Nanocomposite Material Based on Polyvinyl Alcohol Modified with Carbon Nanotubes: Mechanism of Formation and Electronic Energy Structure
Abstract
:1. Introduction
2. Material and Methods
2.1. Investigated Material
2.2. «Polymer Nanofill» System
3. Theory/Calculation
4. Results and Discussion
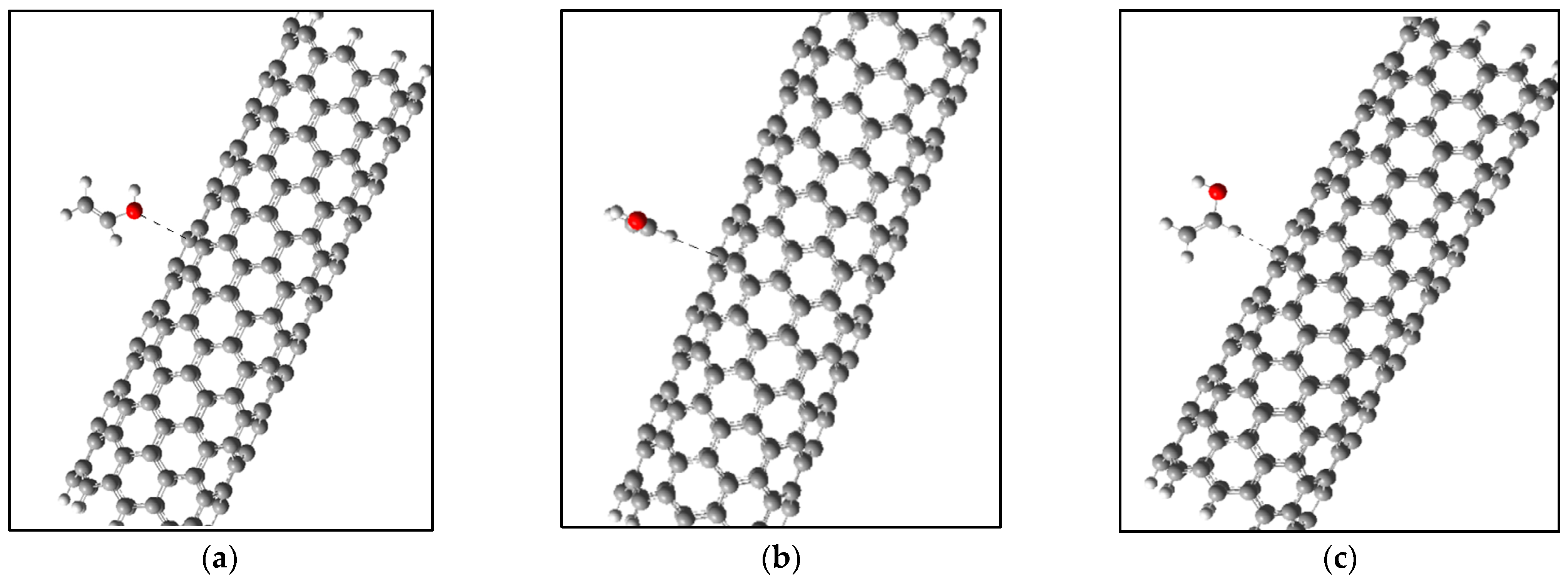
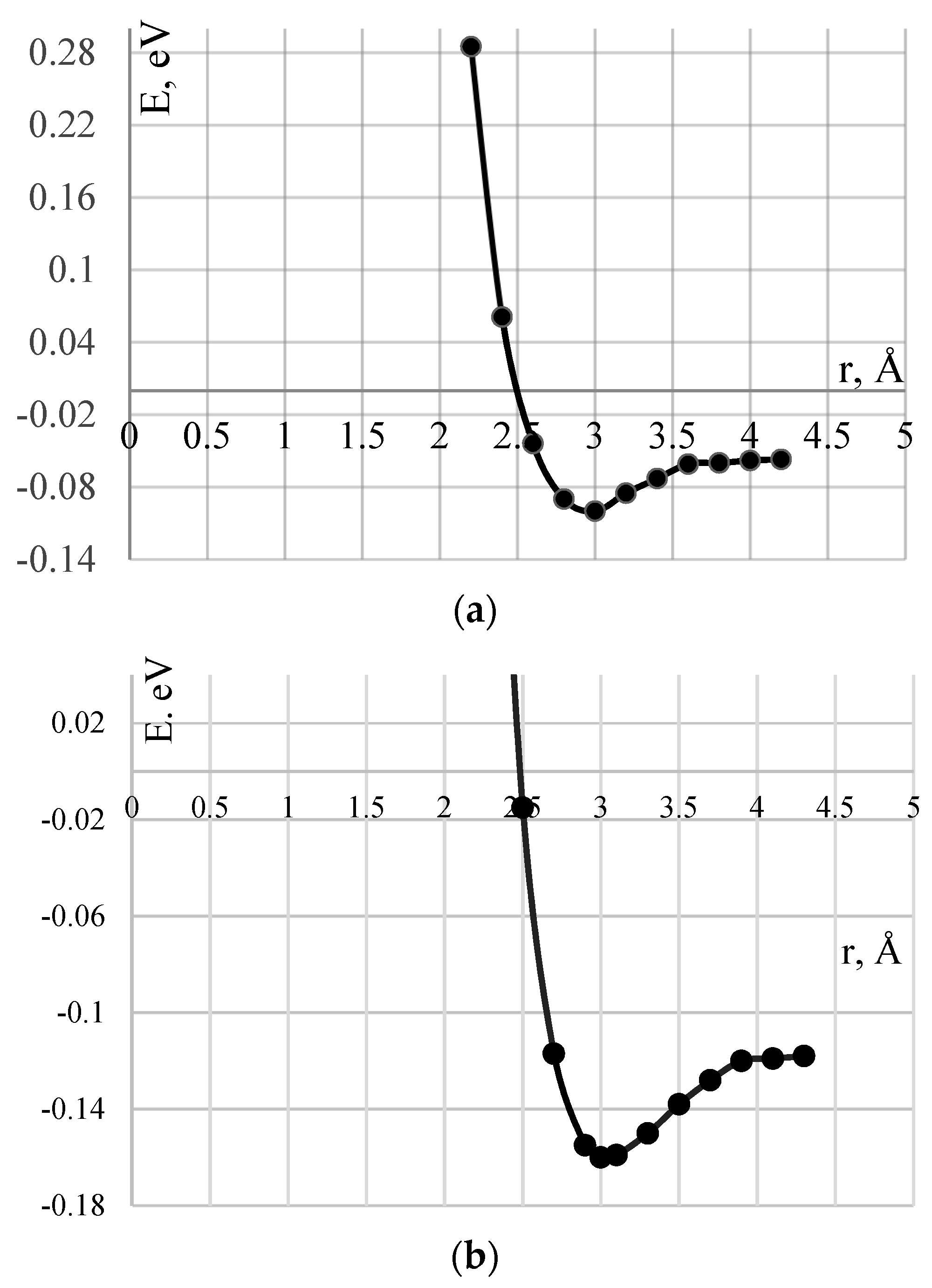
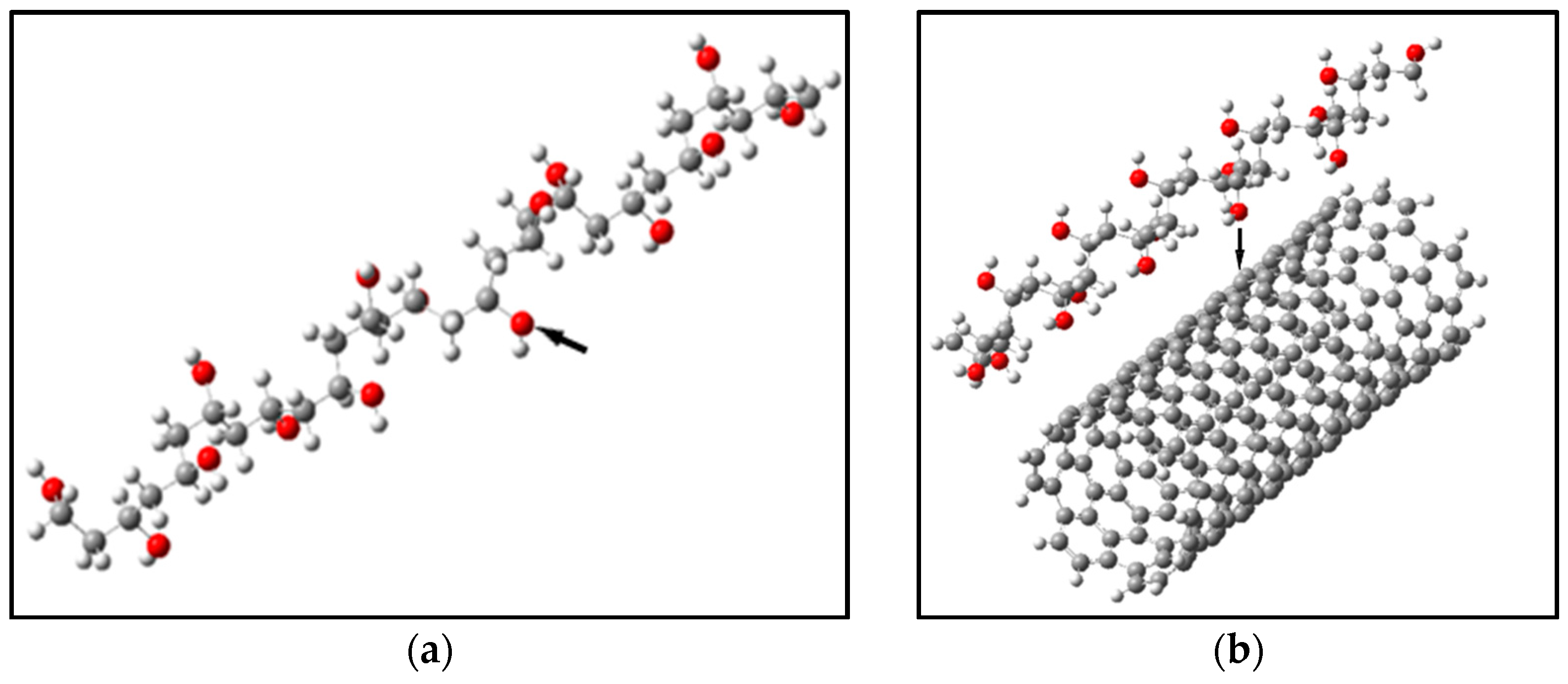
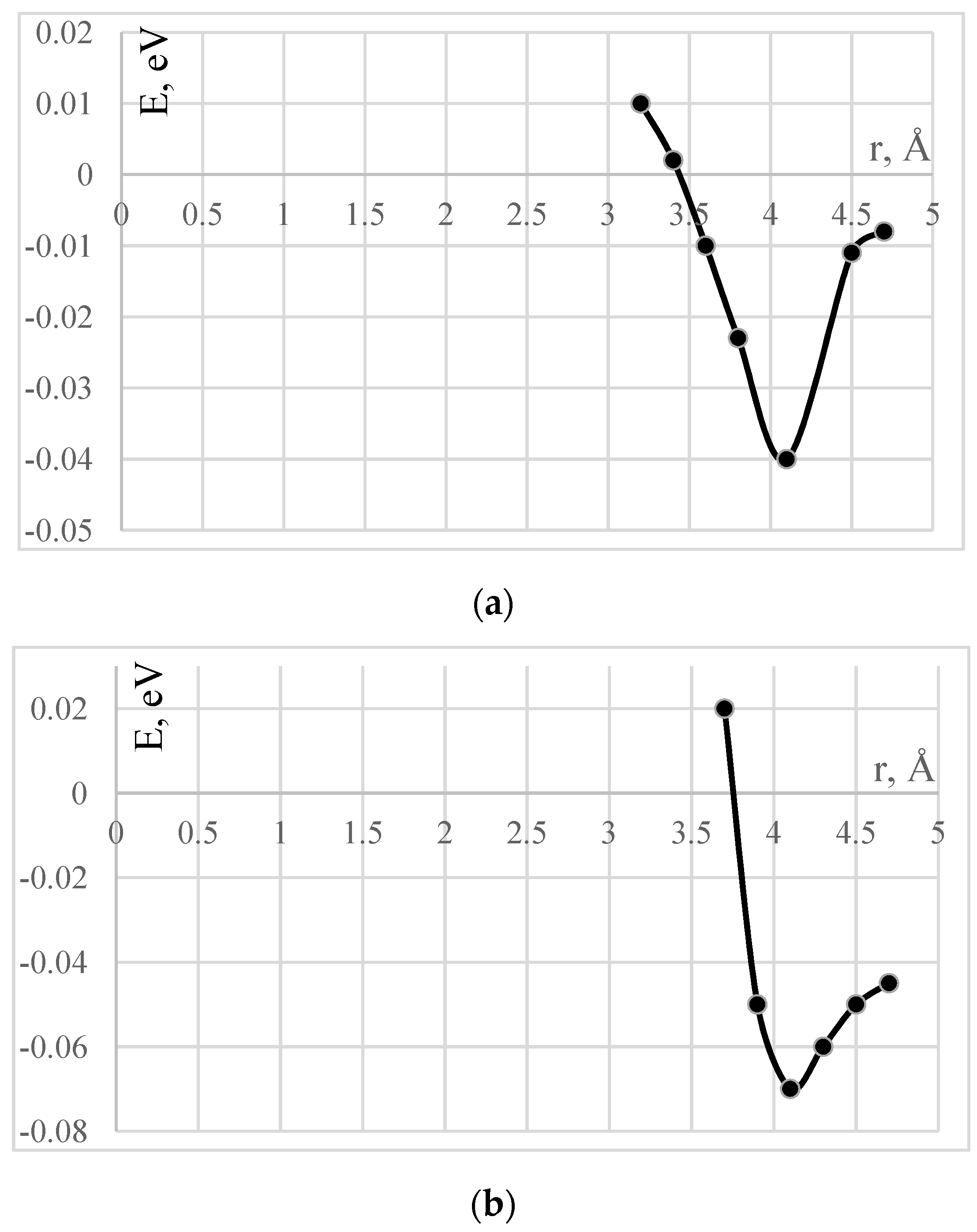
4.1. The Effect of Adsorption of Polyvinyl Alcohol on the Surface of Carbon Nanotubes
4.2. Features of the Electron Energy Structure of Polymer Nanocomposites Based on Polyvinyl Alcohol and Carbon Nanotubes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, D.; Huo, Y.; Jiang, Z.; Zhong, J. Carbon nanotube polymer nanocomposites coated aggregate enabled highly conductive concrete for structural health monitoring. J. Carbon 2023, 206, 340–350. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Lv, K.; Kong, N.; Shao, Y.; Tao, J. Carbon nanotubes boosts the toughness and conductivity of wet-spun MXene fibers for fiber-shaped super capacitors. Carbon 2022, 200, 38–46. [Google Scholar] [CrossRef]
- Kharche, G.; Lokavarapu, B.R. Static buckling analysis of single walled carbon nanotube. Mater. Proc. 2023, in press. [CrossRef]
- Zaporotskova, I.V. Carbon and Non-Carbon Nanomaterials and Composite Structures Based on Them: Structure and Electronic Properties; VolSU Publishing House: Volgograd, Russia, 2009; p. 490. [Google Scholar]
- Dyachkov, P.N. Carbon nanotubes: Structure, properties, applications. Binomial 2005, 196, 36. [Google Scholar]
- Hasnain, M.S.; Nayak, A.K. Nanocomposites for improved orthopedic and bone tissue engineering applications. In Applications of Nanocomposite Materials in Orthopedics; Series in Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–177. [Google Scholar]
- Shi, D.; Guo, Z.; Bedford, N. Nanocomposites. In Nanomaterials and Devices (Micro and Nano Technologies); William Andrew: Norwich, NY, USA, 2015; pp. 293–315. [Google Scholar]
- Kausar, A. Sensing Materials: Nanocomposites. In Encyclopedia of Sensors and Biosensors; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 305–315. [Google Scholar]
- de Oliveira, A.D.; Beatrice, C.A.G. Polymer Nanocomposites with Different Types of Nanofiller. In Nanocomposites—Recent Evolutions; Intechopen: London, UK, 2019; p. 230. [Google Scholar]
- Chebil, A.; Doudou, B.B.; Dridi, C.; Dammak, M. Synthesis characterization, optical and electrical properties of polyvinyl alcohol/multi-walled carbon nanotube nanocomposites: A composition dependence study. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 243, 125–130. [Google Scholar] [CrossRef]
- Tyagi, V.; Thakur, A. Carboxymethyl cellulose-polyvinyl alcohol based materials: A review. Mater. Today Proc. 2023. [CrossRef]
- Khazaei, S.; Mozaffari, S.A.; Ebrahimi, F. Polyvinyl alcohol as a crucial omissible polymer to fabricate an impedimetric glucose biosensor based on hierarchical 3D-NPZnO/chitosan. Carbohydr. Polym. 2021, 266, 118105. [Google Scholar] [CrossRef] [PubMed]
- Pogorelov, T.V. Quantum Chemical Calculations Using the Gaussian Software Package and the Gaussview Graphics Editor. Append. Phys.-Mat. 2000, 76, 2868–2870. [Google Scholar]
- Xie, X.-L.; Mai, Y.-W.; Zhou, X.-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Elbakyan, L.S. Obtaining New Dental Materials Reinforced with Carbon Nanotubes. J. Nano-Electron. Phys. 2014, 6, 03008-1–03008-3. [Google Scholar]
- Elbakyan, L.S.; Zaporotskova, I.V.; Belonenko, M.B. Nanocomposites based on polymethylmethacrylate doped with carbon nanotubes: Some electrophysical studies. J. Perspect. Mater. 2017, 4, 16–22. [Google Scholar]
- Sandler, J.K.W.; Kirk, J.E.; Kinloch, I.A.; Shaffer, M.S.P.; Windle, A.H. Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 2003, 44, 5893–5899. [Google Scholar] [CrossRef]
- Ramasubramaniam, R.; Chen, J.; Liu, H. Homogeneous carbon nanotube/polymer composites for electrical applications. Appl. Phys. Lett. 2003, 83, 2928–2930. [Google Scholar] [CrossRef]
- Scher, H.; Zallen, R. Critical density in percolation processes. J. Chem. Phys. 1970, 53, 3759–3761. [Google Scholar] [CrossRef]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-Consistent Molecular Orbital Methods. 22. Small Split-Valence Basis Sets for Second-Row Elements. J. Am. Chem. Soc. 1982, 104, 2797. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Elbakyan, L.S. Quantum Chemical Calculations Using THE Gaussian Software Package and the Gaussview Graphics Editor: Textbook; Publishing House of the VolSU: Volgograd, Russia, 2022; p. 81. [Google Scholar]


| Types of CNTs | Diameter D, nm | Chirality Angle, α | CNT Cluster Length, nm/Number of Atoms | Type of Conductivity |
|---|---|---|---|---|
| (6,6) | 0.814 | 30 | 24.4/264 | Metal |
| (9,0) | 0.705 | 0 | 23.9/234 | Metal |
| Types of CNTs | Active Center | Adsorption Distance, Rad, Å | Adsorption Energy, ∆Ea, eV |
|---|---|---|---|
| (6,6) | 1 | 3.0 | 0.16 |
| (6,6) | 2 (the plane of the monomer is perpendicular to the CNT axis) | 2.8 | 0.14 |
| (6,6) | 2 (the plane of the monomer is directed along the CNT axis) | 2.7 | 0.07 |
| (9,0) | 1 | 3.0 | 0.1 |
| (9,0) | 2 (the plane of the monomer is perpendicular to the CNT axis) | 2.9 | 0.08 |
| (9,0) | 2 (the plane of the monomer is directed along the CNT axis) | 2.6 | 0.05 |
| Types of CNTs | Active Center | Adsorption Distance, Rad, Å | Adsorption Energy, ∆Ea, eV |
|---|---|---|---|
| (6,6) | 1 | 4.1 | 0.04 |
| (9,0) | 1 | 4.1 | 0.07 |
| Types of CNTs | Connection Option (Active PVA Center) | The Forbidden Zone Width ΔEg, eV |
|---|---|---|
| (6,6) | oxygen atom | 1.175 |
| (9,0) | oxygen atom | 1.307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbakyan, L.; Zaporotskova, I.; Hayrapetyan, D. Nanocomposite Material Based on Polyvinyl Alcohol Modified with Carbon Nanotubes: Mechanism of Formation and Electronic Energy Structure. J. Compos. Sci. 2024, 8, 54. https://doi.org/10.3390/jcs8020054
Elbakyan L, Zaporotskova I, Hayrapetyan D. Nanocomposite Material Based on Polyvinyl Alcohol Modified with Carbon Nanotubes: Mechanism of Formation and Electronic Energy Structure. Journal of Composites Science. 2024; 8(2):54. https://doi.org/10.3390/jcs8020054
Chicago/Turabian StyleElbakyan, Lusine, Irina Zaporotskova, and David Hayrapetyan. 2024. "Nanocomposite Material Based on Polyvinyl Alcohol Modified with Carbon Nanotubes: Mechanism of Formation and Electronic Energy Structure" Journal of Composites Science 8, no. 2: 54. https://doi.org/10.3390/jcs8020054






