The Influence of Activated Carbon Particle Size on the Properties and Performance of Polysulfone Composite Membrane for Protein Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.3. Water Contact Angle Test
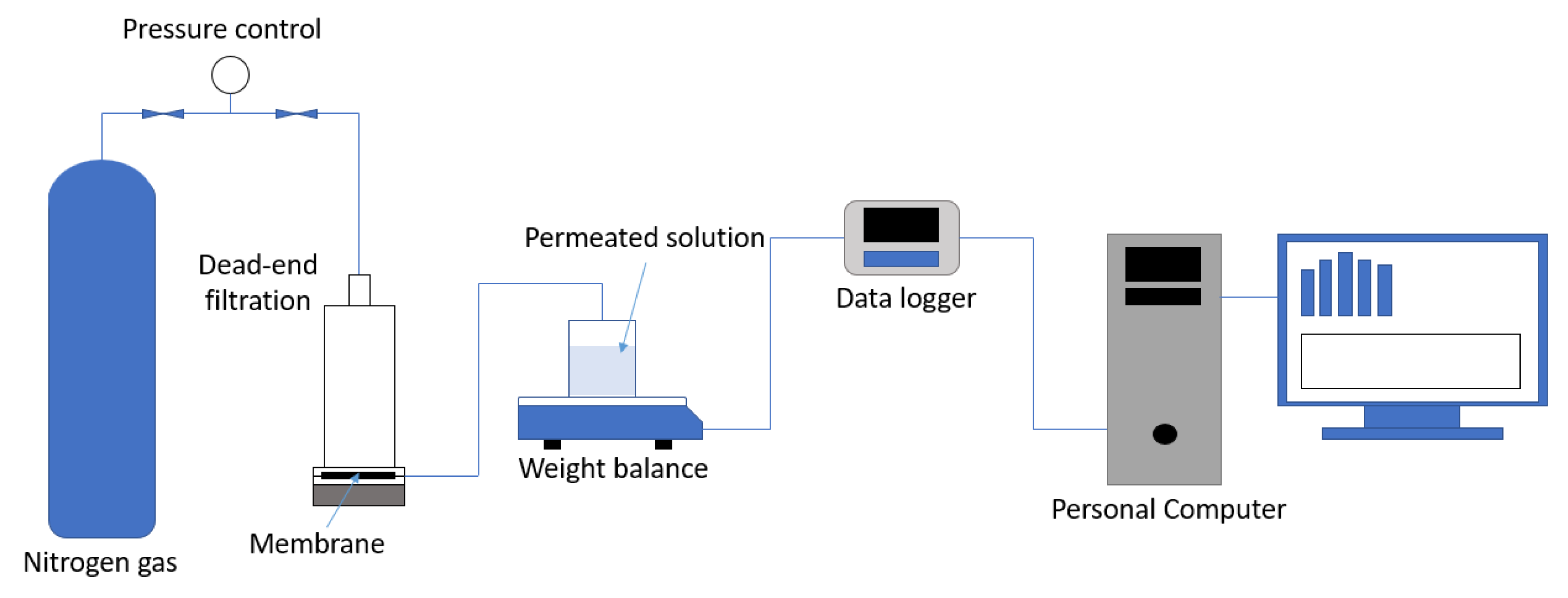
2.4. Ultrafiltration Experiments
2.4.1. Water Flux Test
2.4.2. Protein Separation
2.5. Porosity
2.6. Average Pore Size
2.7. Molecular Weight Cutoff (MWCO)
3. Results
3.1. Contact Angle Analysis
3.2. Membrane Permeability Test
3.3. Effect of AC on Protein Rejection
3.4. Effect of AC on Membranes Porosity
3.5. Measurement of Average Pore Size
3.6. Molecular Weight Cutoff Measurement (MWCO)
3.7. Membrane Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiang, T.; Song, Y.; Zhu, R.; Yuan, W. Biomass material derived hierarchical porous TiO2: Adjustable pore size for protein adsorption. J. Alloys Compd. 2020, 829, 154512. [Google Scholar] [CrossRef]
- Kluszczynska, K.; Peczek, L.; Rozanski, A.; Czernek, L.; Duchler, M. U6/miR-211 expression ratio as a purity parameter for HEK293 cell-derived exosomes. Acta Biochim. Pol. 2022, 69, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Arbita, A.A.; Paul, N.A.; Cox, J.; Zhao, J. Amino acid sequence of two new milk-clotting proteases from the macroalga Gracilaria edulis. Int. J. Biol. Macromol. 2022, 211, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Sakamoto, M.; Yamauchi, N.; Nakazawa, M.; Koizumi, A.; Anazawa, R.; Kurumada, K.; Hidari, K.I.P.J.; Kono, H. Optimization of the conditions for the immobilization of glycopolypeptides on hydrophobic silica particulates and simple purification of lectin using glycopolypeptide-immobilized particulates. Carbohydr. Res. 2022, 519, 108624. [Google Scholar] [CrossRef]
- Chai, M.; Ye, Y.; Chen, V. Separation and concentration of milk proteins with a submerged membrane vibrational system. J. Membr. Sci. 2017, 524, 305–314. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, W.; Ould-Dris, A.; Jaffrin, M.Y.; Tang, B. Concentration of milk proteins for producing cheese using a shear-enhanced ultrafiltration technique. Ind. Eng. Chem. Res. 2016, 55, 11130–11138. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, G.; Rana, D.; Matsuura, T.; Lan, C.Q. Engineering polyvinylidene fluoride membranes using charge tunable dendrimer polyamidoamine to enable microporous membranes for protein separation. Sep. Purif. Technol. 2025, 354 Pt 4, 128887. [Google Scholar] [CrossRef]
- Kassa, S.T.; Hu, C.C.; Keshebo, D.L.; Ang, M.B.M.; Lai, J.Y.; Chu, J.P. Surface modification of high-rejection ultrafiltration membrane with antifouling capability using activated oxygen treatment and metallic glass deposition. Appl. Surf. Sci. 2020, 529, 147131. [Google Scholar] [CrossRef]
- Jin, Z.; Shen, Y.; Chen, X.; Qiu, M.; Fan, Y. Construction of high-performance Ce-doped TiO2 tight UF membranes for protein separation. Appl. Surf. Sci. 2023, 610, 155468. [Google Scholar] [CrossRef]
- Simari, C.; Caprì, A.; Rehman, M.H.U.; Enotiadis, A.; Gatto, I.; Baglio, V.; Nicotera, I. Composite anion exchange membranes based on polysulfone and silica nanoscale ionic materials for water electrolyzers. Electrochim. Acta 2023, 462, 142788. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Feng, M.; Huang, T.; Zhang, N.; Wang, Y. UV-activated superwetting ability of electrospun polysulfone/titanium dioxide membranes toward highly efficient methylene blue removal and oil/water separation. J. Membr. Sci. 2024, 695, 122450. [Google Scholar] [CrossRef]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Yogarathinam, L.T.; Raji, Y.O.; El-badawy, T.H. Recent development in modification of polysulfone membrane for water treatment application. J. Water Process. Eng. 2021, 40, 101835. [Google Scholar] [CrossRef]
- Pagidi, A.; Saranya, R.; Arthanareeswaran, G.; Ismail, A.F.; Matsuura, T. Enhanced oil–water separation using polysulfone membranes modified with polymeric additives. Desalination 2014, 344, 280–288. [Google Scholar] [CrossRef]
- Khan, A.; Sherazi, T.A.; Khan, Y.; Li, S.; Naqvi, S.A.R.; Cui, Z. Fabrication and characterization of polysulfone/modified nanocarbon black composite antifouling ultrafiltration membranes. J. Membr. Sci. 2018, 554, 71–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, P.; Du, T.; Shan, L.; Wang, Y. Development of a sulfated Y-doped nonstoichiometric zirconia/polysulfone composite membrane for treatment of wastewater containing oil. Sep. Purif. Technol. 2009, 70, 153–159. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thakur, R.S. Effect of inorganic particle on the performance of polyethersulfone-cellulose acetate ultrafiltration membranes. In Sustainable Membrane Technology for Energy, Water, and Environment; Ismail, A.F., Matsuura, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 11. [Google Scholar]
- Abid, Z.; Abbas, A.; Mahmood, A.; Rana, N.F.; Khan, S.J.; Duclaux, L.; Deen, K.M.; Ahmad, N.M. Water Treatment Using High Performance Antifouling Ultrafiltration Polyether Sulfone Membranes Incorporated with Activated Carbon. Polymers 2022, 14, 2264. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Hussein, M.A.; Khaleel, M.A.; Khraisheh, M.; Hilal, N. Can carbon-based nanomaterials revolutionize membrane fabrication for water treatment and desalination. Desalination 2016, 391, 69–88. [Google Scholar] [CrossRef]
- Khalid, A.; Al-Juhani, A.A.; Al-Hamouz, O.C.; Laoui, T.; Khan, Z.; Atieh, M.A. Preparation and properties of nanocomposite polysulfone/multi-walled carbon nanotubes membranes for desalination. Desalination 2015, 367, 134–144. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.R.; Hosseini, S.M.; Ray, M.B.; Parvizian, F.; Van der Bruggen, B. Highly hydrophilic and antifouling nanofiltration membrane incorporated with water-dispersible composite activated carbon/chitosan nanoparticles. Chem. Eng. Res. Des. 2018, 132, 812–821. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Amini, S.H.; Khodabakhshi, A.R.; Bagheripour, E.; Van der Bruggen, B. Activated carbon nanoparticles entrapped mixed matrix polyethersulfone based nanofiltration membrane for sulfate and copper removal from water. J. Taiwan Inst. Chem. Eng. 2018, 82, 169–178. [Google Scholar] [CrossRef]
- Sharma, M.; Alves, P.; Gando-Ferreira, L.M. Effect of activated carbon nanoparticles on the performance of PES nanofiltration membranes to separate Kraft lignin from black liquor. J. Water Process Eng. 2023, 52, 103487. [Google Scholar] [CrossRef]
- Noorani, N.; Barzegar, B.; Mehrdad, A.; Aghdasinia, H.; Peighambardoust, S.J.; Kazemian, H. CO2 capture in activated pyrolytic coke/metal oxide nanoparticle composites. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132554. [Google Scholar] [CrossRef]
- Remy, M.; Potier, V.; Temmink, H. Why low powdered activated carbon addition reduces membrane fouling in MBRs. Water Res. 2010, 44, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Zhang, S.; Li, Y.; Liu, W. Effects of powdered activated carbon dosage on the performance of membrane bioreactors treating biochemical tail water. Sci. Total Environ. 2023, 874, 162429. [Google Scholar] [CrossRef] [PubMed]
- Torretta, V.; Urbini, G.; Raboni, M.; Copelli, S.; Viotti, P.; Luciano, A.; Mancini, G. Effect of powdered activated carbon to reduce fouling in membrane bioreactors: A sustainable solution.Case study. Sustainability 2013, 5, 1501–1509. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Nagendran, A.; Rana, D.; Matsuura, T.; Neelakandan, S.; Malarvizhi, K. Effects of Polyvinylpyrrolidone on the permeation and fouling-resistance properties of Polyetherimide ultrafiltration membranes. Ind. Eng. Chem. Res. 2015, 54, 4832–4838. [Google Scholar] [CrossRef]
- Ferreira, I.; Alves, P.; Gil, M.H.; Gando-Ferreira, L.M. Lignin separation from black liquor by mixed matrix polysulfone nanofiltration membrane filled with multiwalled carbon nanotubes. Sep. Purif. Technol. 2021, 260, 118231. [Google Scholar]
- Sarbolouki, M.N. A general diagram for estimating pore size of ultrafiltration and reverse osmosis membranes. Sep. Sci. Technol. 1982, 17, 381–386. [Google Scholar] [CrossRef]
- Nagendran, A.; Mohan, D. Cellulose acetate and polyetherimide blend ultrafiltration membranes: II. Effect of additive. Polym. Adv. Technol. 2008, 19, 24–35. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; Salmon, J.; Ji, J.; Miao, S. Characterisation of the Wetting Behaviour of Poor Wetting Food Powders and the Influence of Temperature and Film Formation. KONA Powder Part. J. 2017, 34, 282–289. [Google Scholar] [CrossRef]
- Wu, C.; Dai, X.; Sun, X.; Zhang, J. Preparation and characterization of fluoroalkyl activated carbons/PVDF composite membranes for water and resources recovery by membrane distillation. Sep. Purif. Technol. 2023, 305, 122519. [Google Scholar] [CrossRef]
- Aziz, M.H.A.; Othman, M.H.D.; Hashim, N.A.; Rahman, M.A.; Jaafar, J.; Hubadillah, S.K.; Tai, Z.S. Pretreated aluminium dross waste as a source of inexpensive alumina-spinel composite ceramic hollow fibre membrane for pretreatment of oily saline produced water. Ceram. Int. 2019, 45, 2069–2078. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Jaber, L.; Chatla, A.; Abushawish, A.; Shanableh, A.; Ali Atieh, M. Unveiling the relationship between MOF porosity, particle size, and polyethersulfone membranes properties for efficient decontamination of dye and organic matter. Chem. Eng. J. 2023, 471, 144616–144634. [Google Scholar] [CrossRef]
- Awang Chee, D.N.; Ismail, A.F.; Aziz, F.; Mohamed Amin, M.A.; Abdullah, N. The influence of alumina particle size on the properties and performance of alumina hollow fiber as support membrane for protein separation. Sep. Purif. Technol. 2020, 250, 117147. [Google Scholar] [CrossRef]
- Tsung-Shou, Y.; Michael, D.S. Effect of particle size distribution on the sintering of alumina. Commun. Am. Ceram. Soc. 1988, 487, 484–487. [Google Scholar]
- Ji, Y.; Ke, J.; Duan, F.; Chen, J. Preparation and characterization of modified activated carbon/polysulfone blended ultrafiltration membrane. Desalination Water Treat. 2017, 98, 78–84. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Rahimpour, A.; Jahamshahi, M.; Peyravi, M.; Khavarpour, M. The effect of silver nanoparticle size on performance and antibacteriality of polysulfone ultrafiltration membrane. Desalination 2012, 306, 41–50. [Google Scholar] [CrossRef]
- Goel, V.; Tanwar, R.; Saikia, A.K.; Mandal, U.K. Separation characteristics of surface modified polysulfone ultrafiltration membrane using oxidative catalytic polymerization of aniline. J. Polym. Mater. 2022, 39, 283–305. [Google Scholar] [CrossRef]
- Erragued, R.; Kujawski, W.; Kujawa, J.; Gando-Ferreira, L.M.; Bouaziz, M. Optimizing operating conditions for olive leaf valorization using activated carbon mixed matrix membrane. J. Water Process Eng. 2024, 59, 105036. [Google Scholar] [CrossRef]





| Iodine Number, mg/g | 850 |
| Moisture by Weight | 2% |
| Effective Size | 0.8–1.0 |
| Uniformity Coefficient | 2.1 |
| Abrasion Number | 75 |
| Screen Size by Weight, US Sieve Series | |
| On 8 mesh | 15% |
| Through 30 mesh | 4% |
| Apparent Density (tamped) | 0.58 g/cc |
| Water Extractables | <1% |
| Non-Wettable | <1% |
| No. | Membrane Code | AC Powder Size (µm) |
|---|---|---|
| 1 | PSF-CA0 | - |
| 2 | PSF-CA1 | 297 |
| 3 | PSF-CA2 | 149 |
| 4 | PSF-CA3 | 74 |
| 5 | PSF-CA4 | 37 |
| Membrane Code | Porosity (%) | Pore Radius, | MWCO (kDa) |
|---|---|---|---|
| PSF-AC0 | 63.41 | 30.12 | 35 |
| PSF-AC1 | 63.46 | 29.88 | 35 |
| PSF-AC2 | 66.02 | 27.88 | 35 |
| PSF-AC3 | 66.61 | 27.66 | 35 |
| PSF-AC4 | 66.92 | 27.83 | 35 |
| Polymer | Modifier | Permeability Flux, LMH/Bar | Pore Size, nm | Rejection (%) | ε (%) | WCA (°) | Ref. |
|---|---|---|---|---|---|---|---|
| PSF | AC | 53.3/116.3 | 34.1/43.2 | ~93.2 (BSA) | - | - | [37] |
| PSF/PVP | Silver-nanoparticle | 12/55 | - | ~48 (BSA) | - | 81.2/60.9 | [38] |
| PSF | PANI-CuCl2, PANI-FeCl3 and PSF/PANI/APS | 30/450 | 92.12/122.94 | 96 (BSA) | - | 41.8–80 | [39] |
| PSF/PEG | AC | 5/9.8 | 6.4/7.2 | 81-93 (total phenolic compounds) | 83–86 | 48.2–66.7 | [40] |
| PSF | CNT | 1/8 | 2.82/10.18 | 98 (lignin) | 74.4/75.5 | 57.6/74.4 | [28] |
| PSF | AC | 15/42.5 | 27.66/30.12 | 98 (BSA) 90 (Pepsin) 73.9 (Lysozyme) | 63.41/66.92 | 57.5/67.6 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prihandana, G.S.; Muthi’ah, A.D.; Sriani, T.; Mahardika, M. The Influence of Activated Carbon Particle Size on the Properties and Performance of Polysulfone Composite Membrane for Protein Separation. J. Compos. Sci. 2024, 8, 483. https://doi.org/10.3390/jcs8110483
Prihandana GS, Muthi’ah AD, Sriani T, Mahardika M. The Influence of Activated Carbon Particle Size on the Properties and Performance of Polysulfone Composite Membrane for Protein Separation. Journal of Composites Science. 2024; 8(11):483. https://doi.org/10.3390/jcs8110483
Chicago/Turabian StylePrihandana, Gunawan Setia, Aisyah Dewi Muthi’ah, Tutik Sriani, and Muslim Mahardika. 2024. "The Influence of Activated Carbon Particle Size on the Properties and Performance of Polysulfone Composite Membrane for Protein Separation" Journal of Composites Science 8, no. 11: 483. https://doi.org/10.3390/jcs8110483
APA StylePrihandana, G. S., Muthi’ah, A. D., Sriani, T., & Mahardika, M. (2024). The Influence of Activated Carbon Particle Size on the Properties and Performance of Polysulfone Composite Membrane for Protein Separation. Journal of Composites Science, 8(11), 483. https://doi.org/10.3390/jcs8110483







