Abstract
Joint damage is a major symptom of osteoarthritis, a degenerative disease that worsens over time. The purpose of this review was to assess the effectiveness and safety of nanomaterials as an alternative to the widely used methods. Due to its poor regenerative and self-healing properties, cartilage repair after lesions or debilitating disease is a major clinical issue. Here, we use the organometallic chemistry identity of chondroitin sulphate to repair cartilage lesions by creating a nano-elemental particle through electrostatic interactions. As an integral part of the extracellular matrix, chondroitin sulphate (CS) is shown to improve osteogenesis in this review. The injectability of hydrated cement products was greatly improved by the addition of CS, but there was no discernible change in their phase, morphology, apparent porosity, or compressive strength. This review article provides a thorough analysis of the results from the use of nanocomposites in orthopaedic drug delivery and bone remodelling engineering.
1. Introduction
In recent years, osteoporosis has become more common worldwide, especially among the elderly. Osteoporotic rupture and bone loss affect 20% of people over 50, and bone deficiencies due to decreased bone healing capacity increase with age [1]. Osteoarthritis (OA) is a prevalent ailment of the joints, especially in industrialised nations. The much more prevalent symptoms include pain and functional limitation likely to result from deleterious damage of bone and cartilage. About 27 million Americans have OA, and the disease is estimated to cost society more than $60 billion annually [2]. One of the most difficult aspects of orthopaedic surgery is repairing or replacing broken bones. Bone grafts are the second most common type of tissue transplant, after blood. There are approximately 500,000 annual bone grafting procedures in the United States and approximately 2.2 million practises worldwide, totalling approximately $2.5 billion in annual sales. This method is also used to address developmental defects and severe damage, relieve joint pain, remove malignancies, and replace or reduce the effects of a failed implant [3]. Bone defects caused by large broken bones, trauma, and rare genetic bone diseases, as well as the effects of ageing, can be difficult to repair and reconstruct due to bone’s complex nature [4]. Despite the widespread use of various methods and biomaterials in orthopaedic care over the past few decades, there is still room for advancement in the area of mechanical strength, which is one of the most pressing issues and worries regarding bone substitutes. We are aware that the scaffolds need to promote cell adhesion and tissue development. Well-distributed pores, low toxicity, and satisfactory biocompatibility, as well as a necessary thermostability, notably a suitable mechanical property, are necessary for this purpose in scaffolds [5]. In order to treat the skeletal deficit, chondroitin sulphate and its derived nanoparticles were employed in recent times. One form of hyaluronan found in abundance in mammalian extracellular matrix (ECM) [6], skeletal, cartilaginous, nerve cells, skin, and blood vessels is chondroitin sulphate (CS) [7]. Both the symptomatic potential and the structure-modifying effect of CS have been documented in various clinical trials [8]. CS is a carbohydrate that can be discovered inside the non-collagenous ECM of skeletal remains. Because of its ability to enhance the configuration of some signalling pathways involved in bone regeneration, CS has shown promise in enhancing bone regeneration [9]. Antioxidation, anti-atherosclerosis, anti-coagulation, anti-thrombosis, and low immunogenicity are just some of the biological properties of CS. CS is a nutraceutical used in the treatment of osteoarthritis as well as other joint cartilage syndromes because of its anti-inflammatory effects [10]. However, a chitosan hyaluronan/nano CS tetragonal composite sponge was recently reported as a promising new material for use in wound dressings [11]. CS has been studied as a matrix phase for colon-specific and melanoma drug delivery systems due to its ability to be demeaned by intestine microflora and its reported tumour localisation by sticking with CD44, which is upregulated on the surfaces of different tumour cells [12]. The connective tissues of nearly all animal species contain CS, a geometric natural biological macromolecule. Wound healing, promoting neurite outgrowth, regulating bioactive molecules, adhesion molecules, and cell division are just a few of the many important roles that CS plays in the human body [13]. Cartilage tissue engineering has recently shifted its focus to CS, a glycosaminoglycan present in the ECM of human bone that lacks collagen, and all the literature search related to the present study is shown in Figure 1. Identified to attach expansion factors involved in the bone healing process, CD is a chondroitin amorphous polymer of linear chains, (1,4)-linked supplement and uronic acid residues that show great promise as transdermal tissue engineering or bone grafts [14]. CS exhibit remarkable potential to boost the bioavailability of hydrogels. As a result of its strong electrostatic attraction to tissue factors like platelet-derived growth factor (TGF-), chondroitin sulphate can stimulate mesenchymal stem cell (MSC) differentiation into chondrocytes. Because of its high negative charge density, CS osmotically retains moisture within the tissue matrix, increasing cartilage’s compressive strength [15]. Synovial fluids, hyaline cartilages, and bones are all common locations for CS because of the structural and biophysical roles it plays in these tissues. Since hyaluronic acid is the most well-known GAG [16], its natural abundance in specialised tissues like cartilage or general joints has been the primary focus of research into CS’s bioactivity [17]. The expression of chondrogenic markers, as well as cytotoxicity and the internalisation of cells, were investigated in vitro. The results provide more evidence that thickening in regenerating cartilage [18]. Based on this trait, it seems like CS could be an effective drug carrier. Nevertheless, natural CS is easily and quickly water-soluble. Because of this property, it cannot be used as a conventional dosage form in the human body. CS is still being debated as a viable option for controlled release systems. Self-assembling and capable of forming nanoparticles, many of these chondroitin sulphate derivatives, can be used for sustained release and targeted therapies of bioactive components [19]. Bone remodelling requires distinct and active progenitor cells to synthesise the ECM, that also claims to support the mineralising process. That is why we will get there with the right blend of polyesters and nanomaterials. Nanostructured TiO2 and its nanocomposites show significant bioactivity, biodegradability, and biocompatibility, as demonstrated in our previous study [20]. Two prevalent inflammatory disorders that might worsen over time are rheumatoid arthritis (RA) and periodontal disease (PD). Research indicated that symptoms and indicators suggesting periodontal infection were prevalent among RA patients. Younger patients with more active disease had the most severe issues with biting, chewing, and toothbrushing [21]. Chronic periodontitis (PD), an inflammatory illness with numerous causes, affects the gums, ligaments, and bone which sustain the teeth. The WHO estimates that 20% of the world’s population has PD [22]. Likewise, the rare disease SAPHO affects the skin, bones, and joints and may be multifactorial. The majority of SAPHO cases are young women. Its most common sign is bone issues. About 10% of jaw lesions are osteitic and sclerotic. SAPHO syndrome (Temporomandibular joint) TMJ treatment resembles systemic illness treatment. SAPHO symptoms in the TMJ do not respond well to non-steroid anti-inflammatory drugs because there is not usually inflammation [23].
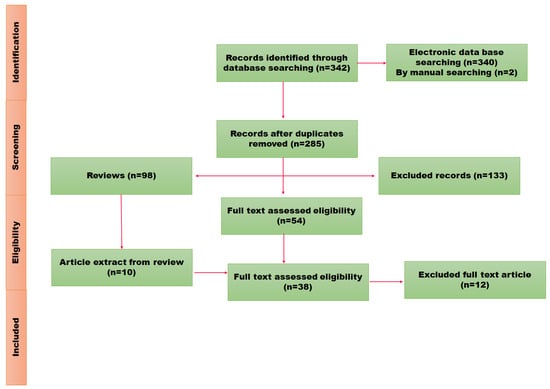
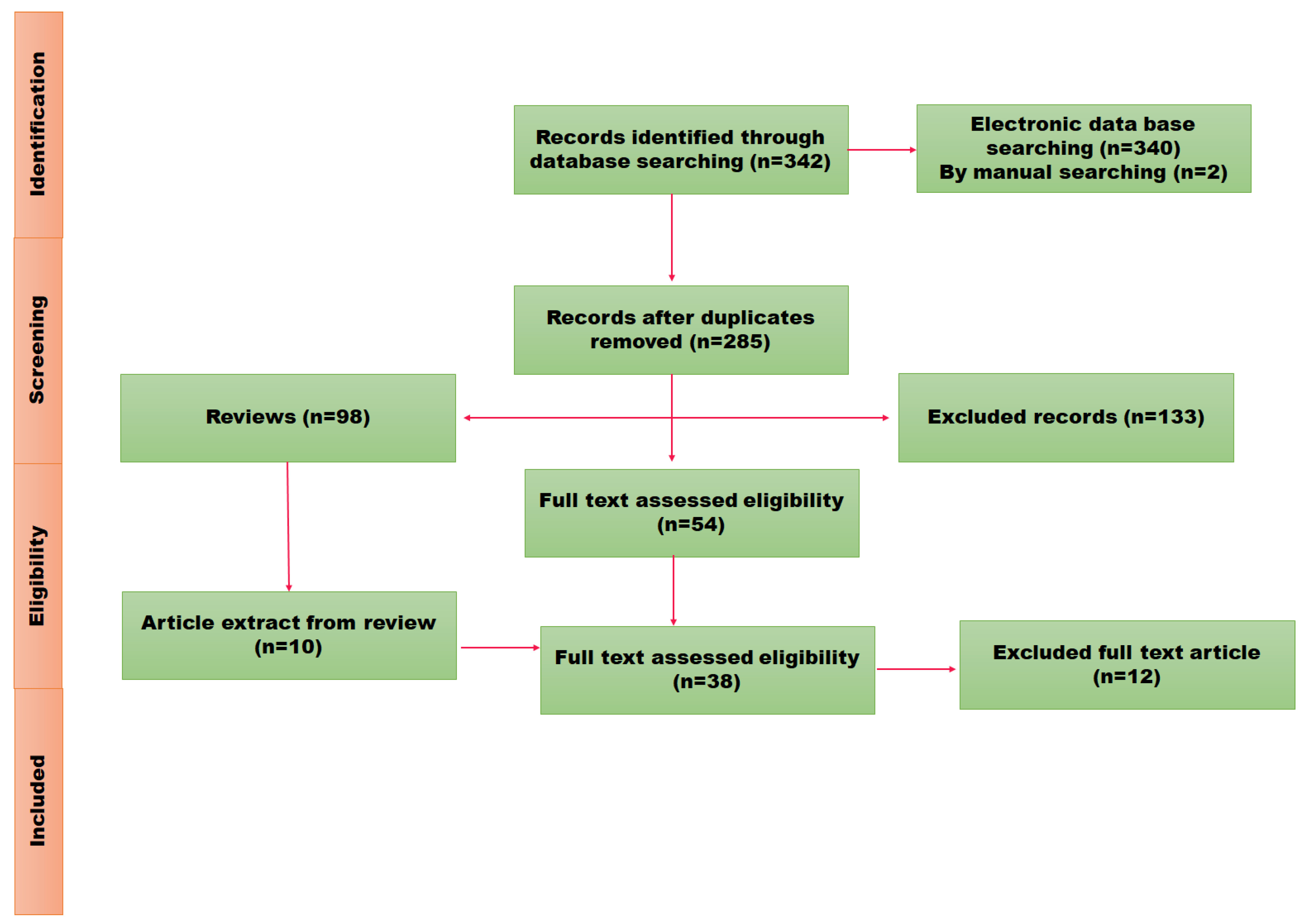
Figure 1.
PRISMA flow representation of schematic review.
Additional reports detailing the fabrication of CS-based amphipathic polymer nanoparticles with drug encapsulation are cited in the aforementioned literature. Following is a discussion of how CS-based nanocarriers transport and release therapeutic organic compounds, nucleic acid drugs, proteins, and peptides [24]. Nano-bioglass was synthesised using a freeze-gelation technique, and it was shown to be both stable and biodegradable biomaterials used in bone tissue regeneration [6]. It is an attempt to restore and mimic natural bone through osteoinduction using nanocomposite materials that are antifungal and toxic-free. Patients with bone conditions like osteoarthritis, osteomyelitis, and bone loss should receive the safest possible therapeutic bone material.
2. Structure of Chondroitin Sulphate
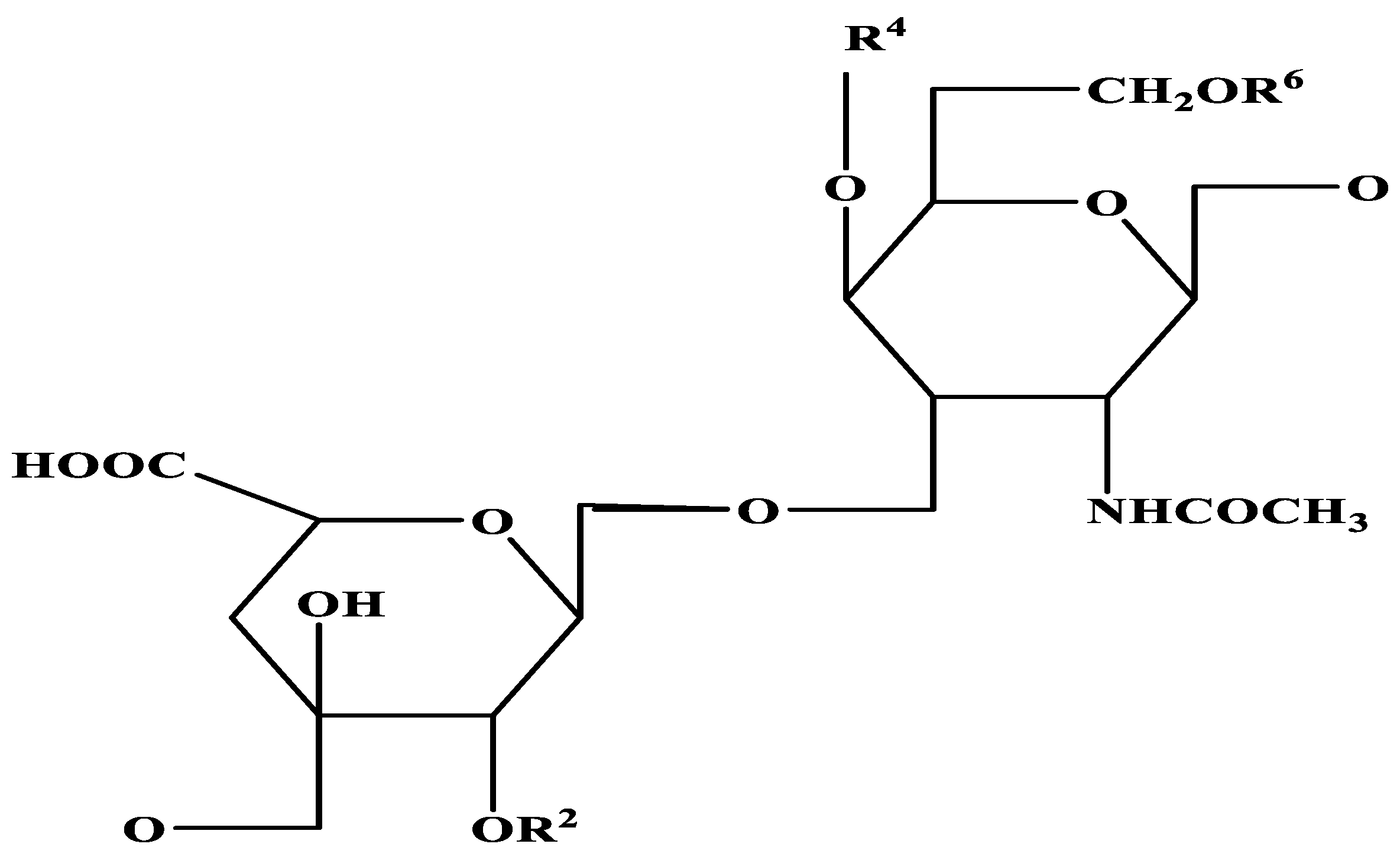
Repeating disaccharide units of glucuronic acid and N-acetyl-D-galactosamine that have been sulphated whether at the 4- or 6-hydroxyl of the galactosamine residues make up chondroitin sulphate (CS). CS consists of a sequence of glucuronic acid (GlcA) and N-acetyl galactosamine (GalNAc) linked by alternating β-(1→4) and β-(1→3) glycosidic bonds (Figure 2) [25]. Different sites of sulphation in both rings give rise to unique units that can coalesce into polymer chains of varying lengths and compositions. How this sulphation affects physical properties like the CS shape and how it plays an influence in deciding biological properties is not well understood [26]. Furthermore, it is puzzling that organisms have not evolved the ability to biosynthesise sulphated hyaluronan, given that it two strands of glucosamine supplements and hyaluronan diverge by only a single stereochemical rearrangement of the 4-hydroxyl [27]. Since CS varies greatly in charge density, sulphation pattern, and molecular weight, this means that the chemical is also very diverse [28].
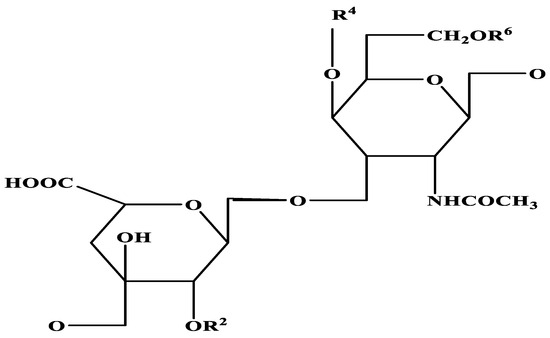
Figure 2.
Structure of chondroitin sulphate [-4GlcAβ1-3GalNAcβ1-]n.
It has recently been reported that chondroitin has potential biological functions apart from being sulphonated by sulphotransferases to produce CS. Cell adhesion and periosteal morphogenesis are two cellular processes affected by osteoarthritis synoviocytes that are also affected by CS. GalNAcT-I is responsible for the transfer of GalNAc residues to the tetrasaccharide complementarity region (GalNAc transferase I). The enzymes GlcAT-II (GlcA transferase II) and GalNAcT-II (GalNAc transferase II) subsequently link GlcA and GalNAc resides in an alternating fashion to form recurrent disaccharide units. The tetrasaccharide-linked CS chain is eventually produced in the body as a proteoglycan and is embedded into the extracellular matrix [29].
3. Chondroitin Sulphate Based Nanoparticle Production
Due to their varied structures and compositions, nanomaterials have enriched many biomedical applications. Nanomaterials have recently been implemented in the study of bone disorders. Biological tumour imaging, crack development detection, arthritis metabolomics sensors, targeted delivery, and complication prevention are just some of the emerging fields of nanomaterials for the diagnoses and treatment of bone-related diseases [30]. Natural-origin polymers like chitosan (CHT) and chondroitin sulphate (CS) are viable alternatives to the organic template because of their structural similarities to the extracellular environment (ECM), chemical versatility, and favourable biocompatibility [31]. Potentially useful for preventing spoilage in refrigerated grass carp, PC encapsulated NPs added to CS bio-films reduced bacterial and oxidation-induced spoilage [32]. Due to the small identity potential of the bone and cartilage tissues, cartilage repair following trauma or degenerative diseases like osteoarthritis (OA) remains a major challenge in modern medicine. Cryo-biomaterials made of marine collagen, chitosan, fucoidan, and chondroitin sulphate are infused with primary human cells for cartilage tissue engineering [33]. Preparation and characterisation of MgCS, a compound containing magnesium and chondroitin sulphate, two chemicals found to be effective in treating OA. It helps by boosting cartilage production and halting its breakdown [34]. It would appear that the delivery system’s design is not particularly straightforward. A collagen-CS-based hydrogel type distribution system was used at the outset of the study because it provides a satisfactory release profile of this bioactive agent, which is necessary to initiate osteogenesis in several clinical applications. Other protein-based drugs that target bone tissue can also be delivered using this system. The mesh worked wonderfully to hold the implant in place and stop it from moving around [35]. In order to speed up the bone healing process, orthopaedic implants were coated with cell surface bone matrix components. Type I collagen coatings on 0.8 mm titanium pins significantly improved bone remodelling during the beginning phases of fracture healing around Ti implants [36]. The Chinese sturgeon (Cart-CS) and the Russian sturgeon (Noto-CS) were used to obtain these particular lipids. Cart-CS had a higher molecular weight than Noto-CS in both sturgeon species, with the highest molecular weight found in Chinese Cart-CS. The spine and notochord of the Russian sturgeon are rich in CS-C and CS-A, and between. Natural biomolecules phenolics and biological biomaterials can be found in high concentrations in sturgeon CS. Future research will elucidate the sturgeon Cart-CS and Noto-additional CS’s functional activities [13]. When compared to a gelatin sponge, the MeCMC/CSS dressing demonstrated superior platelet adhesion. In addition, there was a high level of haemocompatibility and cytocompatibility demonstrated by the Me-CMC/CSS dressing. The findings suggest that composite dressings may be used as quick haemostatic materials [37]. Through enzymatic crosslinking, a biopolymer mimicking ECM component was prepared by incorporating bilayered calcium phosphate (BCP) nanoparticles. These findings suggest that regular member cross-linked CDTA-GTA gel with BCP nanomaterials may be useful for tissue and bone tissue engineering as a means of speeding up the bone repair process [14]. Using itaconylchondroitin sulphate nanogel (ICSNG) as a synergistic agent for reduction and stabilisation, a novel biofunctionalised nanosilver (ICS-Ag) was developed for antibacterial and anti-biofilm applications. By reducing microorganism pathogens and pathogens growth on medical equipment, ICS-Ag film has the potential to significantly enhance patient outcomes and safety [38]. The development of a nano-elemental selenium particle (chondroitin sulphate Aselenium nanoparticle, CSA-SeNP) through the membrane protein identity of Na2SeO3 and opposite charges, chondroitin sulphate A is highly promising as a selenium supplementation preparation for clinical application to tackle the challenge of healing cartilage lesions with exceptional repair effects [39]. Heparan sulphate (HS) and chondroitin sulphate (CS) play critical roles in cartilage development, cartilage development in the zebrafish pharynx, specifically the functions of heparan sulphate (HS) and chondroitin sulphate (CS) proteoglycans. Ext2 and extl3 mutants, predicted to already have defective HS polymerisation, and uxs1 and b3gat3 mutants, estimated to have cognitive impairment biosynthesis of both HS and CS due to damaged establishment of the widely known proteoglycan linkage tetrasaccharide, were examined [40]. Implantable self-crosslinking hybrid Gel-OCS/MBGN biomaterials were developed for bone repair applications. Crosslinking and gelation were both sped up by the addition of MBGNs. In comparison to Gel-CS hydrogels, Gel-OCS/MBGN hydrogels significantly enhanced the propagation and osteogenic difference of BMSCs in vitro and demonstrated efficient bone regeneration in vivo. Since the degradation and gelation behaviour of the hybrid hydrogels can be tuned, and since the hydrogels exhibit favourable mechanical behaviour and osteogenic activity [9]. Using nanostructures with novel properties and exceptional functions has the potential to improve therapeutic efficacy, and this evaluation offers a clear and onward summary of innovative theranostic nanomaterials in bone-related diseases.
3.1. Drug Delivery and Biomedical Application
The biomimetic possibility of inducing CaP precipitation by using multilayers to trap ions has potential for improving the efficiency with which biologically active fibreglass biomaterials are prepared for orthopaedic uses, such as bone tissue engineering [31]. Bone regeneration biomaterial-based structures have been developed thanks to a greater grasp of the specific activities, combined actions, and perhaps even ability to induce effects of growth factors. Bioactive agent-based strategies for modulating cell response may rely on the precise regulation of molecule release via drug delivery systems (Table 1). For example, a biodegradable dosage forms system that allows for the diastolic release of PTH over the course of 21 days offers a promising alternative to the conventional, once-daily injections required to maintain therapeutic levels of this drug [41]. Applying successful methods in the lab to animal models has not always yielded the same positive results. Pilot studies can provide even a basic understanding and the in vivo performance of such techniques, leading to a more streamlined procedure for developing novel, functional tissue engineering solutions. Sulphated polysaccharides have the proper biocompatibility for osteochondral tissue engineering, and the studies discussed here show that they do not trigger any major systemic inflammation when implanted in vivo [42]. To slow the development of osteoarthritis through dual antioxidation, chondroitin sulphate MMP (ChsMA) microspheres grounded with liquiritin (LQ)-loaded liposomes (ChsMA@Lipo) were created. Enzymatic hydrolysis of ChsMA into chondroitin sulphate monomers in vivo can remove reactive oxygen species. Because of its biodegradability and dual antioxidant properties, ChsMA@Lipo holds great promise as a drug delivery platform for the treatment of osteoarthritis [43]. Numerous studies have demonstrated that chitosan’s properties are optimal for use in chondrocytes regeneration and repair when it is mixed with or covalently linked to fibroin, gelatin, collagen, or other safe man-made polymers like PEO and PCL, or when it is used as a polyelectrolyte complex with hyaluronan and chondroitin sulphate [10]. The results of this investigation suggest that a novel biocompatible selenium-chondroitin sulphate (SeCS) may be useful in the treatment of knee cartilage damage (KBD) and osteoarthritis for bone regeneration/repair applications (Figure 3) [44]. Moreover, to aid in wound healing, the developed chitosan-HYA/nCS composite sponge can be subsumed into chitosan-HYA sponge as a transport vehicle for growth factors [45]. Chitosan and hyaluronic acid are used in wound healing, medication transport, tissue engineering, and biomedical device coatings. Besides preventing bacteria from attaching, chitosan may restore injured tissue. Natural hyaluronic acid is found in human skin, joints, and connective tissues. Medical uses include osteoarthritis, ophthalmology, cosmetic surgery, and wound healing. Patient-derived platelet-rich fibrin (PRF) and plasma (PRP) are autologous materials with high platelet concentrations [46]. Several medical specialties use PRF and PRP to speed healing and stimulate tissue regeneration [47]. Cell proliferation, angiogenesis, and tissue repair are promoted by growth factors and bioactives. Wound closure, tissue regrowth, and augmentation surgeries, orthopedics, dentistry, dermatology, and other professions now use them. PRF and PRP can improve wound healing, inflammation, and tissue regeneration when applied topically or intravenously. PRF in oral surgery speeds wound healing, bone regeneration, and extraction socket healing [48]. Although it is possible to bioprint and implant simple connective tissue in animal models, more research and optimisation are needed before complex tissues like cartilage can be printed. Although three-dimensional natural CTE frameworks have been shown to stimulate chondrogenesis in the lab, this success has not yet been transferred to clinical use [49]. Opportunities abound for researchers in chemistry, physics, materials, engineering, physiology, and clinical medicine to work together to find lasting solutions to the pressing problems we have outlined above.
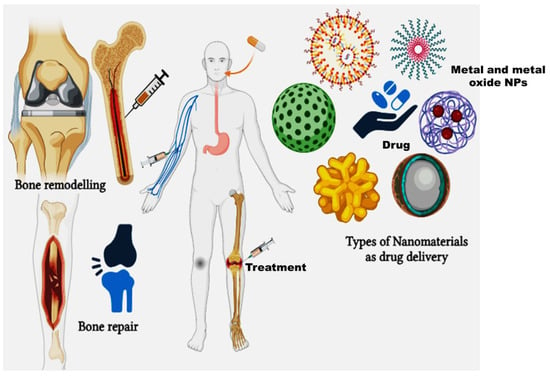
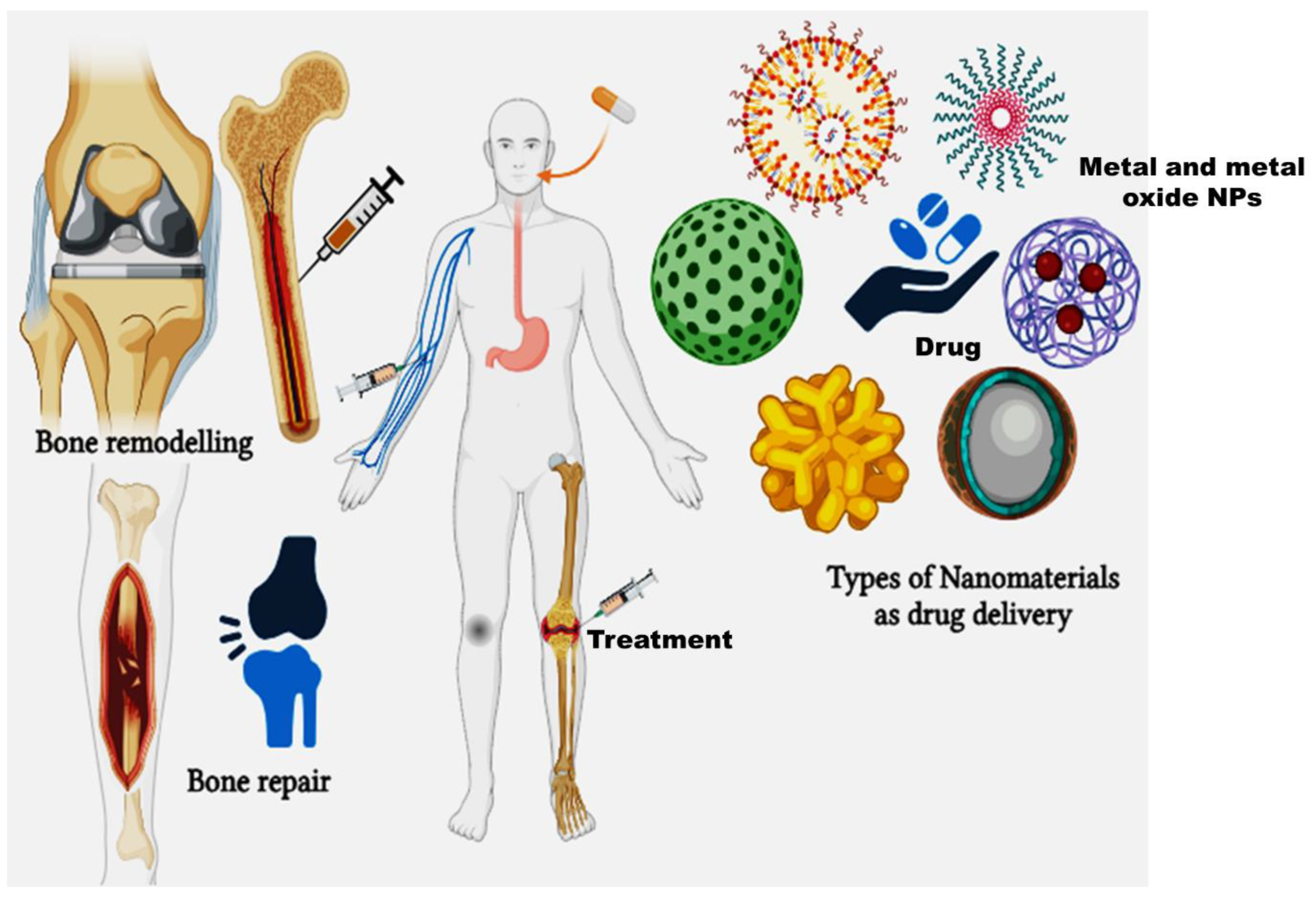
Figure 3.
Types of nanomaterials as drug delivery systems in bone repair and remodelling.
Overall, if the aforementioned obstacles can be overcome, nanomaterials may one day replace many of the conventional drugs used to treat RA [50]. The pros and cons of CS-based nanostructures for the dispatch of biopharmaceuticals, as well as stimuli-sensitive delivery systems like HAase and ROS sensitive nanocarriers for tumor-targeted delivery, are discussed in depth. The manuscript also discusses the use of CS-based tissue engineering in synthetic biology and wound healing [24]. The G10-F@Mc composite scaffold was made by loading CSA microspheres into a 3D printed framework. This scaffold has the chance of growing as a biomaterial scaffold for filling bone defects because of its ability to effectively connect the conversation of cells and considerations in bone tissue microenvironment [51]. The complex formation rather than dual modes of action on pathogenesis of degenerative cartilage may account for improvement in efficacy. These findings demonstrated the therapeutic potential of drug targeting with this ChS moving targets to articular cartilage for the treatment of OA [52]. In search of a more cost-effective and environmentally friendly source for chondroitin sulphate (CS) isolation for potential applications in tissue engineering and the pharmaceutical industry, chicken keel skeletal cartilage was investigated. Use of this technology in nanomedicine to create a reliable transportation vehicle for natural compounds, improve its specificity, and achieve controlled drug release are all potential future obstacles [53]. In general, natural polymers may help autoimmune disease patients repair their bones. They are interesting options for bone regeneration and autoimmune response management due to their flexibility, immunomodulatory effects, biodegradability, resemblance to the extracellular matrix, and biocompatibility. A microporous membrane made from a novel blend of strontium chondroitin sulphate and silk fibroin (SrCS/SF) was created. Thus, it was reasoned that the SrCS/SF membranes developed could serve as bioactive GBR membranes with multiple functions [54].

Table 1.
Types of biomaterials with chondroitin sulphate and its applications.
Table 1.
Types of biomaterials with chondroitin sulphate and its applications.
| S.No | Combination of Biomaterials and Chondroitin Sulphate | Application | References |
|---|---|---|---|
| 1. | Titanium coated type I collagen and chondroitin sulphate | Cellular reaction and New Bone Formation | [55] |
| 2. | Glucosamine and Chondroitin sulphate | Cartilage regeneration microfracture or autologous chondrocyte transplantation | [56] |
| 3. | Carboxymethylated Pullulan/Chondroitin Sulphate Hydrogel | Regenerating cartilage tissue | [57] |
| 4. | N-acetyl glucosamine and chondroitin sulphate | Japanese adults with knee pain and/or stiffness | [58] |
| 5. | Chondroitin sulphate and hyaluronic acid | Clinical trial studies in tendon ruptures | [59] |
| 6. | Bedaquiline fumarate BDQ loaded Chondroitin Sulphate Modified PLGA NPs | Invitro drug release | [60] |
| 7. | Gelatin-chondroitin sulphate/polycaprolactone nanofibrous scaffolds | Chondrogenesis differentiation, cartilage tissue engineering application | [61] |
| 8. | Chondroitin sulphate micro granules embedded with oligochitosan-calcium complexes | Osteoporosis in OVX rats | [62] |
| 9. | photo-cross-linkable methacryloyl groups microgels anchored with liquiritin loaded liposomes | Osteoarthritis treatment | [43] |
| 10. | Extracellular matrices based on collagen and chondroitin sulphate | Bone remodelling and de novo bone formation in vivo | [63] |
| 11. | Silk fibroin/chondroitin sulphate/hyaluronic acid ternary scaffolds | Dermal tissue regeneration | [64] |
| 12. | Hyaluronic acid, chondroitin 6 sulphate and dermatan sulphate | Healing bone regenerate bone in defect model | [65] |
| 13. | Electrospun fiber membranes by LbL-collagen/ chondroitin sulphate nanocoating | Bone repair and regeneration | [66] |
| 14. | Biomimetic synthesis of chondroitin sulphate-analogue hydrogels | Potential bone and cartilage regeneration | [67] |
| 15. | Chondroitin sulphate/poly(vinyl alcohol)/bovine bone powder porous biocomposite | Potential skin tissue and bone tissue engineering | [68] |
3.2. Bone Repair and Remodelling
It is a clinical challenge to repair broken bones triggered by trauma, infection, tumours, or inherited conditions that cause abnormal skeletal development. Spinal fusions and osteolysis-related defects around implants also call for bone regeneration [69]. Osteoclastic resorption is the first step in bone replacement, which is quickly followed by osteoblastic formation. Frost, who coined the term “basic metabolising units”, first described the intimate relationship between resorption and formation within temporary anatomic structures (BMUs) [70]. Because the remodelling system is structured to allow for projects to be prioritised, a limited amount of effector cells can be deployed most efficiently with the most pressing needs, such as the targeted genesis of new BMUs for earlier findings repair [71]. Bone remodelling is primarily carried out by osteoclasts, osteoblasts, and osteocytes. Bone resorption is mediated by osteoclasts. They are associated to myeloid cells and developed from hematopoietic stem cells (Figure 4) [72]. Tissue engineering scaffolds can also be made through photopolymerisation. Hydrogels of this type are typically easy to inject or print. It has been demonstrated that implantable MA-modified HA containing the small molecule drug kartogenin can gel in situ and repair cartilage defects [73]. Mitochondrial content, cellular structure, and matrix remodelling rate are all drastically different between fibrocartilage and subchondral bone. These variations in stress distribution between fibrocartilage and subchondral bone serve to safeguard cartilage in its natural state [74].
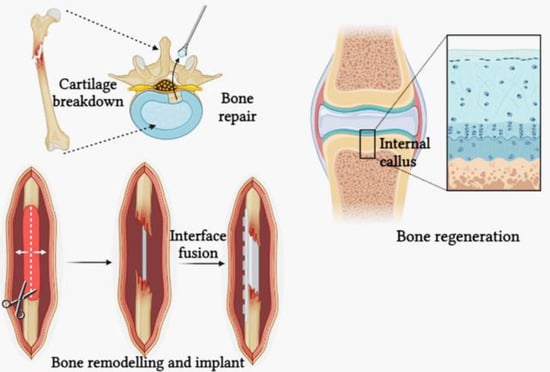
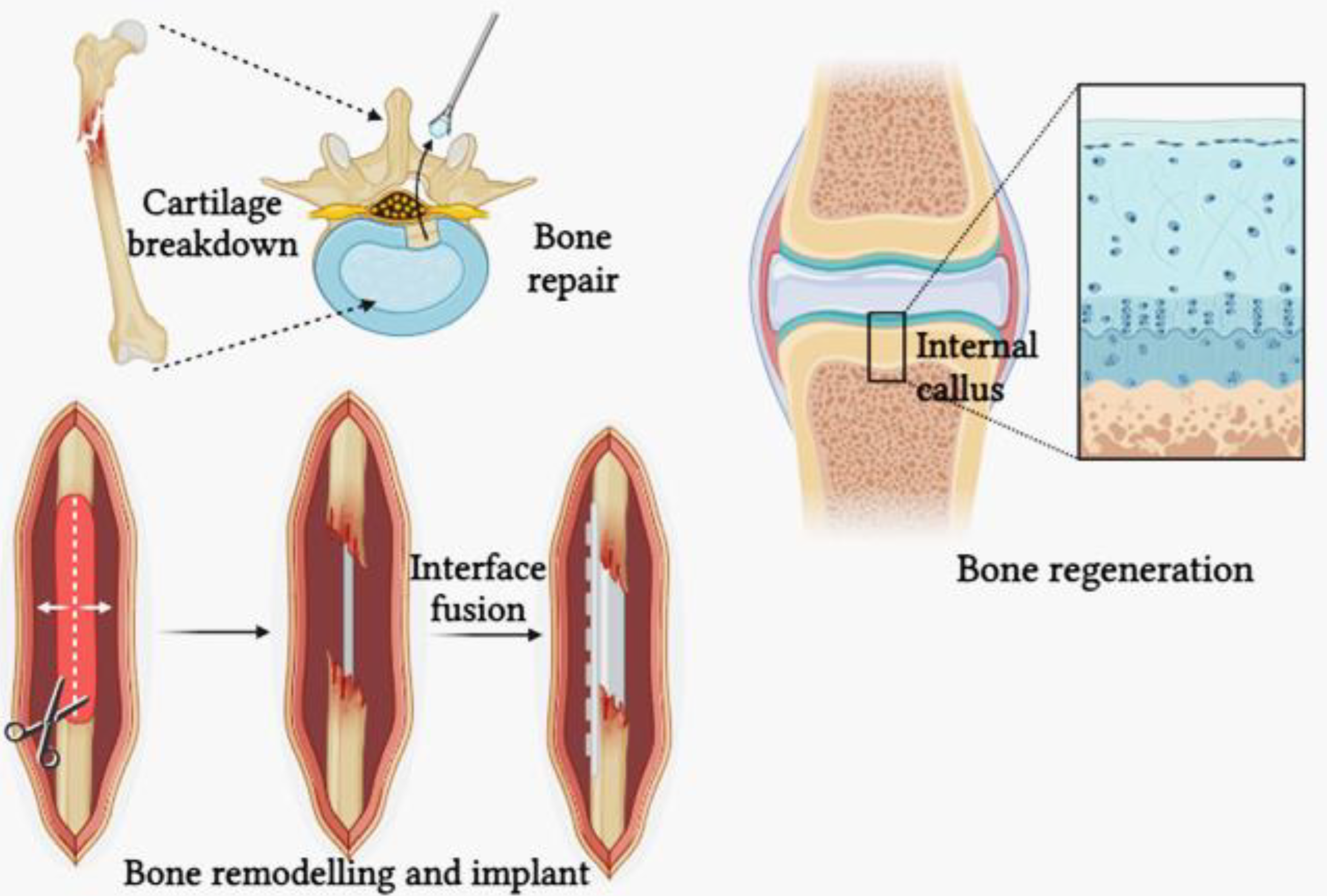
Figure 4.
Bone remodelling and bone repair.
Multiple kinds of bone transplants are accessible for bone repair. Auto grafts are determined to be superior among the others due to their remarkable osteoconductive and osteogenic possibilities. Autografts have >90% effectiveness in bones defect therapy and typically 200.000 transplanted tissue are harvested in the United States alone [75]. For a self-healing structure, bone is notoriously difficult to regrow. Calcium homeostasis and stress and age-related damage repair both necessitate bone remodelling for optimal bone health. Bone is broken down by osteoclasts and rebuilt by osteoblasts. The amount of new bone formed is always exactly the same as the amount of bone resorbed, so any bone that is lost is always replaced [76]. Long bones of critical size do not unite, fusion does not occur, and there are calvarial deficiencies. Given the observation that healthy persons are incapable of self-healing lateral nodules beyond a specific threshold, it is plausible to explore the efficacy of systems for treating minor injuries by conducting experiments on rodents such as rabbits and hamsters. Many fractures can be readily repaired by utilizing sophisticated fail-safe systems that enable the examination of animals of human size through intricate models and state-of-the-art methods [77]. The study examined the accuracy and appropriateness of cone beam computed tomography (CBCT) grayscale values for evaluating radiographic bone mineral density compared to CT Hounsfield units (HU). To convert CBCT grayscale data into gold standard HU, the study determined conversion ratios and a standardised density scale resulted [78]. Bone modelling begins in the foetal stage and continues throughout life, and bone restoration is necessary for the creation and preservation of bone function. In fact, bone restoration is the process by which bone cells adapt their configuration and materials in response to mechanical stimuli. When a bone is broken, it loses its ability to support the body mechanically, prompting the body to take corrective measures [79]. Regenerating new bone and improving its basic mechanical properties through repair is an amazing process. Trauma causes damage to the skeletal and circulatory systems. A fibrin clot forms as part of the inflammatory response that kicks off the healing process after a fracture. The immune system at this point relies on “active” cells. The next step is for mesenchymal stem cells (MSCs) to migrate to the fracture site from their primary locations in the periosteum and bone marrow. Granular ECM is formed by MSCs [80]. One of the key characteristics of bone scaffolds is their capacity to endorse or intensify bone differentiation, a process that ultimately results in the generation of new mineral bone tissue. Bone extracellular matrix (ECM) synthesis and mineralisation are linked to this procedure [81]. Since rat osteogenic repair is higher than human levels, future studies using this model of osteoporosis should concentrate on animals whose bone structure is similar to that of humans. Furthermore, in order to fully understand how bone marrow-mesenchymal stem cells (BM-MSCs) repair osteoporosis, additional research is required [82]. The best way to treat osteoporosis using BM-MSCs in humans requires more clinical trials. Most of the processes involved in primary bone healing occur during bone remodelling. It is only in the later stages of secondary bone healing that the modelling and remodelling processes become active. Secondary bone healing begins with a period of reconstruction of the fracture site through intramembranous and/or endochondral ossification. Bone and cartilage are woven together to create this stability. Then, the bone undergoes a process of modelling and remodelling to restore its original shape by replacing the damaged tissue with healthy lamellar bone [83].
4. Summary
The prevalence of OA and the resulting societal and economic costs are expected to rise over the next five to ten years due to the ageing of the world’s population. Therefore, new safe and effective medications to treat OA must be developed immediately. In humans, CS taken orally is absorbed and moves on to the joint. The most common method of administering CS now is orally, but absorption is significantly affected by the digestive process. Other delivery routes, such as intra-articular immunisation, should be explored to convey elevated numbers of undamaged CS without hydrolysation in order to achieve the desired benefits of CS. In this article, we have summarised chondroitin sulphate and its applications that are believed to mimic the organic and inorganic phases of bone. Polysaccharide-bioceramic composites are discussed in this article as a novel implant material for bone tissue engineering (BTE) applications that is thought to imitate the carbonaceous phases of bone.
5. Future Trends
Prospective medicinal interventions for osteoarthritis (OA) may prioritise the use of chondroprotective medications such as CS, which exhibit both safety and efficacy. In the future, therapy guidelines for osteoarthritis may recommend the use of chondroitin sulphate in combination with other substances such as glucosamine. Quality scores were calculated as a percentage of a perfect 100 by applying the assessment developed by Chalmers et al. (1981) [84] to the trials evaluated by McAlindon et al. The average quality score was 35.5%, with a range of 12.3% to 55.4% [56,84]. Even the gold standard of medicine today has some very undesirable side effects. Reducing the use of nonsteroidal anti-inflammatory drugs (NSAIDs) or analgesics (APMs) in OA treatment is one example of the clinical side effects [2,85]. The biomaterials enhance the performance of conventional biomaterials by leveraging their additional capabilities. Intelligent biomaterials must not only be used in BTE, but also tackle challenges such as microbial biofilm formation, medication resistance, and cancer cell growth. Composites have physicochemical and mechanical properties that are similar to natural bone, and they also have the added benefit of biological activity [86].
6. Pros and Cons of the Study
The development of such systems for bone healing applications must carefully consider the obstacles that come with working with nanomaterials, such as concerns related to toxicity, stability, and scalability. To summarise, chondroitin sulphate’s biocompatibility, effects on cartilage growth and inflammation, and usage with nanomaterials could provide controlled release and improved delivery in bone repair and remodelling. However, there are challenges related to the stability and possible toxicity of nanomaterials that must be taken into account.
Advantages of Chondroitin Sulphate in Bone Repair and Remodeling:
Biocompatibility: Chondroitin sulphate exhibits biocompatibility and is typically well-tolerated by the human body, rendering it acceptable for use in biomedical applications. It has been noted to enhance cartilage regeneration with the synthesis of extracellular matrix components, facilitating the formation and restoration of cartilage [87]. Chondroitin sulphate possesses anti-inflammatory properties, which can be advantageous in diminishing inflammation at the site of injuries or in sick bone structures. It has increased cell proliferation and differentiation capacity to augment the growth and specialisation of osteoblasts, the cells accountable for the creation of bone [88].
Disadvantages of Chondroitin Sulphate in Bone Repair and Remodelling:
Chondroitin sulphate exhibits poor stability and a shorter half-life in the body, necessitating regular administration or adjustments to maintain its sustained efficacy. The efficacy of chondroitin sulphate in bone healing can be influenced by factors such as the source, purity, and molecular weight of the compound [89]. Interactions with other substances or medications may arise, potentially impacting its efficacy or resulting in undesirable effects.
7. Limitations of the Study
Research into the potential of chondroitin sulphate combined with nanomaterials for use in bone remodelling and healing is an exciting area of study. But there are caveats to this field of study that must be noted with any research, they are as follows; Nanomaterials in biological systems may raise issues about their toxicity and compatibility. Nanoparticles and chondroitin sulphate must be tested in vivo to ensure safety. Understanding the long-term effects of these medications is crucial. Nanomaterials may cause unanticipated impacts on bone and adjacent tissues after prolonged exposure [30]. Finding the right dose and concentration of chondroitin sulphate and nanoparticles is essential for bone repair without adverse effects. A high dosage can cause complications, while a low concentration may not be beneficial. These compounds must degrade efficiently without causing byproducts or irritation. To ensure reproducibility and comparability in research efforts, these materials must be synthesised, characterised, and used according to standard protocols [90]. It is crucial to understand how chondroitin sulphate interacts with nanomaterials to affect bone repair and remodelling. These pathways must be well studied to maximise therapeutic efficacy. Though preclinical research is promising, transferring these findings to clinical settings requires human testing and confirmation. Variations in people, immunological reactions, and complex human physiology must be considered. Economic viability and expansion of nanomaterial production for medicinal purposes may be challenging. To enhance accessibility, cost-effective and high-quality production methods are needed [87]. Nanoparticle medical applications must meet safety and effectiveness standards, making regulatory regulations and licensing complicated.
Author Contributions
M.D.: Conceptualization, original draft preparation, methodology and formal analysis. S.V.: Software, validation, data curation, review and editing, visualization. J.C.: Supervision, investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The author Sekar Vijayakumar would like to thank Shandong University for providing the opportunity for a post-doctoral fellowship. Sekar Vijayakumar would also like to thank the China Postdoctoral Science Foundation (2022M721922) for supporting this study.
Conflicts of Interest
The author declares no conflict of interest.
References
- Xu, L.; Ma, F.; Leung, F.K.L.; Qin, C.; Lu, W.W.; Tang, B. Chitosan-strontium chondroitin sulfate scaffolds for reconstruction of bone defects in aged rats. Carbohydr. Polym. 2021, 273, 118532. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Ando, K.; Mimura, T.; Matsusue, Y.; Mori, K. Chondroitin sulfate for the treatment of hip and knee osteoarthritis: Current status and future trends. Life Sci. 2009, 85, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.; Sampath, S.K.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, K.; Venkatachalam, R.; Wang, C.; Valiyaveettil, S.; Ganesan, K. In vitro and preliminary in vivo toxicity screening of high-surface-area TiO2-chondroitin-4-sulfate nanocomposites for bone regeneration application. Colloids Surf. B Biointerfaces 2015, 128, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic mineralized hierarchical hybrid scaffolds based on in situ synthesis of nano-hydroxyapatite/chitosan/chondroitin sulfate/hyaluronic acid for bone tissue engineering. Colloids Surf. B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- Cheng, K.M.; Hung, Y.W.; Chen, C.C.; Liu, C.C.; Young, J.J. Green synthesis of chondroitin sulfate-capped silver nanoparticles: Characterization and surface modification. Carbohydr. Polym. 2014, 110, 195–202. [Google Scholar] [CrossRef]
- Henrotin, Y.; Marty, M.; Mobasheri, A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas 2014, 78, 184–187. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Zhang, F.M.; Jiang, Y.; Cai, M.; Dai, C.; Luo, Y.A.; Tu, L.J.; Zhou, Z.N.; Li, X.J.; et al. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 2021, 6, 890–904. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef]
- Young, J.J.; Cheng, K.M.; Young, Y.A.; Chen, X.A.; Chen, Y.H.; Chang, T.Y.; Yen, H.J.; Chen, C.C. Chondroitin sulfate-stabilized silver nanoparticles: Improved synthesis and their catalytic, antimicrobial, and biocompatible activities. Carbohydr. Res. 2018, 457, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, M.; Wang, J.; Zhai, G. Chondroitin sulfate-based nanocarriers for drug/gene delivery. Carbohydr. Polym. 2015, 133, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Leng, X.; Zhang, Y.; Luo, J.; Du, H.; Takagi, Y.; Dai, Z.; Wei, Q. Comparation of the structural characteristics and biological activities of chondroitin sulfates extracted from notochord and backbone of Chinese sturgeon (Acipenser sinensis). Carbohydr. Res. 2022, 522, 108685. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Dang, L.H.; Nguyen, P.; Pham, T.L.B.; Le, H.K.; Nguyen, M.T.; Nhi, T.T.Y.; Feng, S.; Chen, J.; Tran, N.Q. Dual composition Chondroitin Sulfate and gelatin biomimetic hydrogel based on tyramine crosslinking for tissue regenerative medicine. Eur. Polym. J. 2023, 189, 111975. [Google Scholar] [CrossRef]
- Nair, M.B.; Baranwal, G.; Vijayan, P.; Keyan, K.S.; Jayakumar, R. Composite hydrogel of chitosan-poly(hydroxybutyrate-co-valerate) with chondroitin sulfate nanoparticles for nucleus pulposus tissue engineering. Colloids Surf. B Biointerfaces 2015, 136, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; do Bronze, M.R. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef]
- Cimini, D.; Bedini, E.; Schiraldi, C. Biotechnological advances in the synthesis of modified chondroitin towards novel biomedical applications. Biotechnol. Adv. 2023, 67, 108185. [Google Scholar] [CrossRef]
- Wytrwal, M.; Szmajnta, K.; Kucharski, M.; Nowak, J.; Oclon, E.; Kepczynski, M. Kartogenin-loaded liposomes coated with alkylated chondroitin sulfate for cartilage repair. Int. J. Pharm. 2023, 646, 123436. [Google Scholar] [CrossRef]
- Xi, J.; Zhou, L.; Dai, H. Drug-loaded chondroitin sulfate-based nanogels: Preparation and characterization. Colloids Surf. B Biointerfaces 2012, 100, 107–115. [Google Scholar] [CrossRef]
- Kandiah, K.; Duraisamy, N.; Amirthalingam, V.; Ramasamy, B. Scavenging free radicals and soaring osteoinduction by extra cellular matrix protein–based nanocomposites on degenerative bone treatments. Mater. Sci. Eng. C 2017, 77, 1189–1195. [Google Scholar] [CrossRef]
- Radwan-Oczko, M.; Dus-Ilnicka, I.; Richards, P.; Thomsen, A.M.; Rasmussen, C. Evaluation of oral health status and oral care of patients with rheumatoid arthritis. Int. J. Dent. 2020, 2020, 8896766. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Oczko, M.; Duś-Ilnicka, I.; Richards, P.; Thomsen, A.M.; Rasmussen, C. Rheumatoid arthritis patients’ oral health and disease activity. Int. J. Rheum. Dis. 2019, 22, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Wiȩckiewicz, M.; Paradowska, A.; Kawala, B.; Wiȩckiewicz, W. SAPHO syndrome as a possible cause of masticatory system anomalies—A review of the literature. Adv. Clin. Exp. Med. 2011, 20, 521–525. [Google Scholar]
- Sharma, R.; Kuche, K.; Thakor, P.; Bhavana, V.; Srivastava, S.; Mehra, N.K.; Jain, S. Chondroitin Sulfate: Emerging biomaterial for biopharmaceutical purpose and tissue engineering. Carbohydr. Polym. 2022, 286, 119305. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, A.; Rabbani, S.A.; Neupane, Y.R.; Pandey, S.; Ahmad, A.; Khan, M.A.; Gupta, N.; Madaan, A.; Jaggi, M.; Sandal, N.; et al. Facile functionalization of Teriflunomide-loaded nanoliposomes with Chondroitin sulphate for the treatment of Rheumatoid arthritis. Carbohydr. Polym. 2020, 250, 116926. [Google Scholar] [CrossRef] [PubMed]
- Suganya, P.; Vaseeharan, B.; Vijayakumar, S.; Balan, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer zein-coated gold nanoparticles: Synthesis, antibacterial potential, toxicity and histopathological effects against the Zika virus vector Aedes aegypti. J. Photochem. Photobiol. B Biol. 2017, 173, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Sattelle, B.M.; Shakeri, J.; Roberts, I.S.; Almond, A. A 3D-structural model of unsulfated chondroitin from high-field NMR: 4-sulfation has little effect on backbone conformation. Carbohydr. Res. 2010, 345, 291–302. [Google Scholar] [CrossRef]
- Valcarcel, J.; García, M.R.; Sampayo, L.F.; Vázquez, J.A. Marine chondroitin sulfate of defined molecular weight by enzymatic depolymerization. Carbohydr. Polym. 2020, 229, 115450. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, R.; Chen, J.; Xiong, H.; Wang, Y.; Pang, B.; Du, G.; Kang, Z. Advances and challenges in biotechnological production of chondroitin sulfate and its oligosaccharides. Int. J. Biol. Macromol. 2023, 253, 126551. [Google Scholar] [CrossRef]
- Zheng, K.; Bai, J.; Yang, H.; Xu, Y.; Pan, G.; Wang, H.; Geng, D. Nanomaterial-assisted theranosis of bone diseases. Bioact. Mater. 2023, 24, 263–312. [Google Scholar] [CrossRef]
- Leite, Á.J.; Sher, P.; Mano, J.F. Chitosan/chondroitin sulfate multilayers as supports for calcium phosphate biomineralization. Mater. Lett. 2014, 121, 62–65. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, D.; Dong, J.; Xia, W. Ultrasound-reinforced encapsulation of proanthocyanidin by chitosan-chondroitin sulfate nanosystem. Food Hydrocoll. 2022, 132, 107872. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gelinsky, M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Marine collagen-chitosan-fucoidan/chondroitin sulfate cryo-biomaterials loaded with primary human cells envisaging cartilage tissue engineering. Int. J. Biol. Macromol. 2023, 241, 124510. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, F.; Pang, X.; Tang, B.; Lin, L. Synthesis of chondroitin sulfate magnesium for osteoarthritis treatment. Carbohydr. Polym. 2019, 212, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.S.; Tezcaner, A.; Korkusuz, P.; Korkusuz, F.; Hasirci, V. Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2 delivery system for bone repair. Biomaterials 2005, 26, 4023–4034. [Google Scholar] [CrossRef]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef]
- Wang, S.; Ji, X.; Chen, S.; Zhang, C.; Wang, Y.; Lin, H.; Zhao, L. Study of double-bonded carboxymethyl chitosan/cysteamine-modified chondroitin sulfate composite dressing for hemostatic application. Eur. Polym. J. 2022, 162, 110875. [Google Scholar] [CrossRef]
- Yahya, R.; Alharbi, N.M. Biosynthesized silver nanoparticles-capped chondroitin sulfate nanogel targeting microbial infections and biofilms for biomedical applications. Int. J. Biol. Macromol. 2023, 253, 127080. [Google Scholar] [CrossRef]
- Han, J.; Deng, H.; Li, Y.; Qiao, L.; Jia, H.; Zhang, L.; Wang, L.; Qu, C. Nano-elemental selenium particle developed via supramolecular self-assembly of chondroitin sulfate A and Na2SeO3 to repair cartilage lesions. Carbohydr. Polym. 2023, 316, 121047. [Google Scholar] [CrossRef]
- Holmborn, K.; Habicher, J.; Kasza, Z.; Eriksson, A.S.; Filipek-Gorniok, B.; Gopal, S.; Couchman, J.R.; Ahlberg, P.E.; Wiweger, M.; Spillmann, D.; et al. On the roles and regulation of chondroitin sulfate and heparan sulfate in zebrafish pharyngeal cartilage morphogenesis. J. Biol. Chem. 2012, 287, 33905–33916. [Google Scholar] [CrossRef]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef] [PubMed]
- Dinoro, J.; Maher, M.; Talebian, S.; Jafarkhani, M.; Mehrali, M.; Orive, G.; Foroughi, J.; Lord, M.S.; Dolatshahi-Pirouz, A. Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 2019, 214, 119214. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.; Wang, J.; Yang, X.; Lin, C.; Ge, L.; Ying, C.; Xu, K.; Liu, A.; Wu, L. Chondroitin sulfate microspheres anchored with drug-loaded liposomes play a dual antioxidant role in the treatment of osteoarthritis. Acta Biomater. 2022, 151, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Guo, X.; Lei, Y.; Dennis, B.S.; Wu, S.; Wu, C. Synthesis and characterization of selenium-chondroitin sulfate nanoparticles. Carbohydr. Polym. 2012, 90, 122–126. [Google Scholar] [CrossRef]
- Anisha, B.S.; Sankar, D.; Mohandas, A.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Chitosan-hyaluronan/nano chondroitin sulfate ternary composite sponges for medical use. Carbohydr. Polym. 2013, 92, 1470–1476. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Perez, A.G.M.; Galdames, S.E.M.; Brissac, I.C.S.; Santana, M.H.A. Stabilization of porous chitosan improves the performance of its association with platelet-rich plasma as a composite scaffold. Mater. Sci. Eng. C 2016, 60, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Raeissadat, S.A.; Babaee, M.; Rayegani, S.M.; Hashemi, Z.; Hamidieh, A.A.; Mojgani, P.; Vanda, H.F. An overview of platelet products (PRP, PRGF, PRF, etc.) in the Iranian studies. Futur. Sci. OA 2017, 3, FSO231. [Google Scholar] [CrossRef] [PubMed]
- KhaliliJafarabad, N.; Behnamghader, A.; Khorasani, M.T.; Mozafari, M. Platelet-rich plasma-hyaluronic acid/chondrotin sulfate/carboxymethyl chitosan hydrogel for cartilage regeneration. Biotechnol. Appl. Biochem. 2022, 69, 534–547. [Google Scholar] [CrossRef]
- Thomas, V.; Mercuri, J. In vitro and in vivo Efficacy of Naturally Derived Scaffolds for Cartilage Repair and Regeneration. Acta Biomater. 2023, 171, 1–18. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, T.; Liu, M.; Wang, S.; Liu, S.; Yang, Y.; Yang, Y.; Nan, Y.; Huang, Q.; Ai, K. Rheumatoid arthritis microenvironment insights into treatment effect of nanomaterials. Nano Today 2022, 42, 101358. [Google Scholar] [CrossRef]
- Guo, L.; Chen, H.; Li, Y.; Zhou, J.; Chen, J. Biocompatible scaffolds constructed by chondroitin sulfate microspheres conjugated 3D-printed frameworks for bone repair. Carbohydr. Polym. 2023, 299, 120188. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mishra, S.K.; Vuddanda, P.R.; Singh, S.K.; Singh, R.; Singh, S. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomed. Nanotechnol. Biol. Med. 2014, 10, e1031–e1040. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Baruah, R.; Goyal, A. Physicochemical, antioxidant and biocompatible properties of chondroitin sulphate isolated from chicken keel bone for potential biomedical applications. Carbohydr. Polym. 2017, 159, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fenbo, M.; Xingyu, X.; Bin, T. Strontium chondroitin sulfate/silk fibroin blend membrane containing microporous structure modulates macrophage responses for guided bone regeneration. Carbohydr. Polym. 2019, 213, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Heck, C.; Bernhardt, R.; Bierbaum, S.; Scharnweber, D.; Goebbels, J.; Ziegler, J.; Biewener, A.; Zwipp, H. In vivo effects of coating loaded and unloaded Ti implants with collagen, chondroitin sulfate, and hydroxyapatite in the sheep tibia. J. Orthop. Res. 2007, 25, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- DiNubile, N.A. Glucosamine and chondroitin sulfate in the management of osteoarthritis. Postgrad. Med. 2009, 121, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Yan, D. An Injectable Enzymatically Crosslinked Carboxymethylated Pullulan/Chondroitin Sulfate Hydrogel for Cartilage Tissue Engineering. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Tsuji, T.; Yoon, J.; Kitano, N.; Okura, T.; Tanaka, K. Effects of N-acetyl glucosamine and chondroitin sulfate supplementation on knee pain and self-reported knee function in middle-aged and older Japanese adults: A randomized, double-blind, placebo-controlled trial. Aging Clin. Exp. Res. 2016, 28, 197–205. [Google Scholar] [CrossRef]
- Yudistira, A.; Risantoso, T.; Asmiragani, S.; Basunanda, T.A.; Putera, M.A. Combination of chondroitin sulfate and hyaluronic acid increases amount of fibroblast, collagen and decreases adhesion of achilles tendon after repair. J. Arthrosc. Jt. Surg. 2020, 7, 211–215. [Google Scholar] [CrossRef]
- Mazahir, F.; Sahoo, R.K.; Gupta, U.; Yadav, A.K. Chondroitin sulfate anchored biodegradable nanoparticles: Design, synthesis, and in-vitro anti-tubercular efficacy. Mater. Today Commun. 2023, 34, 105364. [Google Scholar] [CrossRef]
- Sharifi, F.; Irani, S.; Azadegan, G.; Pezeshki-Modaress, M.; Zandi, M.; Saeed, M. Co-electrospun gelatin-chondroitin sulfate/polycaprolactone nanofibrous scaffolds for cartilage tissue engineering. Bioact. Carbohydr. Diet. Fibre 2020, 22, 100215. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Bi, X.; Ji, J.; Wang, L.; Cheng, J. Chondroitin sulfate micro granules embedded with oligochitosan-calcium complexes for potential osteoporosis prevention. J. Funct. Foods 2022, 90, 104984. [Google Scholar] [CrossRef]
- Dudeck, J.; Rehberg, S.; Bernhardt, R.; Schneiders, W.; Zierau, O.; Inderchand, M.; Goebbels, J.; Vollmer, G.; Fratzl, P.; Scharnweber, D.; et al. Increased bone remodelling around titanium implants coated with chondroitin sulfate in ovariectomized rats. Acta Biomater. 2014, 10, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, Q.; Wang, J.; Liu, Y.; Lu, S.; Li, M.; Kaplan, D.L. Silk fibroin/chondroitin sulfate/hyaluronic acid ternary scaffolds for dermal tissue reconstruction. Acta Biomater. 2013, 9, 6771–6782. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, P.; Lagarde, N.; Uguen, A.; Marcorelles, P. Mixture of hyaluronic acid, chondroitin 6 sulphate and dermatan sulphate used to completely regenerate bone in rat critical size defect model. J. Cranio-Maxillofac. Surg. 2012, 40, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Rahman, N.; Li, L.; Zhang, J.; Tan, H.; Xue, Y.; Zhao, Y.; Zhai, J.; Zhao, N.; Xu, F.; et al. Biofunctionalization of electrospun fiber membranes by LbL-collagen/chondroitin sulfate nanocoating followed by mineralization for bone regeneration. Mater. Sci. Eng. C 2021, 128, 112295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhu, Y.; Wang, D.; Li, Y.; Xu, X.; Cai, H.; Chu, H.; Li, J.; Zhang, D. Biomimetic synthesis of chondroitin sulfate-analogue hydrogels for regulating osteogenic and chondrogenic differentiation of bone marrow mesenchymal stem cells. Mater. Sci. Eng. C 2020, 117, 111368. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.T.; Voss, G.T.; Kaplum, V.; Nakamura, C.V.; Wilhelm, E.A.; Luchese, C.; Fajardo, A.R. Development, characterization and biocompatibility of chondroitin sulfate/poly(vinyl alcohol)/bovine bone powder porous biocomposite. Mater. Sci. Eng. C 2017, 72, 526–535. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Brown, A.; Zaky, S.; Ray, H.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543–553. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.; Wang, W.; Zhang, Y.; Wan, P.; Yang, K.; Han, Y. Evaluation of the osteo-inductive potential of hollow three-dimensional magnesium-strontium substitutes for the bone grafting application. Mater. Sci. Eng. C 2017, 73, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Song, J.H.; Li, S.; Zhu, Y.N.; Liu, M.Y.; Wan, M.C.; Mu, Z.; Tay, F.R.; Niu, L.N. Advances in materials-based therapeutic strategies against osteoporosis. Biomaterials 2023, 296, 122066. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L.; et al. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, S.; Chen, W.; Hu, Y.; Geng, Z.; Su, J. Bone/cartilage targeted hydrogel: Strategies and applications. Bioact. Mater. 2023, 23, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Malliappan, S.P.; Yetisgin, A.A.; Sahin, S.B.; Demir, E.; Cetinel, S. Bone tissue engineering: Anionic polysaccharides as promising scaffolds. Carbohydr. Polym. 2022, 283, 119142. [Google Scholar] [CrossRef]
- Henriksen, K.; Neutzsky-Wulff, A.V.; Bonewald, L.F.; Karsdal, M.A. Local communication on and within bone controls bone remodeling. Bone 2009, 44, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H. Advances in regenerative orthopedics. Mayo Clin. Proc. 2013, 88, 1323–1339. [Google Scholar] [CrossRef]
- Gaur, A.; Dhillon, M.; Puri, N.; Ahuja, U.S.; Rathore, A. Questionable accuracy of CBCT in determining bone density: A comparative CBCT–CT in vitro study. Dent. Med. Probl. 2022, 59, 413–419. [Google Scholar] [CrossRef]
- Parfitt, A.M. Targeted and nontargeted bone remodeling: Relationship to basic multicellular unit origination and progression. Bone 2002, 30, 5–7. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Li, F.; Pan, Z.; Ni, X.; Shen, Y.; Xu, H.; Huang, Q. Bioactive calcium sulfate/magnesium phosphate cement for bone substitute applications. Mater. Sci. Eng. C 2014, 35, 70–76. [Google Scholar] [CrossRef]
- Rayat Pisheh, H.; Ansari, M.; Eslami, H. How is mechanobiology involved in bone regenerative medicine? Tissue Cell 2022, 76, 101821. [Google Scholar] [CrossRef] [PubMed]
- Soliman, T.; Ali, Z.; Zayed, M.; Sabry, D.; Abubakr, N. Assessing the bone-healing potential of bone marrow mesenchymal stem cells in jawbone osteoporosis in albino rats. Dent. Med. Probl. 2022, 59, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Burr, D.B. Bone Modeling and Remodeling. In Basic and Applied Bone Biology; Academic Press: Cambridge, MA, USA, 2014; pp. 75–90. [Google Scholar] [CrossRef]
- Chalmers, T.C.; Smith, H.; Blackburn, B.; Silverman, B.; Schroeder, B.; Reitman, D.; Ambroz, A. A method for assessing the quality of a randomized control trial. Control. Clin. Trials 1981, 2, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, S.; Sterchi, R.; Scherer, M.; Trelle, S.; Bürgi, E.; Bürgi, U.; Dieppe, P.A.; Jüni, P. Meta-analysis: Chondroitin for osteoarthritis of the knee or hip. Ann. Intern. Med. 2007, 146, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.M.; Yetisgin, A.A.; Demir, E.; Sahin, S.B.; Cetinel, S. Polysaccharide-bioceramic composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2023, 250, 126237. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Xu, L.; Tang, J.; Wang, Q.; Lim, K.S.; Hooper, G.; Woodfield, T.B.F.; Liu, G.; Tian, K.; Zhang, W.; et al. The advances in nanomedicine for bone and cartilage repair. J. Nanobiotechnol. 2022, 20, 141. [Google Scholar] [CrossRef]
- Shen, Q.; Guo, Y.; Wang, K.; Zhang, C.; Ma, Y. A Review of Chondroitin Sulfate’s Preparation, Properties, Functions, and Applications. Molecules 2023, 28, 7093. [Google Scholar] [CrossRef]
- Wen, J.; Li, H.; Dai, H.; Hua, S.; Long, X.; Li, H.; Ivanovski, S.; Xu, C. Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater. Today Bio 2023, 19, 100597. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, F.; Cao, J.; Dou, Q.; Wang, J.; Wang, J.; Yang, L.; Chen, W. Research advances of nanomaterials for the acceleration of fracture healing. Bioact. Mater. 2024, 31, 368–394. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).