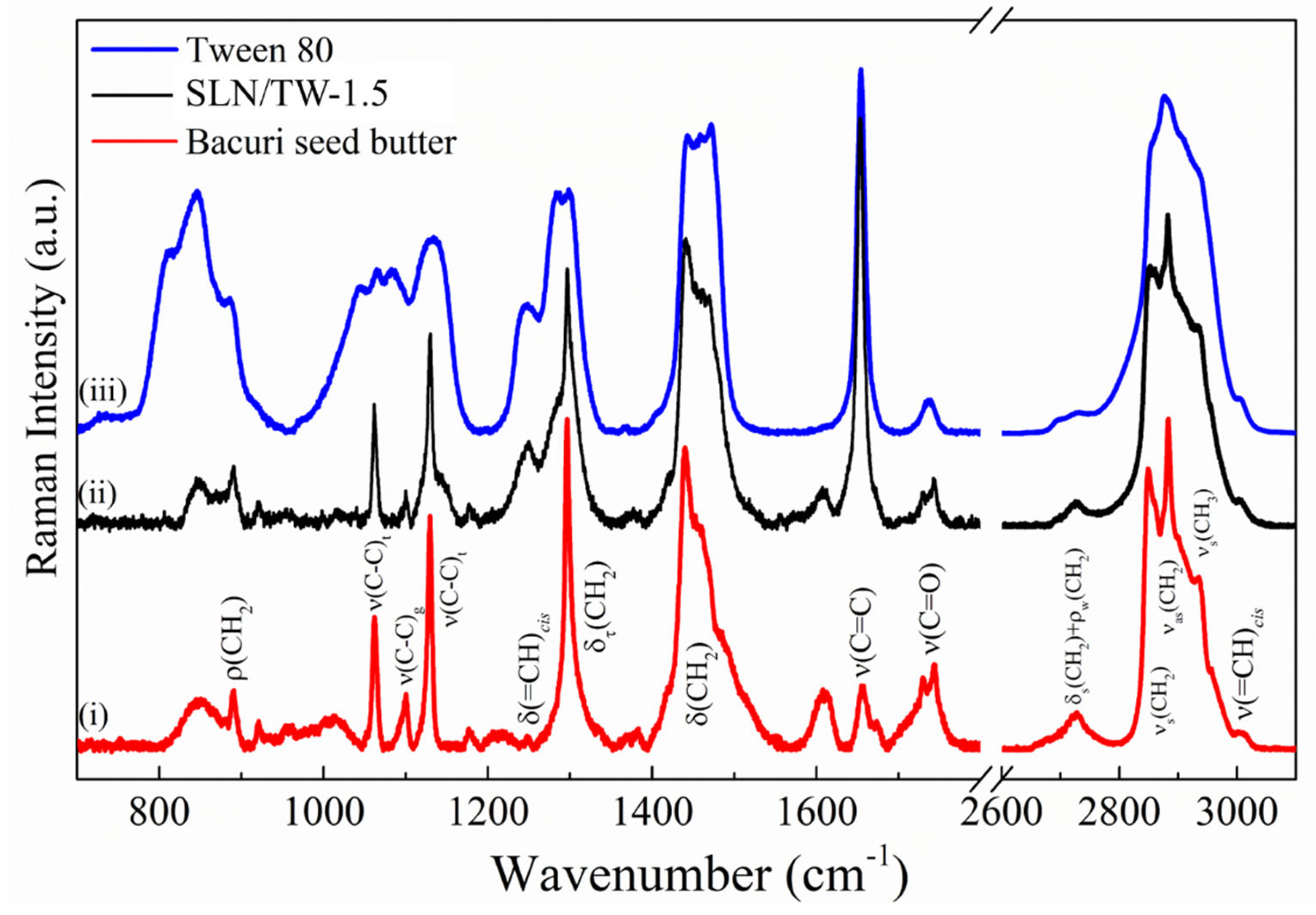
3.4. Raman Spectroscopy
3.5. In Vitro Antioxidant Evaluation by Electron Paramagnetic Resonance Spectroscopy (EPR)
3.6. Morphological Analysis of the SLN/TW-1.5 by Atomic Force Microscopy
3.7. Cytotoxicity Evaluation of Solid Lipid Nanoparticles
3.8. The Evaluation of SLN/TW-1.5 Toxicity in Zophobas Morio Larvae
3.9. The Evaluation of the Antioxidant Activity in Zophobas Morio Larvae
3.9.1. Reduced Non-Protein Sulfhydryl Groups—Reduced Glutathione (GSH)
3.9.2. Nitrite Evaluation by Griess Method
3.9.3. Superoxide Dismutase (SOD) Enzyme Activity
3.9.4. Evaluation of Myeloperoxidase (MPO) Enzyme Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durazzo, A.; Lucarini, M. Editorial: The State of Science and Innovation of Bioactive Research and Applications, Health, and Diseases. Front. Nutr. 2019, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I-Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II-Production Scales and Clinically Compliant Production Methods. Nanomaterials 2020, 10, 455. [Google Scholar] [CrossRef]
- Blanco-Llamero, C.; Fonseca, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Señoráns, F.J.; Souto, E.B. Nutraceuticals and Food-Grade Lipid Nanoparticles: From Natural Sources to a Circular Bioeconomy Approach. Foods 2022, 11, 2318. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Ugazio, E. Lipid nano-and microparticles: An overview of patent- related research. J. Nanomater. 2019, 2019, 2834941. [Google Scholar] [CrossRef]
- Gomes, G.V.L.; Sola, M.R.; Marostegan, L.F.P.; Jange, C.G.; Cazado, C.P.S.; Pinheiro, A.C.; Vicente, A.A.; Pinho, S.C. Physico-chemical stability and in vitro digestibility of beta- carotene-loaded lipid nanoparticles of cupuacu butter (Theobroma grandiflorum) produced by the phase inversion temperature (PIT) method. J. Food Eng. 2017, 192, 93–102. [Google Scholar] [CrossRef]
- Rodrigues Lima, S.K.; Pereira, E.J.d.A.L.; Machado, G.d.O.; Silva, R.A.d.; Lucarini, M.; Durazzo, A.; Diele-Viegas, L.M.; Arcanjo, D.D.R. Systematic Mapping of the Production Chain of “Bacuri” (Platonia insignis Mart.) in Brazil. Sustainability 2022, 14, 15051. [Google Scholar] [CrossRef]
- Lima, S.K.R.; Coêlho, A.G.; Lucarini, M.; Durazzo, A.; Arcanjo, D.D.R. The Platonia insignis Mart. as the Promising Brazilian ‘Amazon Gold’: The State-of-the-Art and Prospects. Agriculture 2022, 12, 1827. [Google Scholar] [CrossRef]
- Jacomino, A.P.; Pinto, P.M.; Gallon, C.Z. Bacuri-Platonia insignis. In Exotic Fruits—Reference Guide; Rodrigues, S., Silva, E.O., Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 1, Chapter 7; pp. 49–52. [Google Scholar] [CrossRef]
- Lima, G.M.; Brito AK, D.S.; de Farias, L.M.; Rodrigues LA, R.L.; Pereira CF, C.; Lima SK, R.; Frota KM, G.; Rizzo MD, S.; Nunes PH, M.; Lucarini, M.; et al. Effects of "Bacuri" Seed Butter (Platonia insignis Mart.) on Metabolic Parameters in Hamsters with Diet-Induced Hypercholesterolemia. Evid. Based Complement. Altern. Med. Ecam 2021, 2021, 5584965. [Google Scholar] [CrossRef]
- Lindoso, J.V.D.S.; Alencar, S.R.; Santos, A.A.D.; Mello Neto, R.S.; Mendes, A.V.D.S.; Furtado, M.M.; Silva, M.G.D.; Brito, A.K.D.S.; Batista, E.K.F.; Baêta, S.A.F.; et al. Effects of ‘’Bacuri’’ Seed Butter (Platonia insignis Mart.), a Brazilian Amazon Fruit, on Oxidative Stress and Diabetes Mellitus-Related Parameters in STZ-Diabetic Rats. Biology 2022, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.C.; Russo, H.M.; Fraige, K.; Zeraik, M.L.; Nogueira, C.R.; da Silva, P.B.; Codo, A.C.; Calixto, G.M.F.; de Medeiros, A.I.; Chorilli, M.; et al. Bioactive Bioflavonoids from Platonia insignis (Bacuri) Residues as Added Value Compounds. J. Braz. Chem. Soc. 2021, 32, 786–799. [Google Scholar] [CrossRef]
- Feitosa, J.M.; Silva, T.S.A.; Xavier, A.E.F.; Rodrigues, W.C.S.; Silva, A.C.E.; Corrêa, C.V.P.; Aguiar, F.S.; Mourão, R.H.V.; Oliveira, E.G.; Nunes, K.M. Evaluation of the quality of Amazonian butters as sustainable raw materials for applications in bioproducts. Rev. De Ciências Farm. Básica E Apl. 2021, 42, e708. [Google Scholar] [CrossRef]
- Costa Júnior, J.S.; Ferraz, A.B.; Sousa, T.O.; Silva, R.A.; De Lima, S.G.; Feitosa, C.M.; Citó, A.M.; Melo Cavalcante, A.A.; Freitas, R.M.; Moura Sperotto, A.R.; et al. Investigation of biological activities of dichloromethane and ethyl acetate fractions of Platonia insignis Mart. seed. Basic Clin. Pharmacol. Toxicol. 2013, 112, 34–41. [Google Scholar] [CrossRef]
- Nascimento, J.L.; Coelho, A.G.; Barros YS, O.; Silva, O.A.; Freitas, R.M.; Rocha, M.S.; David, J.M.; Costa Junior, J.S.; Arcanjo, D.D.R.; Oliveira, R.C.M.; et al. Evaluation of the in vitro antioxidant activity of the hexanic extract of bacuri (Platonia insignis Mart.) seed and its inclusion complex with β-cyclodextrin. Bol. Inf. Geum 2014, 5, 44–53. [Google Scholar]
- Marques Fortes Lustosa, A.; Alves Bezerra, É.; Franca Rodrigues, K.; Amorim, L.; Sousa Lima-Neto, J.; Quirino Araújo, B.; Soares da Costa-Júnior, J.; Nogueira Mendes, A.; Amorim Carvalho, F.; Dias Rufino Arcanjo, D.; et al. Antileishmanial effect of fruit seeds from Platonia insignis against macrophage-internalized amastigote forms of Leishmania amazonensis. Rev. Cuba. Plantas Med. 2018, 23. Available online: https://revplantasmedicinales.sld.cu/index.php/pla/article/view/639 (accessed on 3 August 2023).
- Coêlho, E.S.; Lopes, G.L.N.; Pinheiro, I.M.; Holanda, J.N.P.; Alves, M.M.M.; Carvalho, N.N.; Carvalho, F.A.A.; Carvalho, A.L.M. Emulgel based on amphotericin B and bacuri butter (Platonia insignis Mart.) for the treatment of cutaneous leishmaniasis: Characterization and in vitro assays. Drug Dev. Ind. Pharm. 2018, 44, 1713–1723. [Google Scholar] [CrossRef]
- Bezerra, É.A.; de Alves, M.M.M.; Lima, S.K.R.; Pinheiro, E.E.A.; Amorim, L.V.; Lima Neto, J.d.S.; Carvalho, F.A.d.A.; Citó, A.M.d.G.L.; Arcanjo, D.D.R. Biflavones from Platonia insignis Mart. Flowers Promote In Vitro Antileishmanial and Immunomodulatory Effects against Internalized Amastigote Forms of Leishmania amazonensis. Pathogens 2021, 10, 1166. [Google Scholar] [CrossRef]
- Arcanjo, D.D.; da Costa-Júnior, J.S.; Moura, L.H.; Ferraz, A.B.; Rossatto, R.R.; David, J.M.; Quintans-Júnior, L.J.; Oliveira, R.d.e.C.; Citó, A.M.; de Oliveira, A.P. Garcinielliptone FC, a polyisoprenylated benzophenone from Platonia insignis Mart., promotes vasorelaxant effect on rat mesenteric artery. Nat. Prod. Res. 2014, 28, 923–927. [Google Scholar] [CrossRef]
- Lima Nascimento, J.; Coelho, A.G.; Oliveira Barros, Y.S.; Sousa Oliveira, I.; Vieira da Silva, F.; Custódio Viana, A.F.S.; Araújo, B.Q.; dos Santos Rocha, M.; das Chagas Pereira de Andrade, F.; de Oliveira Barbosa, C.; et al. Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia insignis Seed Extract as a Proposal for a Gastroprotective System. Appl. Sci. 2023, 13, 58. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Thuy, V.N.; Van, T.V.; Dao, A.H.; Lee, B.J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano 2022, 8, 100064. [Google Scholar] [CrossRef]
- Coelho, A.G.; Santos, W.R.P.; Santos, A.A.; Silva, M.G.; Cunha, F.V.M.; Mendes, A.N.; Arcanjo, D.D.R. Plant-Derived Butters as Lipid Nanocarriers: A Systematic and Prospective Review. Recent Pat. Nanotechnol. 2020, 14, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-by-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Araújo, G.L.; Campos, M.A.A.; Valente, M.A.S.; Silva, S.C.T.; França, F.D.; Chaves, M.M.; Tagliati, C.A. Alternative methods in toxicity testing: The current approach. Braz. J. Pharm. Sci. 2014, 50, 55–62. [Google Scholar] [CrossRef]
- Ridolfi, D.M.; Marcato, P.D.; Machado, D.; Silva, R.A.; Justo, G.Z.; Durán, N. In vitro cytotoxicity assays of solid lipid nanoparticles in epithelial and dermal cells. J. Phys. Conf. Ser. 2011, 304, 012032. [Google Scholar] [CrossRef]
- Rodrigues, D.A.; Pereira, G.A.M.; Silva, A.A.; Santos, M.H.; Demuner, A.J.; Oliveira, P.M. Phytochemical profile of pasture weeds in the Brazilian Cerrado. Planta Daninha 2019, 37, e019181108. [Google Scholar] [CrossRef]
- Botelho, M.G.L.; Homma, A.K.O.; Furtado, L.G.; Lima, M.D.C.S.; Costa, M.D.S.S. Productive and market potential of bacuri fruit (Platonia insignis Mart.) in Pará, Brazil. Res. Soc. Dev. 2020, 9, e989975124. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.L.; Dias, D.S.; Lamarão, C.V.; Castelo, K.F.A.; Lima, M.S.; Antonio, A.S.; Converti, A.; Lima, E.S.; Veiga-Junior, V.F. Amazonian Bacuri (Platonia insignis Mart.) Fruit Waste Valorisation Using Response Surface Methodology. Biomolecules 2021, 11, 1767. [Google Scholar] [CrossRef]
- Hartman, L.; Lago, R.C. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract. 1973, 22, 475–476. [Google Scholar]
- Neves, A.R.; Lucio, M.; Martins, S.; Lima, J.L.C.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef]
- Soldati, P.P. Desenvolvimento de Nanopartículas Lipídicas Sólidas Utilizando Manteiga Natural Para Aplicação Tópica. Master’s Thesis, Universidade Federal de Juiz de Fora, Juiz de Fora, MG, Brazil, 2015. Available online: https://repositorio.ufjf.br/jspui/handle/ufjf/1551 (accessed on 4 August 2023).
- Durazzo, A. Study Approach of Antioxidant Properties in Foods: Update and Considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M. A Current Shot and Re-thinking of Antioxidant Research Strategy. Braz. J. Anal. Chem. 2018, 5, 9–11. [Google Scholar] [CrossRef]
- Ferreira-Nunes, R.; Silva, S.M.M.; Souza, P.E.N.; Magalhães, P.O.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Incorporation of Eugenia dysenterica extract in microemulsions preserves stability, antioxidant effect and provides enhanced cutaneous permeation. J. Mol. Liq. 2018, 265, 408–415. [Google Scholar] [CrossRef]
- Arcanjo, D.D.R.; Mafud, A.C.; Vasconcelos, A.G.; Silva-Filho, J.C.; Amaral, M.P.M.; Brito, L.M.; Bemquerer, M.P.; Kückelhaus, S.A.S.; Plácido, A.; Delerue-Matos, C.; et al. In Silico, In Vitro and In Vivo Toxicological Assessment of BPP-BrachyNH2, A Vasoactive Proline-Rich Oligopeptide from Brachycephalus ephippium. Int. J. Pept. Res. Ther. 2017, 23, 323–331. [Google Scholar] [CrossRef]
- Ribeiro, I.M.M.; De Sousa, V.C.; Melo, E.C.S.; Carvalho, R.C.V.; Santos, M.S.; Nery Neto, J.A.O.; Melo, D.S.; Teixeira, L.S.A.; Citó, A.M.G.L.; Moura, A.K.S.; et al. Antileishmania and immunomodulatory potential of cashew nut shell liquid and cardanol. Toxicol. Vitr. 2023, 87, 105524. [Google Scholar] [CrossRef]
- Habeeb, A.F.S.A. Reaction of protein sulfhydryl groups with Ellman’s reagent. In Methods in Enzymology. Enzyme Structure, Part B; Hirs, G.H.W., Timashef, S.N., Eds.; Academic Press: New York, NY, USA, 1972; Volume 25, Chapter 37; pp. 457–464. [Google Scholar]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Moshage, H.; Kok, B.; Huizenga, J.R.; Jansen, P.L.M. Nitrite and Nitrate Determinations in Plasma: A Critical Evaluation. Clin. Chem. 1995, 41 Pt 1, 892–896. [Google Scholar] [CrossRef]
- Romitelli, F.; Santini, S.A.; Chierici, E.; Pitocco, D.; Tavazzi, B.; Amorini, A.M.; Lazzarino, G.; Di Stasio, E. Comparison of nitrite/nitrate concentration in human plasma and serum samples measured by the enzymatic batch Griess assay, ion- pairing HPLC and ion-trap GC-MS: The importance of a correct removal of proteins in the Griess assay. J. Chromatogr B 2007, 851, 257–267. [Google Scholar] [CrossRef]
- Mendes Furtado, M.; Lima Rocha, J.É.; da Silva Mendes, A.V.; Mello Neto, R.S.; Brito, A.K.d.S.; Sena de Almeida, J.O.C.; Rodrigues Queiroz, E.I.; de Sousa França, J.V.; Cunha Sales, A.L.d.C.; Gomes Vasconcelos, A.; et al. Effects of ω-3 PUFA-Rich Oil Supplementation on Cardiovascular Morphology and Aortic Vascular Reactivity of Adult Male Rats Submitted to an Hypercholesterolemic Diet. Biology 2022, 11, 202. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Pavia Donald, L.; Lampman Gary, M.; Kriz George, S.; Vyvyan James, R. Introdução à Espectroscopia; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Muller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Bentes, M.H.S.; Serruya, H.; Rocha, F.G.N.; Godoy, R.L.O.; Cabral, J.A.S.; Maia, J.G.S. Estudo químico das sementes de bacuri. Acta Amaz. 1986, 16, 363–368. [Google Scholar] [CrossRef]
- Lima Rocha, J.É.; Mendes Furtado, M.; Mello Neto, R.S.; da Silva Mendes, A.V.; Brito, A.K.d.S.; Sena de Almeida, J.O.C.; Rodrigues Queiroz, E.I.; de Sousa França, J.V.; Silva Primo, M.G.; Cunha Sales, A.L.d.C.; et al. Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats. Nutrients 2022, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Dinicolantonio, J.J.; O’Keefe, J.H. Effects of dietary fats on blood lipids: A review of direct comparison trials. Open Heart 2018, 5, e000871. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Evaluation of the physical stability of NLS and NLC before and after incorporation into hydrogel formulations. Eur. J. Pharm. Biopharm. 2004, 58, 83–90. [Google Scholar] [CrossRef]
- Müller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (NLS) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Padilha, M.E.S.; Augusto-Ruiz, W. Obtenção de ácidos graxos por cristalização do óleo de pescado fracionado por hidrólise enzimática. Ciência E Tecnol. Aliment. 2010, 30, 35–41. [Google Scholar] [CrossRef]
- Ribeiro, A.P.B.; De Moura, J.M.L.N.; Grimaldi, R.; Gonçalves, L.A.G. Interesterificação química: Alternativa para obtenção de gorduras zero trans. Química Nova 2007, 30, 1295–1300. [Google Scholar] [CrossRef]
- Attama, A.A.; Schicke, B.C.; Paepenmuller, T.; Muller-Goymann, C.C. Solid lipid nanodispersions containing mixed lipid core and a polar heterolipid: Characterization. Eur. J. Pharm. Biopharm. 2007, 67, 48–57. [Google Scholar] [CrossRef]
- Kim, B.; Na, K.; Choi, H. Preparation and characterization of solid lipid nanoparticles (NLS) made of cacao butter and curdlan. Eur. J. Pharm. Sci. 2005, 24, 199–205. [Google Scholar] [CrossRef]
- Jahurul, M.H.; Zaidul, I.S.; Nik Norulaini, N.A.; Sahena, F.; Abedin, M.Z.; Mohamed, A.; Mohd Omar, A.K. Hard cocoa butter replacers from mango seed fat and palm stearin. Food Chem. 2014, 154, 323–329. [Google Scholar] [CrossRef]
- Hielsher, T. Ultrasonic production of nano-size dispersions and emulsions. In Proceedings of the European Nanosystems Conference, Paris, France, 14–16 December 2005; pp. 14–16. Available online: https://hal.science/hal-00166996 (accessed on 3 August 2023).
- Couvreur, P.; Barratt, G.; Fattal, E.; Vauthier, C. Nanocapsule Technology: A Review. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 99–134. [Google Scholar] [CrossRef]
- Lippacher, A.; Muller, R.H.; Mader, K. Semisolid NLS dispersions for topical application: Influence of formulation and production parameters on viscoelastic properties. Eur. J. Pharm. Biopharm. 2002, 53, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Experts Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef]
- Soddu, E.; Rassu, G.; Cossu, M.; Giunchedi, P.; Cerri, G.; Gavini, E. The effect of formulative parameters on the size and physical stability of NLS based on “green” components. Pharm. Dev. Technol. 2016, 21, 98–107. [Google Scholar] [CrossRef]
- Gref, R.; Couvreur, P. Nanocapsules: Preparation, characterization and therapeutic application. In Nanoparticulates as Drug Carriers; Torchilin, V.P., Ed.; Imperial College Press: London, UK, 2006; Chapter 12; pp. 255–276. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Alves, K.L.d.M. Desenvolvimento e Avaliação de Estabilidade Preliminar de Emulsões Cosméticas Utilizando Nanopartículas Lipídicas Sólidas de MURU Muru (Astrocaryum Murumuru) e Ucuúba (Virola Surinamensis). Bachelor’s Dissertation, University of Brasília, Brasília, DF, Brazil, 2018. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Guia de Controle de Qualidade de Produtos Cosméticos, 2nd ed.; Anvisa: Brasília, Brazil, 2008. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/cosmeticos/manuais-e-guias/guia-de-controle-de-qualidade-de-produtos-cosmeticos.pdf/@@download/file (accessed on 4 August 2023).
- Tang, E.S.K.; Huang, M.; Lim, L.Y. Ultrasonication of chitosan and chitosan nanoparticles. Int. J. Pharm. 2003, 265, 103–114. [Google Scholar] [CrossRef]
- Zimmermann, E.; Müller, R.H. Electrolyte- and pH-stabilities of aqueous solid lipid nanoparticles (NLS) dispersions in artificial gastrointestinal media. Eur. J. Pharm. Biopharm. 2001, 52, 203–210. [Google Scholar] [CrossRef]
- Liu, W.; Tian, M.; Kong, Y.; Lu, J.; Li, N.; Han, J. Multilayered vitamin C nanoliposomes by self-assembly of alginate and chitosan: Long- term stability and feasibility application in mandarin juice. LWT–Food Sci. Technol. 2017, 75, 608–615. [Google Scholar] [CrossRef]
- Klang, M.; McLymont, V.N.G.N.; Ng, N. Osmolality, pH, and Compatibility of Selected Oral Liquid Medications With an Enteral Nutrition Product. J. Parenter. Enter. Nutr. 2013, 37, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Villanova, J.C.D.; Sá, V.R. Excipients. Practical Guide for Standardization, 2nd ed; Pharmabooks: São Paulo, Brazil, 2010. [Google Scholar]
- Shtay, R.; Tan, C.P.; Schwarz, K. Development and characterization of solid lipid nanoparticles (NLSs) made of cocoa butter: A factorial design study. J. Food Eng. 2018, 231, 30–41. [Google Scholar] [CrossRef]
- Salminen, H.; Stübler, A.S.; Weiss, J. Preparation, characterization, and physical stability of cocoa butter and tristearin nanoparticles containing β-carotene. Eur. Food Res. Technol. 2020, 246, 599–608. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; Freitas, L.L.; Pohlmann, A.R. Physicochemical characterization and stability of the polymeric nanoparticle systems for drug administration. Química Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- de Sousa, M. Preparação de Nanopartículas Lipídicas Sólidas NLS Para Liberação Modificada/Prolongada de Fármacos Antiretrovirais (Nevirapina, Saquinavir e Efavirenz). Master’s Dissertation, Institute of Chemistry, State University of Campinas, Campinas, SP, Brazil, 2013. Available online: https://hdl.handle.net/20.500.12733/1621741 (accessed on 4 August 2023).
- Meyagusku, V.M. Development and Characterization of Solid Lipid Nanoparticles Containing Ciprofloxacin. Master’s Thesis, Graduate Program in Pharmaceutical Sciences, Paulista State University, Araraquara, SP, Brazil, 2014. Available online: https://www2.fcfar.unesp.br/Home/Pos-graduacao/CienciasFarmaceuticas/vanessa-maria-meyagusku---me.pdf (accessed on 4 August 2023).
- de Oliveira, J.L.; Campos, E.V.; Gonçalves da Silva, C.M.; Pasquoto, T.; Lima, R.; Fraceto, L.F. Solid lipid nanoparticles co-loaded with simazine and atrazine: Preparation, characterization, and evaluation of herbicidal activity. J. Agric. Food Chem. 2015, 63, 422–432. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Da Silva, E.; Bresson, S.; Rousseau, D. Characterization of the three major polymorphic forms and liquid state of tristearin by Raman spectroscopy. Chem. Phys. Lipids 2009, 157, 113–119. [Google Scholar] [CrossRef]
- Akita, C.; Kawaguchi, T.; Kaneko, F. Structural study on polymorphism of cis- unsaturated triacylglycerol: Triolein. J. Phys. Chem. B 2006, 110, 4346–4353. [Google Scholar] [CrossRef]
- Collard, L.; Sinjab, F.; Notingher, I. Raman spectroscopy study of curvature-mediated lipid packing and sorting in single lipid vesicles. Biophys. J. 2019, 117, 1589–1598. [Google Scholar] [CrossRef]
- Víctor-Ortega, M.D.; Martins, R.C.; Gando-Ferreira, L.M.; Quinta-Ferreira, R.M. Recovery of phenolic compounds from wastewaters through micellar enhanced ultrafiltration. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 18–24. [Google Scholar] [CrossRef]
- Yamaguchi, K.K.L.; Pereira, C.V.L.; Lima, E.S.; Veiga Júnior, V.F. Chemistry and pharmacology of bacuri (Platonia insignis). Sci. Amazon. 2014, 3, 39–46. [Google Scholar]
- Silva, A.G.A.; Moreira, R.A.; Sousa, R.P.; MFilho, E.S.; Veras, M.D.A.; Chaves, M.H.; Freitas, S.D.L. Chemical Composition and Photoprotective and Antiradical Activities of the Branches of Platonia Insignis (Clusiaceae). Química Nova 2021, 44, 954–962. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Jorge, A.J.; de Heliodoro, L.G.; Alejandro, Z.C.; Ruth, B.C.; Noé, A.C. The optimization of phenolic compounds extraction from cactus pear (Opuntia ficus-indica) skin in a reflux system using response surface methodology. Asian Pac. J. Trop. Biomed. 2013, 3, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lombardi-Boccia, G.; Santini, A.; Lucarini, M. Dietary Antioxidants and Metabolic Diseases. Int. J. Mol. Sci. 2021, 22, 12558. [Google Scholar] [CrossRef] [PubMed]
- Bidzińska, E. Thermally generated radicals as indicators of the starch modification studied by EPR spectroscopy: A review. Carbohydr. Polym. 2015, 124, 139–149. [Google Scholar] [CrossRef]
- zur Mühlen, A.; zur Mühlen, E.; Niehus, H.; Mehnert, W. Atomic force microscopy studies of solid lipid nanoparticles. Pharm. Res. 1996, 13, 1411–1416. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Mazuryk, J.; Deptuła, T.; Polchi, A.; Gapiński, J.; Giovagnoli, S.; Magini, A.; Emiliani, C.; Kohlbrecher, J.; Patkowski, A. Rapamycin-loaded solid lipid nanoparticles: Morphology and impact of the drug loading on the phase transition between lipid polymorphs. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 54–65. [Google Scholar] [CrossRef]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; de Almeida, M.P.; Pereira, E.; West, P. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Scholer, N.; Hahn, H.; Muller, R.; Liesenfeld, O. Effect of lipid matrix and size of lipid solid nanoparticles (SLN) on the viability and cytokine production of macrophages. Int. J. Pharm. 2002, 231, 167–176. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, 17–71. [Google Scholar] [CrossRef]
- Gordon, S. Pattern Recognition Receptors: Doubling up for the Innate Immune Response. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef]
- Faraji, A.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Louhana, M.R.; Alexandre, C.C.S.; Nilce, V.G.; de Tamara, G.A.; de Fátima, C.E.d.O.P.; Rinaldo, S.A.; Emília, M.A.S.; Nágila, M.P.S.R. Linseed Oil Nanoemulsion with Pluronic® F127 Loaded with Betulinic Acid: Preparation, Rheology, MTT Assay and in vitro Release Kinetics. J. Braz. Chem. Soc. 2022, 33, 1319–1331. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Sobral, M.V. Ensaio de redução do MTT. In Cultivo de Células da Teoria à Bancada; Goncalves, J.C.R., Sobral, M.V., Eds.; Editora UFPB: João Pessoa, Brazil, 2020; Chapter 12; pp. 123–132. Available online: http://www.editora.ufpb.br/sistema/press5/index.php/UFPB/catalog/view/669/839/7054-1 (accessed on 4 August 2023).
- Lustosa, A.K.M.F.; Arcanjo, D.D.R.; Ribeiro, R.G.; Rodrigues, K.A.F.; Passos, F.F.B.; Piauilino, C.A.; Silva-Filho, J.C.; Araújo, B.Q.; Lima-Neto, J.S.; Costa-Júnior, J.S.; et al. Immunomodulatory and toxicological evaluation of the fruit seeds from Platonia insignis, a native species from Brazilian Amazon Rainforest. Rev. Bras. Farmacogn. 2016, 26, 77–82. [Google Scholar] [CrossRef]
- Bruggisser, R.; Von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of Plant Extracts, Phytoestrogens and Antioxidants with the MTT Tetrazolium Assay. Planta Medica 2002, 68, 445–448. [Google Scholar] [CrossRef]
- Henderson, M.I.; Eygeris, Y.; Jozic, A.; Herrera, M.; Sahay, G. Leveraging Biological Buffers for Efficient Messenger RNA Delivery via Lipid Nanoparticles. Mol. Pharm. 2022, 11, 4275–4285. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.I.; Siva-Jothy, M.T. Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera:Tenebrionidae): Cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. London. Ser. B Biol. Sci. 2000, 267, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Reichhart, J.M.; Hetru, C. Innate immunity in higher insects. Curr. Opin. Immunol. 1996, 8, 8–13. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Li, X.; Wang, Y.; Zhang, H. Activities of four enzymes in Galleri a mellonella larvae infected with entomopathogenic nematode Heterorhabditis beicherriana n. sp. Afr. J. Agricltural Res. 2013, 8, 3245–3250. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant Systems in Insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12. [Google Scholar]
- Formariz, T.P.; Urban, M.C.C.; Silva-Júnior, A.A.; Gremião, M.D.; Oliveira, A.G. Microemulsions and liquid crystalline phases as drug delivery systems. Braz. J. Pharm. Sci. 2005, 41, 301–313. [Google Scholar] [CrossRef]
- Gaba, B.; Fazil, M.; Khan, S.; Ali, A.; Baboota, S.; Ali, J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 147–159. [Google Scholar] [CrossRef]
- Zarena, A.S.; Udaya Sankar, K. Supercritical carbon dioxide extraction of xanthones with antioxidant activity from Garcinia mangostana: Characterization by HPLC/LC-ESI-MS. J. Supercrit. Fluids 2009, 49, 330–337. [Google Scholar] [CrossRef]
- Csonka, C.; Páli, T.; Bencsik, P.; Görbe, A.; Ferdinandy, P.; Csont, T. Measurement of NO in biological samples. Br. J. Pharmacol. 2015, 172, 1620–1632. [Google Scholar] [CrossRef]
- Bhatia, S.; Shukla, R.; Madhu, S.V.; Gambhir, J.K.; Prabhu, K.M. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 2003, 36, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Q.; Lewis, E.E.; Tarasco, E. Activity changes of antioxidant and detoxifying enzymes in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae infected by the entomopathogenic nematode Heterorhabditis beicherriana (Rhabditida: Heterorhabditidae). Parasitol. Res. 2016, 115, 4485–4494. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]







| Abbreviation | Surfactant Type | Surfactant Concentration |
|---|---|---|
| SLN/TW-1.5 | Tween 80 | 1.5 |
| SLN/PL-1.0 | Pluronic 127 | 1.0 |
| SLN/PL-1.5 | Pluronic 127 | 1.5 |
| Peak | Chemical Constituents | Molecular Formula | [M+•] | Retention Time (min) | Relative Intensity (%) |
|---|---|---|---|---|---|
| 1 | Methyl (Z)-hexadec-9-enoate | C17H32O2 | 268 | 27.245 | 6.29 |
| 2 | Methyl hexadecanoate (palmitic acid) | C16H32O2 | 270 | 27.635 | 62.08 |
| 3 | (9Z,12Z)-Octadec-9,12-methyl dienoate | C19H34O2 | 294 | 30.470 | 0.86 |
| 4 | Methyl (Z)-octadec-9-enoate (oleic acid) | C18H34O2 | 296 | 30.580 | 28.33 |
| 5 | (E)-octadec-9-methyl acetate | C19H36O2 | 296 | 30.660 | 0.89 |
| 6 | Methyl octadecenoate (stearic acid) | C19H38O2 | 298 | 30.970 | 1.55 |
| Sample | PSD 1 (nm) | Intensity (%) | PSD 2 (nm) | Intensity (%) |
|---|---|---|---|---|
| SLN/PL-1.0 | 75.0 ± 37.2 | 33.0 ± 4.6 | 563.2 ± 292.1 | 67.0 ± 12.8 |
| SLN/PL-1.5 | 44.9 ± 6.7 | 33.7 ± 11.5 | 456.8 ± 69.8 | 66.3 ± 10.3 |
| SLN/TW-1.5 | 20.7 ± 3.5 | 44.0 ± 8.7 | 134.4 ± 32.5 | 56.0 ± 8.7 |
| Scheme | PdI | ZP (mV) |
|---|---|---|
| SLN/PL-1.0 | 0.536 ± 0.017 | −38.37 ± 1.45 |
| SLN/PL-1.5 | 0.5955 ± 0.047 | −24.89 ± 0.70 |
| SLN/TW-1.5 | 0.532 ± 0.036 | −30.25 ± 0.70 |
| Group | Melanized Larval Rate (%) |
|---|---|
| TWEEN 80 | 5 |
| MF | 15 |
| MF 1:2 | 15 |
| MF 1:4 | 5 |
| SLN | 25 |
| SLN 1:2 | 25 |
| SLN 1:4 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coêlho, A.G.; de Almeida, J.O.C.S.; Santos, A.A.d.; Santos, W.R.P.d.; da Rocha Sousa, L.; Viana, N.R.; Batista, F.A.; de Sousa Brito Neta, M.; Santos, A.S.; da Silva, S.W.; et al. Solid Lipid Nanoparticles from Platonia insignis Seeds, a Brazilian Amazon Fruit: Characterization, In Vitro and In Vivo Toxicological and Antioxidant Activities. J. Compos. Sci. 2023, 7, 368. https://doi.org/10.3390/jcs7090368
Coêlho AG, de Almeida JOCS, Santos AAd, Santos WRPd, da Rocha Sousa L, Viana NR, Batista FA, de Sousa Brito Neta M, Santos AS, da Silva SW, et al. Solid Lipid Nanoparticles from Platonia insignis Seeds, a Brazilian Amazon Fruit: Characterization, In Vitro and In Vivo Toxicological and Antioxidant Activities. Journal of Composites Science. 2023; 7(9):368. https://doi.org/10.3390/jcs7090368
Chicago/Turabian StyleCoêlho, Angélica Gomes, José Otávio Carvalho Sena de Almeida, Andressa Amorim dos Santos, Webysten Ronny Pereira dos Santos, Leonardo da Rocha Sousa, Nildomar Ribeiro Viana, Felipe Alves Batista, Maria de Sousa Brito Neta, Alexandre Silva Santos, Sebastião William da Silva, and et al. 2023. "Solid Lipid Nanoparticles from Platonia insignis Seeds, a Brazilian Amazon Fruit: Characterization, In Vitro and In Vivo Toxicological and Antioxidant Activities" Journal of Composites Science 7, no. 9: 368. https://doi.org/10.3390/jcs7090368
APA StyleCoêlho, A. G., de Almeida, J. O. C. S., Santos, A. A. d., Santos, W. R. P. d., da Rocha Sousa, L., Viana, N. R., Batista, F. A., de Sousa Brito Neta, M., Santos, A. S., da Silva, S. W., de Souza, P. E. N., Rodrigues de Araújo-Nobre, A., de Sousa Lima-Neto, J., das Graças Lopes Citó, A. M., de Amorim Carvalho, F. A., de Moraes Alves, M. M., Lucarini, M., Durazzo, A., Nogueira Mendes, A., & Arcanjo, D. D. R. (2023). Solid Lipid Nanoparticles from Platonia insignis Seeds, a Brazilian Amazon Fruit: Characterization, In Vitro and In Vivo Toxicological and Antioxidant Activities. Journal of Composites Science, 7(9), 368. https://doi.org/10.3390/jcs7090368









