Effect of Sintering Temperature on the Physical and Mechanical Characteristics of Fabricated ZrO2–Cr–Ni–Ce–Y Composite
Abstract
:1. Introduction
2. Materials and Methods
3. Characterization and Testing Technique
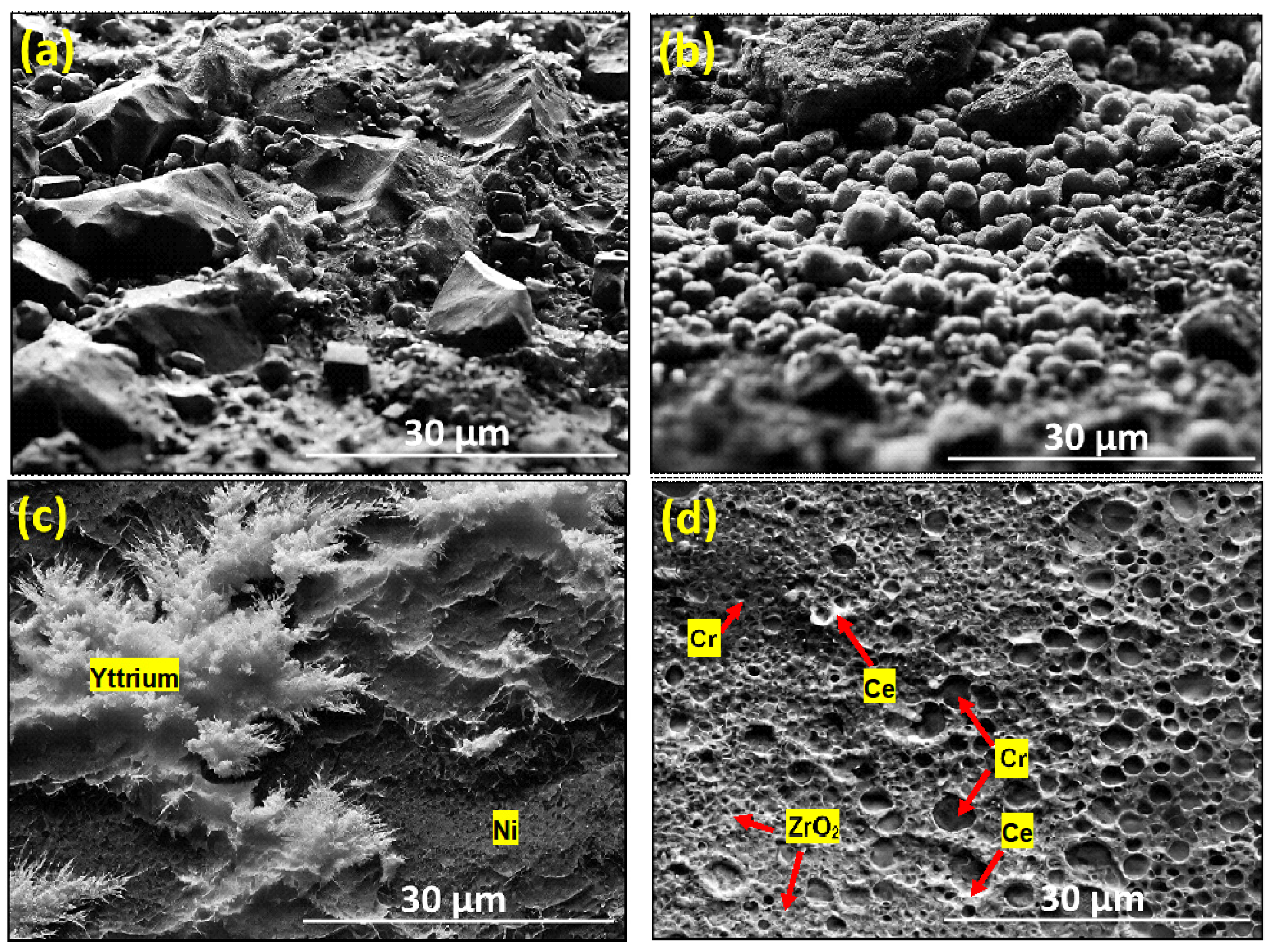
3.1. SEM Analysis
3.2. Measurement of Density
- ρex: density of sintered sample;
- Wa: weight in air;
- Ww: weight in water;
3.3. Mechanical Testing
4. Results and Discussion
4.1. Phase Analysis and Microstructural Features of ZrO2 Metal Composite
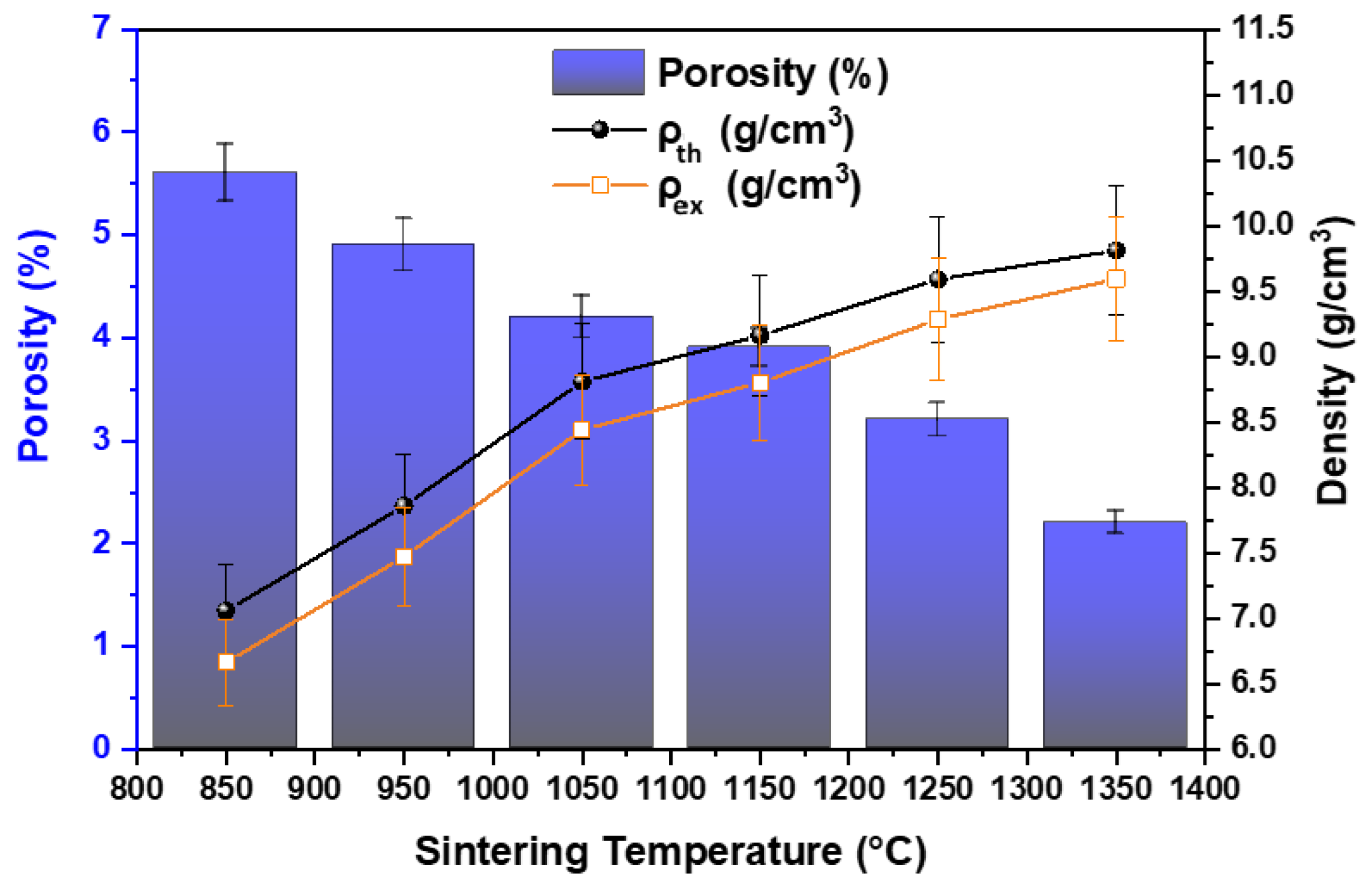
4.2. Variation in Density and Porosity of Composite with Sintering Temperature
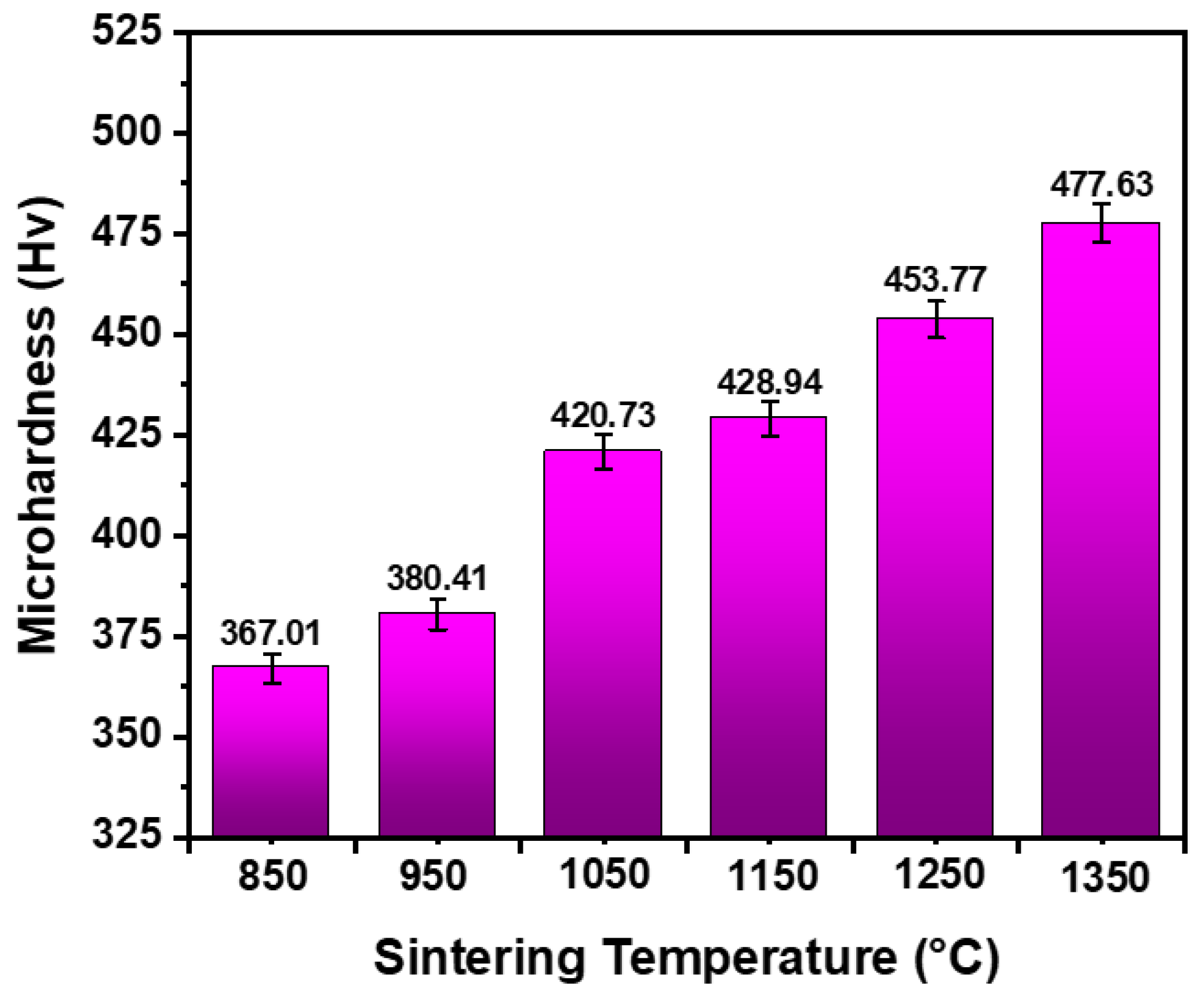
4.3. Hardness
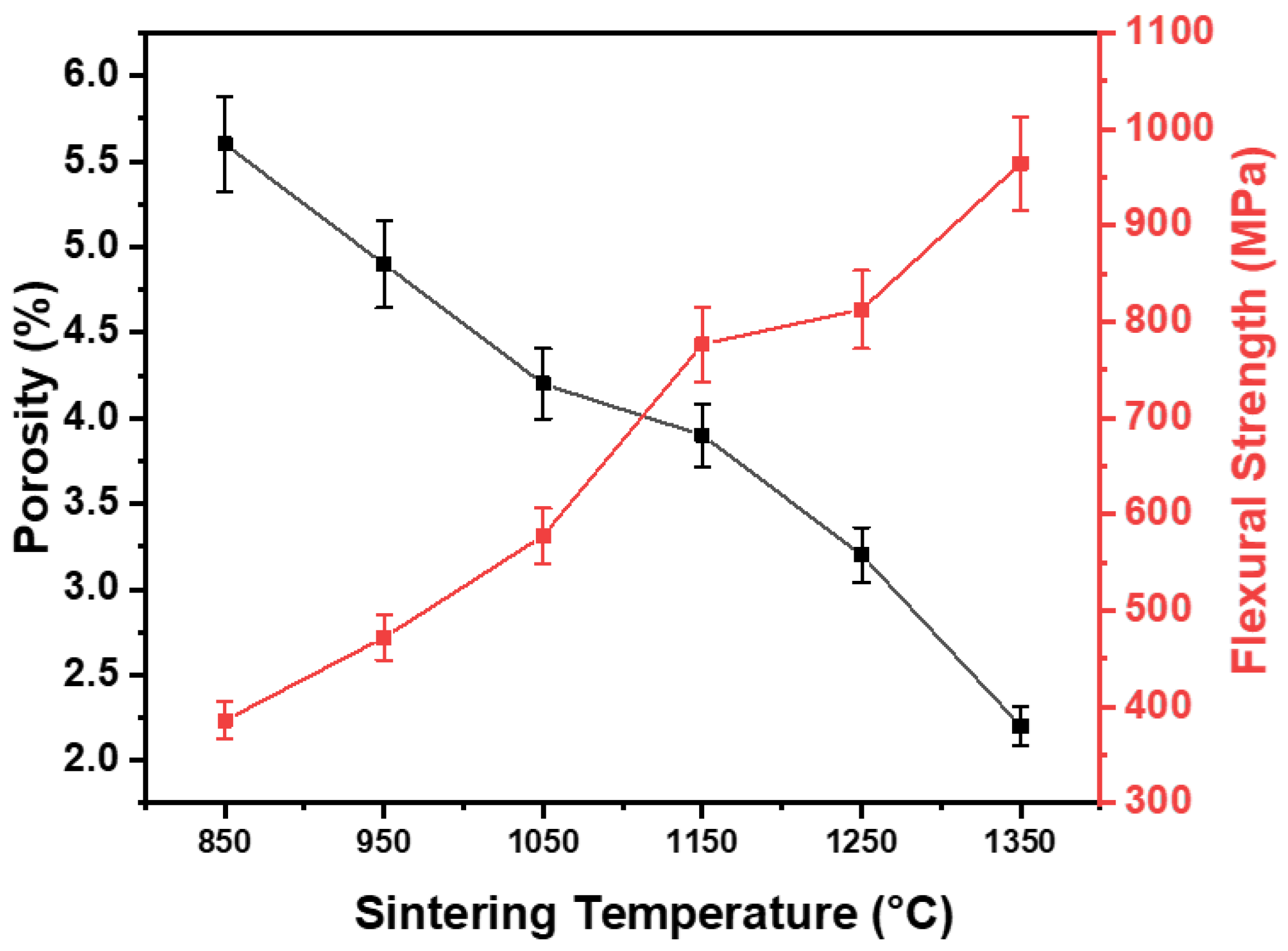
4.4. Flexural Strength
5. Conclusions
- The SEM micrographs depict a homogeneous distribution of ZrO2, Cr, Ni, Ce, and Y phases, indicating the effective fabrication of the composite.
- At lower sintering temperatures (850 °C to 950 °C), the microstructure features stone-shaped structures due to slow fusion rates, which evolve into smaller forms between 1050 °C and 1150 °C.
- The microstructure of the composite has been refined from a coarse, stone-shaped structure at lower temperatures to finer, layered formations at higher temperatures, culminating in a dense, compact structure at 1350 °C.
- The density of the composite increased with temperature from 6.7 gm/cm3 at 850 °C to 9.7 gm/cm3 at 1350 °C, primarily due to filling of pores by liquid Ce phase.
- The micro-hardness was found to be 367 HV at 850 °C and 478 HV at 1350 °C. This is due to the solid solution strengthening and the grain refinement.
- The flexural strength steadily increased with temperature, from a value of 386 MPa at 850 °C and reaching a peak value of 964 MPa at 1350 °C, demonstrating the composite’s potential for high-performance applications.
- The addition of cerium (Ce) and yttrium (Y) played a crucial role in enhancing the mechanical properties of the composite through solid-solution strengthening and densification.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cho, J.; Li, J.; Wang, H.; Li, Q.; Fan, Z.; Mukherjee, A.K.; Rheinheimer, W.; Wang, H.; Zhang, X. Study of deformation mechanisms in flash-sintered yttria-stabilized zirconia by in-situ micromechanical testing at elevated temperatures. Mater. Res. Lett. 2019, 7, 194–202. [Google Scholar] [CrossRef]
- Li, J.; Hao, Z.; Shu, Y.; He, J. Fabrication of spherical Ti–6Al–4V powder for additive manufacturing by radio frequency plasma spheroidization and deoxidation using calcium. J. Mater. Res. Technol. 2020, 9, 14792–14798. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Chen, Y.; Zhang, G.; Sun, J. Effect of nano Y2O3 addition on microstructure and room temperature tensile properties of Ti–48Al–2Cr–2Nb alloy. Vacuum 2019, 170, 108779–108788. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, Q.; Zhang, X. Wear characteristics of Fe-based diamond composites with cerium oxide (CeO2) reinforcements. Int. J. Refract. Met. Hard Mater. 2020, 86, 105093–105103. [Google Scholar] [CrossRef]
- Zhu, B.; Duke, M.; Dumée, L.; Merenda, A.; Ligneris, E.D.; Kong, L.; Hodgson, P.; Gray, S. Short Review on Porous Metal Membranes—Fabrication, Commercial Products, and Applications. Membranes 2018, 8, 83. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Liao, M.; Dai, W.; Wang, Z.; Yan, C.; Li, H.; Lin, C.-T.; Jiang, N.; Yu, J. Constructing a “pea-pod-like” alumina-graphene binary architecture for enhancing thermal conductivity of epoxy composite. Chem. Eng. J. 2020, 381, 122690–122697. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Li, J.; Wu, J. Transformation of Oxide Inclusions in Stainless Steel Containing Yttrium during Isothermal Heating at 1473 K. Metals 2019, 9, 961. [Google Scholar] [CrossRef]
- Jian, S.-Y.; Tzeng, Y.-C.; Ger, M.-D.; Chang, K.-L.; Shi, G.-N.; Huang, W.-H.; Chen, C.-Y.; Wu, C.-C. The study of corrosion behavior of manganese-based conversion coating on LZ91 magnesium alloy: Effect of addition of pyrophosphate and cerium. Mater. Des. 2020, 192, 108707–108721. [Google Scholar] [CrossRef]
- Ikoma, Y.; Toyota, T.; Ejiri, Y.; Saito, K.; Guo, Q.; Horita, Z. Allotropic phase transformation and photoluminescence of germanium nanograins processed by high-pressure torsion. J. Mater. Sci. 2016, 51, 138–143. [Google Scholar] [CrossRef]
- Kumar, R.; Jordan, E.; Gell, M.; Roth, J.; Jiang, C.; Wang, J.; Rommel, S. CMAS behavior of yttrium aluminum garnet (YAG) and yttria-stabilized zirconia (YSZ) thermal barrier coatings. Surf. Coat. Technol. 2017, 327, 126–138. [Google Scholar] [CrossRef]
- Sobolev, A.; Musin, A.; Whyman, G.; Borodianskiy, K.; Krichevski, O.; Kalashnikov, A.; Zinigrad, M. Stabilization of cubic phase in scandium-doped zirconia nanocrystals synthesized with sol-gel method. J. Am. Ceram. Soc. 2019, 102, 3236–3243. [Google Scholar] [CrossRef]
- Deng, X.; Wang, Y.; Chen, Y.; Cui, Z.; Shi, C. Yttrium-doped TiO2 compact layers for efficient perovskite solar cells. J. Solid State Chem. 2019, 275, 206–209. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.; Li, S.; Jin, B.; Yue, Q.; Wang, Z. Cerium oxide doped nanocomposite membranes for reverse osmosis desalination. Chemosphere 2019, 218, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Y.; Yang, Y.; Kim, D.S.; Yuan, A.; Tian, X.; Ophus, C.; Sun, F.; Schmid, A.K.; Nathanson, M.; et al. Observing crystal nucleation in four dimensions using atomic electron tomography. Nature 2019, 570, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Shakirzyanov, R.I.; Volodina, N.O.; Kozlovskiy, A.L.; Zdorovets, M.V.; Shlimas, D.I.; Borgekov, D.B.; Garanin, Y.A. Study of the Structural, Electrical, and Mechanical Properties and Morphological Features of Y-Doped CeO2 Ceramics with Porous Structure. J. Compos. Sci. 2023, 7, 411. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Xu, G.R.; Zou, J.L.; Li, G.B. Effect of sintering temperature on the characteristics of sludge ceramsite. J. Hazard. Mater. 2008, 150, 394–400. [Google Scholar] [CrossRef]
- McDonald, S.A.; Holzner, C.; Lauridsen, E.M.; Reischig, P.; Merkle, A.P.; Withers, P.J. Microstructural evolution during sintering of copper particles studied by laboratory diffraction contrast tomography (LabDCT). Sci. Rep. 2017, 7, 5251–5262. [Google Scholar] [CrossRef]
- Wang, L.; Ma, B.; Ren, X.; Yu, C.; Tian, J.; Liu, C.; Deng, C.; Hu, C.; Liu, Z.; Yu, J.; et al. Phase-engineering strategy of ZrO2 for enhancing the mechanical properties of porous cordierite ceramics. Mater. Today Commun. 2022, 30, 103032–103042. [Google Scholar] [CrossRef]
- Khalil, A.M.; Pozdniakov, A.V.; Solonin, A.N.; Mahmoud, T.S.; Alshah, M.; Mosleh, A.O. The Effects of Zirconium and Yttrium Addition on the Microstructure and Hardness of AlCuMgMn Alloy when Applying In Situ Heating during the Laser Melting Process. Materials 2023, 16, 5477. [Google Scholar] [CrossRef]
- Chang, L.; Jia, B.; Li, S.; Zhu, X.; Feng, R.; Shang, X. Influence of cerium on solidification, recrystallization and strengthening of Cu-Ag alloys. J. Rare Earths 2017, 35, 1029–1034. [Google Scholar] [CrossRef]
- Shen, Z.; Zhen, Y.; Wang, K.; Li, J. Influence of Sintering Temperature on Grain Growth and Phase Structure of Compositionally Optimized High-Performance Li/Ta-Modified (Na,K)NbO3 Ceramics. J. Am. Ceram. Soc. 2009, 92, 1748–1752. [Google Scholar] [CrossRef]
- MAlmomani, A.; Shatnawi, A.M.; Alrashdan, M.K. Effect of Sintering Time on the Density, Porosity Content and Microstructure of Copper—1 wt.% Silicon Carbide Composites. Adv. Mater. Res. 2014, 1064, 32–37. [Google Scholar]
- Sharma, S.C.; Gokhale, N.M.; Dayal, R.; Lal, R. Synthesis, microstructure and mechanical properties of ceria stabilized tetragonal zirconia prepared by spray drying technique. Bull. Mater. Sci. 2002, 25, 15–20. [Google Scholar] [CrossRef]


| Powder | Supplier | Purity (%) | Particle Size (μm) | Apparent Density (g/cm3) | Density (ρ) (g/cm3) |
|---|---|---|---|---|---|
| ZrO2 | Sood Chemicals Haryana, India | 95.01 | 1–5 | 5.5 | 5.89 |
| Cr | Sood Chemicals Haryana, India | 95.16 | 1–5 | 7.10 | 7.19 |
| Ni | Sood Chemicals Haryana, India | 95.10 | 1–5 | 8.02 | 8.91 |
| Yttrium (Y) | Nano laboratories Punjab, India | 98.20 | 1–5 | 4.4 | 6.96 |
| Cerium (Ce) | Nano Laboratories Punjab, India | 99.02 | 40 | 6.23 | 6.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, B.C.; Jain, N.; Rao, D.K.; Singhal, V.; Verma, A.; Goudar, D.M.; Raju, K.; Pinto, D.G. Effect of Sintering Temperature on the Physical and Mechanical Characteristics of Fabricated ZrO2–Cr–Ni–Ce–Y Composite. J. Compos. Sci. 2024, 8, 446. https://doi.org/10.3390/jcs8110446
Saini BC, Jain N, Rao DK, Singhal V, Verma A, Goudar DM, Raju K, Pinto DG. Effect of Sintering Temperature on the Physical and Mechanical Characteristics of Fabricated ZrO2–Cr–Ni–Ce–Y Composite. Journal of Composites Science. 2024; 8(11):446. https://doi.org/10.3390/jcs8110446
Chicago/Turabian StyleSaini, Brajesh Chandra, Naman Jain, Dinesh Kumar Rao, Varun Singhal, Akarsh Verma, Dayanand M. Goudar, Kandavalli Raju, and Deesy G. Pinto. 2024. "Effect of Sintering Temperature on the Physical and Mechanical Characteristics of Fabricated ZrO2–Cr–Ni–Ce–Y Composite" Journal of Composites Science 8, no. 11: 446. https://doi.org/10.3390/jcs8110446
APA StyleSaini, B. C., Jain, N., Rao, D. K., Singhal, V., Verma, A., Goudar, D. M., Raju, K., & Pinto, D. G. (2024). Effect of Sintering Temperature on the Physical and Mechanical Characteristics of Fabricated ZrO2–Cr–Ni–Ce–Y Composite. Journal of Composites Science, 8(11), 446. https://doi.org/10.3390/jcs8110446








