Enhancement of Polyacrylic Acid/Silicon Carbide Nanocomposites’ Optical Properties for Potential Application in Renewable Energy
Abstract
:1. Introduction
2. Experimental Setup
2.1. Preparation of PAA−SiC Nanocomposites
2.2. Measurement of Optical Features and Energy Absorption
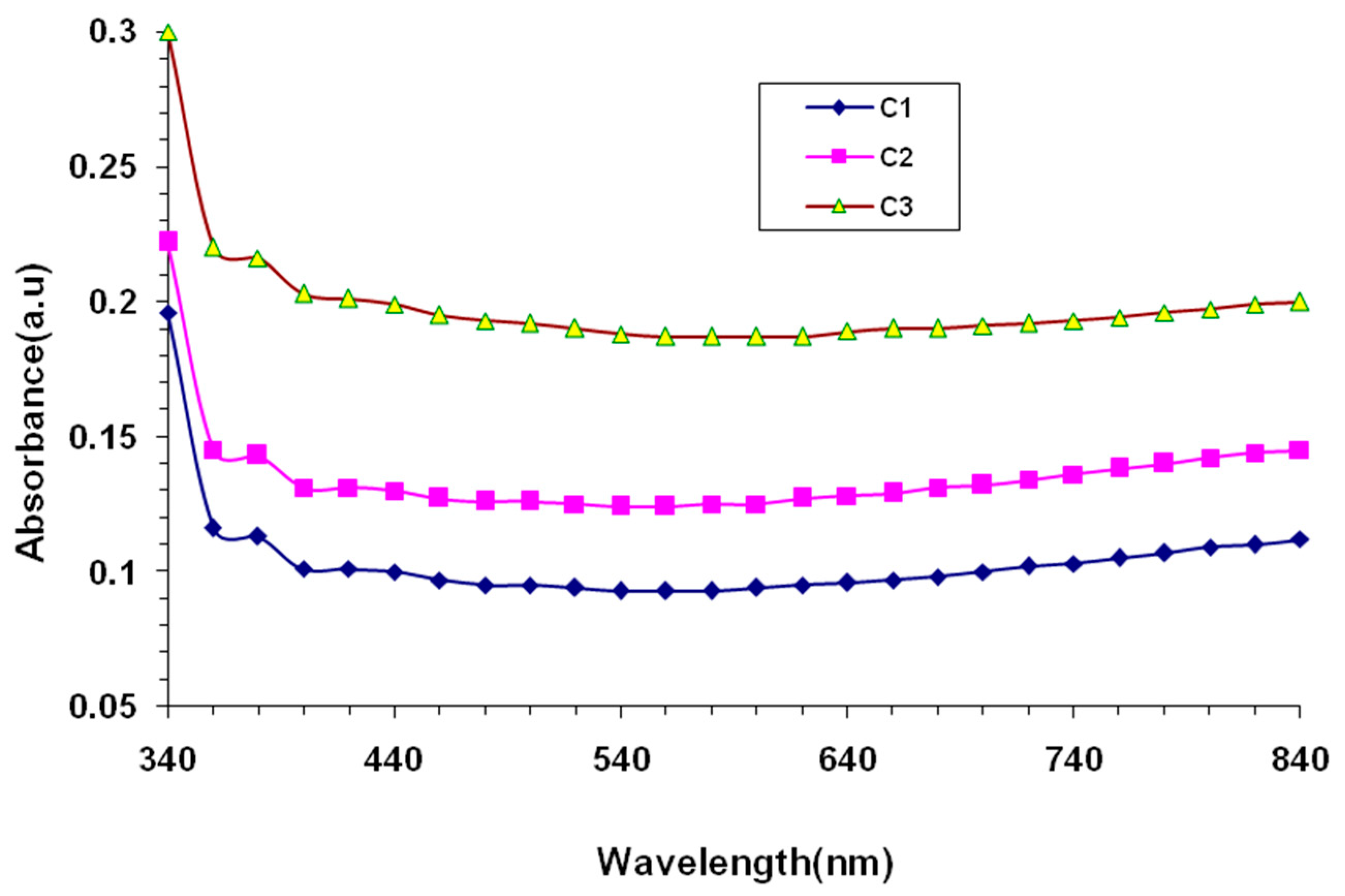
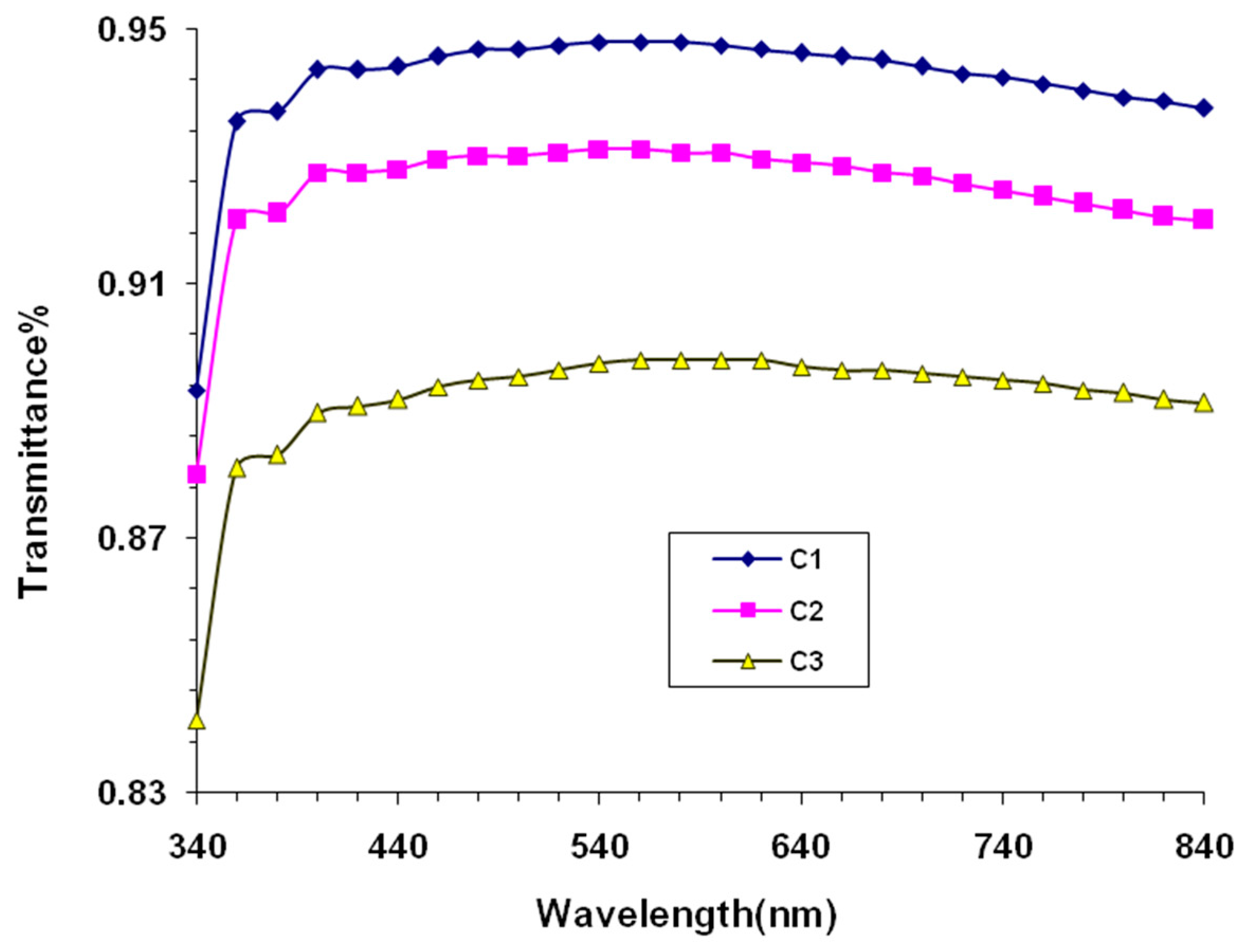
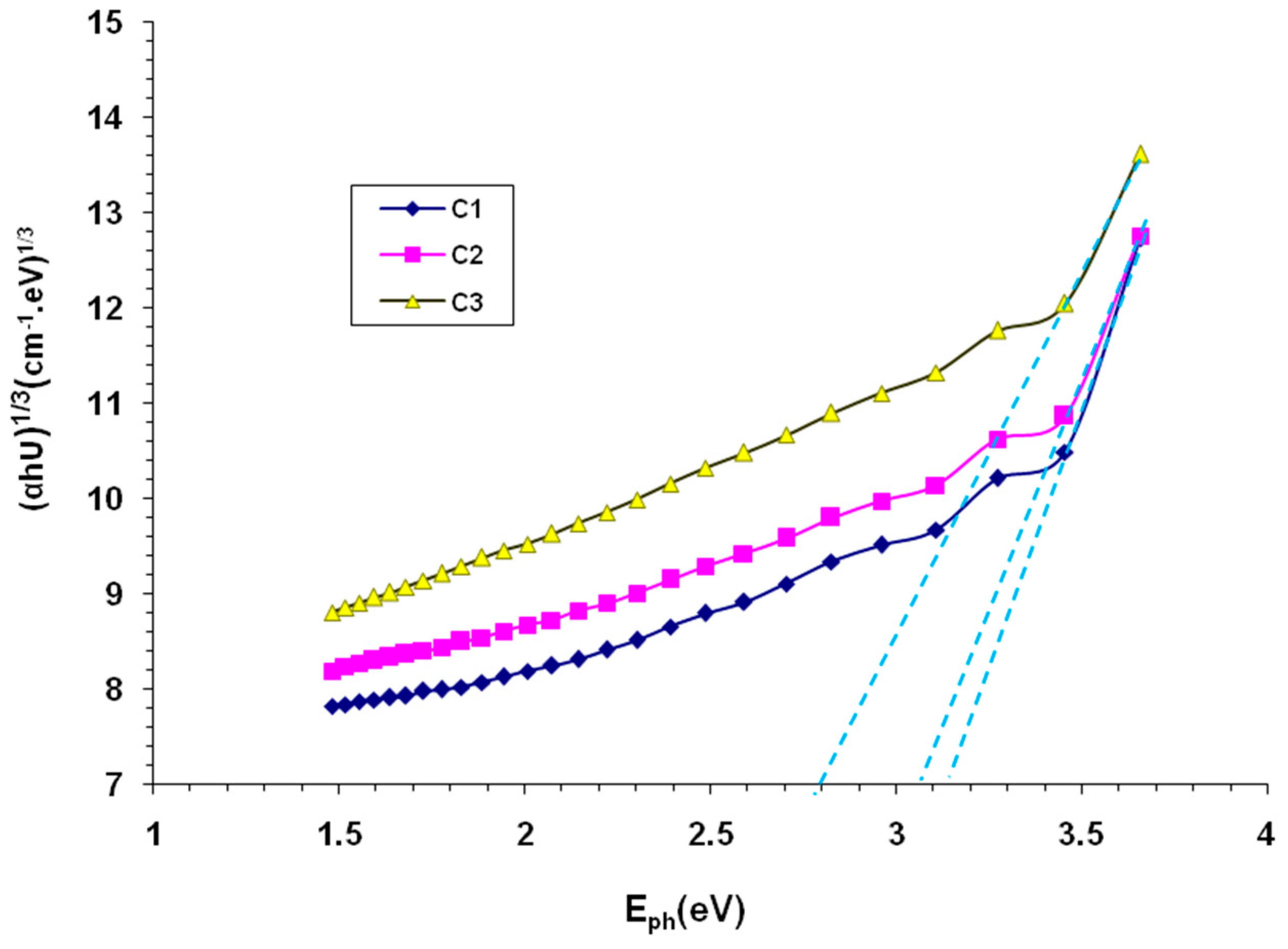
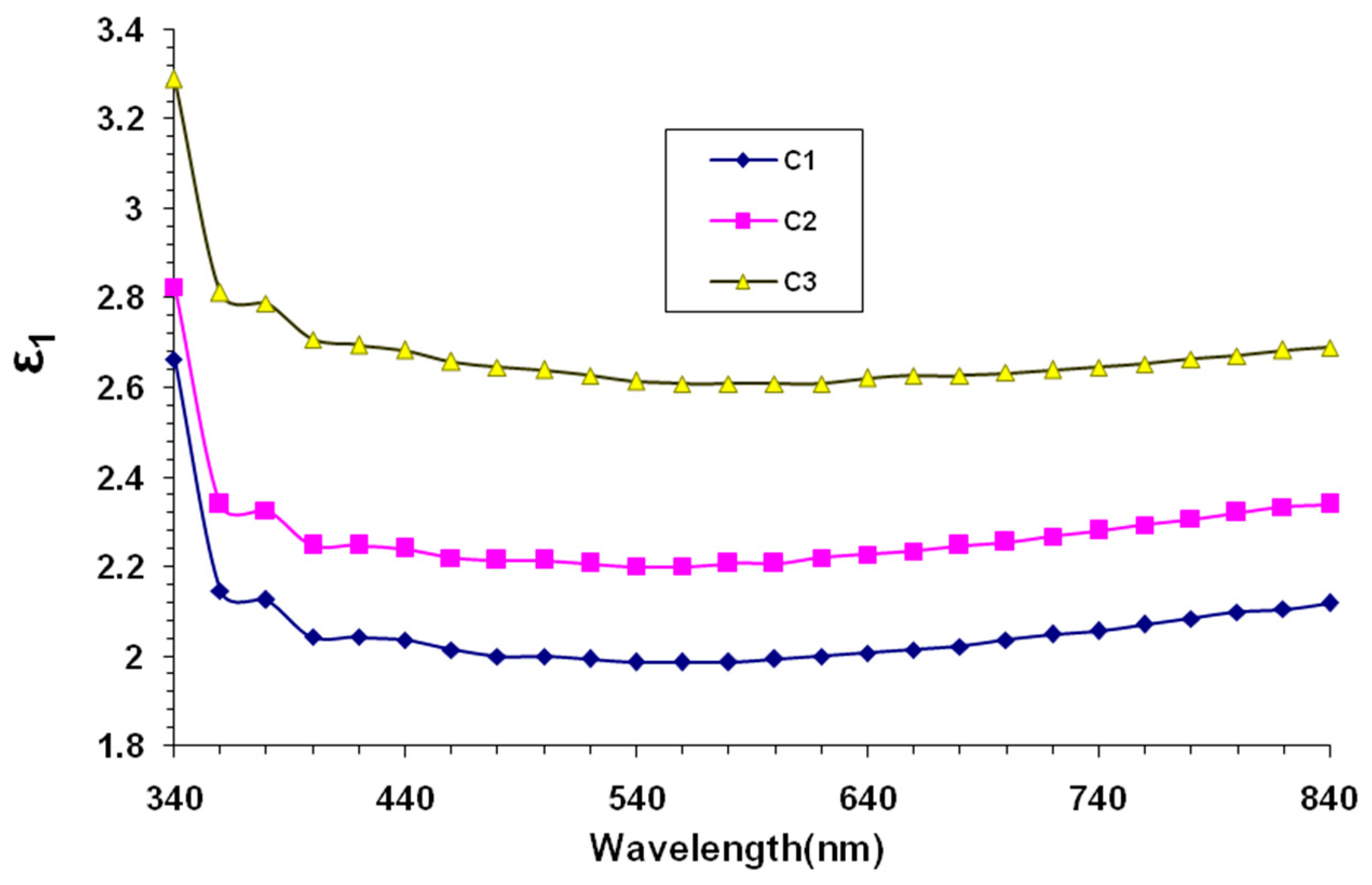
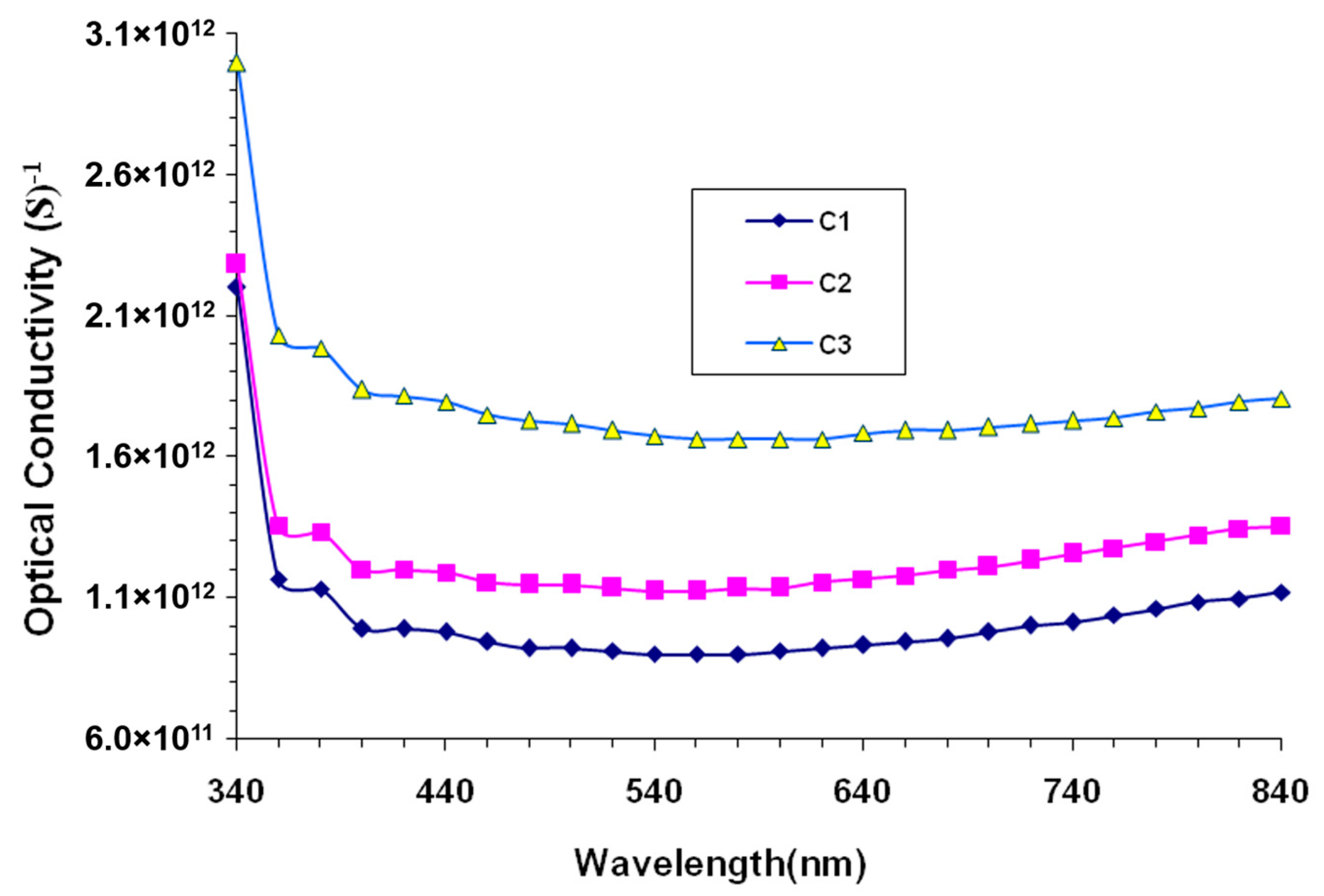
3. Results and Discussion
4. Conclusions
5. Recommendations for Further Research
- As demonstrated in the current research, there is room to utilise the synthesised polyacrylic acid/silicon carbide nanocomposites in renewable energy systems due to an enhancement of optical properties. However, a useful assessment of their suitability for renewable energy systems should be addressed by integrating the improved nanocomposites into the components of solar cells and gauge how they affect the effectiveness of energy conversion.
- A specific investigation is important to make polyacrylic acid/silicon carbide nanocomposites as efficient as possible. Specifically, this should consider the best ratio of polyacrylic acid to silicon carbide nanoparticles that enables the best optical characteristics.
- It would be interesting to examine how the shape and size of silicon carbide nanoparticles affect their optical characteristics. Greater light scattering or absorption can be achieved using smaller nanoparticles and regulated shapes.
- Since there is a possible utilisation of polyacrylic acid/silicon carbide nanocomposites in renewable energy systems, evaluating their stability and durability under a variety of environmental factors, including temperature, humidity, and UV exposure, is vigorous.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Symbol | Definition |
| NIR | Near-infrared |
| PAA | Polyacrylic acid |
| PCM | Phase change material |
| PMMA | Poly-methyl methacrylate |
| SiC | Silicon carbide |
| TES | Thermal energy storage |
| UV | Ultraviolet |
| VIS | Visible |
References
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Sen, S.; Ganguly, S.; Das, A.; Sen, J.; Dey, S. Renewable energy scenario in India: Opportunities and challenges. J. Afr. Earth Sci. 2016, 122, 25–31. [Google Scholar] [CrossRef]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Maxmut O’g’li, X.F. Renewable Energy Sources: Advancements, Challenges, And Prospects. Int. J. Adv. Sci. Res. 2023, 3, 14–25. [Google Scholar]
- Al-Obaidi, M.A.; Zubo, R.H.A.; Rashid, F.L.; Dakkama, H.J.; Abd-Alhameed, R.; Mujtaba, I.M. Evaluation of Solar Energy Powered Seawater Desalination Processes: A Review. Energies 2022, 15, 6562. [Google Scholar] [CrossRef]
- Amberkar, T.; Mahanwar, P. Phase Change Material Nanocomposites for Thermal Energy Storage Applications. Mater. Proc. 2022, 9, 8. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.K.; Ansu, A.K.; Goyal, R.; Sarı, A.; Tyagi, V.V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
- Qian, T.; Li, J. Octadecane/C-decorated diatomite composite phase change material with enhanced thermal conductivity as aggregate for developing structural–functional integrated cement for thermal energy storage. Energy 2018, 142, 234–249. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Chen, X.; Zhang, Y.; Li, A.; Wang, G. Advanced multifunctional composite phase change materials based on photo-responsive materials. Nano Energy 2021, 80, 105454. [Google Scholar] [CrossRef]
- Abdeali, G.; Bahramian, A.R.; Abdollahi, M. Review on nanostructure supporting material strategies in shape-stabilized phase change materials. J. Energy Storage 2020, 29, 101299. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Rahman, S.; Yadav, A.; Samykano, M.; Tyagi, V.V. Graphene–Silver Hybrid Nanoparticle Based Organic Phase Change Materials for Enhanced Thermal Energy Storage. Sustainability 2022, 14, 13240. [Google Scholar] [CrossRef]
- Ates, M.; Karazehir, T.; Sezai Sarac, A.J. Conducting polymers and their applications. Curr. Phys. Chem. 2012, 2, 224–240. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Almoadi, A.; AlAbdulaal, T.H.; Alqahtani, M.S.; Harraz, F.A.; Al-Assiri, M.S.; Yahia, I.S.; Zahran, H.Y.; Mohammed, M.I.; Abdel-Wahab, M.S. Structural, Optical, and Electrical Investigations of Nd2O3-Doped PVA/PVP Polymeric Composites for Electronic and Optoelectronic Applications. Polymers 2023, 15, 1351. [Google Scholar] [CrossRef]
- Ichinose, A.; Kuwata, K.; Esaki, T.; Matsuda, T.; Kobayashi, N. Chemical Heat Storage Using an SiC Honeycomb Packed with CaCl2 Powder. J. Mater. Sci. Chem. Eng. 2020, 8, 23–32. [Google Scholar]
- Li, X.; Wang, Z.; Li, W.; Sun, J. Superstrong water-based supramolecular adhesives derived from poly (vinyl alcohol)/poly (acrylic acid) complexes. ACS Mater. Lett. 2021, 3, 875–882. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Al-Kayiem, H.H.; Lin, S.C.; Lukmon, A. Review on nanomaterials for thermal energy storage technologies. Nanosci. Nanotechnol.-Asia 2013, 3, 60–71. [Google Scholar] [CrossRef]
- Vu, M.C.; Choi, W.K.; Lee, S.G.; Park, P.J.; Kim, D.H.; Islam, M.A.; Kim, S.R. High thermal conductivity enhancement of polymer composites with vertically aligned silicon carbide sheet scaffolds. ACS Appl. Mater. Interfaces 2020, 12, 23388. [Google Scholar] [CrossRef]
- Ibrahim, I.D.; Jamiru, T.; Sadiku, E.R.; Hamam, Y.; Alayli, Y.; Eze, A.A. Application of nanoparticles and composite materials for energy generation and storage. IET Nanodielectrics 2019, 2, 115–122. [Google Scholar] [CrossRef]
- Ahmed, H.; Hashim, A. Fabrication of Novel (PVA/NiO/SiC) Nanocomposites, Structural, Electronic and Optical Properties for Humidity Sensors. Int. J. Sci. Technol. Res. 2019, 8, 1014–1031. [Google Scholar]
- Obaid, W.O.; Hashim, A. Synthesis and Augmented Optical Properties of PC/SiC/TaC Hybrid Nanostructures for Potential and Photonics Fields. Silicon 2022, 14, 11199–11207. [Google Scholar] [CrossRef]
- Hashim, A.; Abbas, M.H.; Al-Aaraji, N.A.-H.; Hadi, A. Facile Fabrication and Developing the Structural, Optical and Electrical Properties of SiC/Y2O3 Nanostructures Doped PMMA for Optics and Potential Nanodevices. Silicon 2023, 15, 1283–1290. [Google Scholar] [CrossRef]
- Hashim, A.; Abbas, M.H.; Al-Aaraji, N.A.-H.; Hadi, A. Controlling the Morphological, Optical and Dielectric Characteristics of PS/SiC/CeO2 Nanostructures for Nanoelectronics and Optics Fields. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1–9. [Google Scholar] [CrossRef]
- Mahdi, H.H. Black Carbon Incorporation Effect on Optical Properties of Poly-Methyl Methacrylate Films. Eur. J. Adv. Eng. Technol. 2017, 4, 773–778. [Google Scholar]
- Sheha, E.; Khoder, H.; Shanap, T.S.; El-Shaarawy, M.G.; El Mansy, M.K. Structure, Dielectric and Optical Properties of p-Type (PVA/CuI) Nanocomposite Polymer Electrolyte for Photovoltaic Cells. Optik 2012, 123, 1161–1166. [Google Scholar] [CrossRef]
- Mahalakshmi, K.; Lakshmi, V.; Anitha, S.D.C.; MaryJenila, R. Optical, Structural and Morphological Analysis of RGO Decorated CoSe2 Nanocomposites. Int. J. Innov. Sci. Eng. Technol. 2021, 8, 180–192. [Google Scholar]
- Heiba, Z.K.; Mohamed, M.B.; Ahmed, S.I. Exploring the Physical Properties of PVA/PEG Polymeric Material upon Doping with Nano Gadolinium Oxide. Alex. Eng. J. 2022, 61, 3375–3383. [Google Scholar] [CrossRef]
- Amin, P.O.; Ketuly, K.A.; Saeed, S.R.; Muhammadsharif, F.F.; Symes, M.D.; Paul, A.; Sulaiman, K. Synthesis, Spectroscopic, Electrochemical and Photophysical Properties of High Band Gap Polymers for Potential Applications in Semi-Transparent Solar Cells. BMC Chem. 2021, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, C.; Devi, A. Alteration of Structural, Optical and Electrical Properties of CdSe Incorporated Polyvinyl Pyrrolidone Nanocomposite for Memory Devices. J. Adv. Dielectr. 2018, 8, 1850020. [Google Scholar] [CrossRef]
- Al-Shawabkeh, A.F.; Elimat, Z.M.; Abushgair, K.N. Effect of Non-Annealed and Annealed ZnO on the Optical Properties of PVC/ZnO Nanocomposite Films. J. Thermoplast. Compos. Mater. 2023, 36, 899–915. [Google Scholar] [CrossRef]
- Gaabour, L.H. Analysis of Spectroscopic, Optical and Magnetic Behaviour of PVDF/PMMA Blend Embedded by Magnetite (Fe3O4) Nanoparticles. Opt. Photon. J. 2020, 10, 197. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Ahmad, A.A.; Alsaad, A.M.; Telfah, A.D. Optical Characterizations of PMMA/Metal Oxide Nanoparticles Thin Films: Bandgap Engineering Using a Novel Derived Model. Heliyon 2021, 7, e05952. [Google Scholar] [CrossRef] [PubMed]
- Said, Z.; Sajid, M.H.; Saidur, R.; Mahdiraji, G.A.; Rahim, N.A. Evaluating the optical properties of TiO2 nanofluid for a direct absorption solar collector. Numer. Heat Transf. Part A Appl. 2015, 67, 1010–1027. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.J.; Du, X.; Wen, D. Photothermal conversion characteristics of gold nanoparticle dispersions. Sol. Energy 2014, 100, 141–147. [Google Scholar] [CrossRef]
- Verma, K.C.; Kotnala, R.K.; Ram, M.; Negi, N.S. Optical Characteristics of Nanocrystalline Pb1–x Sr x TiO3 Thin Films Using Transmission Measurements. Adv. Sci. Focus 2013, 1, 13–19. [Google Scholar] [CrossRef]
- Gayitri, H.M.; Al-Gunaid, M.; Prakash, A.P.G. Optical, Structural and Thermal Properties of Hybrid PVA/CaAl2ZrO6 Nanocomposite Films. Indian J. Eng. Mater. Sci. 2020, 27, 320–332. [Google Scholar]
- Atta, A.; Abdelhamied, M.M.; Abdelreheem, A.M.; Berber, M.R. Flexible Methyl Cellulose/Polyaniline/Silver Composite Films with Enhanced Linear and Nonlinear Optical Properties. Polymers 2021, 13, 1225. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Omer, K.M.; Salih, Y.M. Reducing the optical band gap of polyvinyl alcohol (PVA) based nanocomposite. J. Mater. Sci. Mater. Electron. 2015, 26, 5303–5309. [Google Scholar] [CrossRef]
- Makino, T.; Segawa, Y.; Kawasaki, M.; Koinuma, H. Optical properties of excitons in ZnO-based quantum well heterostructures. Semicond. Sci. Technol. 2005, 20, S78. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Magesh, S.; Kalaiselvam, S. Preparation and thermal energy storage behaviour of stearic acid–TiO2 nanofluids as a phase change material for solar heating systems. Thermochim. Acta 2013, 565, 137–145. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, F.L.; Hashim, A.; Dulaimi, A.; Hadi, A.; Ibrahim, H.; Al-Obaidi, M.A.; Ameen, A. Enhancement of Polyacrylic Acid/Silicon Carbide Nanocomposites’ Optical Properties for Potential Application in Renewable Energy. J. Compos. Sci. 2024, 8, 123. https://doi.org/10.3390/jcs8040123
Rashid FL, Hashim A, Dulaimi A, Hadi A, Ibrahim H, Al-Obaidi MA, Ameen A. Enhancement of Polyacrylic Acid/Silicon Carbide Nanocomposites’ Optical Properties for Potential Application in Renewable Energy. Journal of Composites Science. 2024; 8(4):123. https://doi.org/10.3390/jcs8040123
Chicago/Turabian StyleRashid, Farhan Lafta, Ahmed Hashim, Anmar Dulaimi, Aseel Hadi, Hamed Ibrahim, Mudhar A. Al-Obaidi, and Arman Ameen. 2024. "Enhancement of Polyacrylic Acid/Silicon Carbide Nanocomposites’ Optical Properties for Potential Application in Renewable Energy" Journal of Composites Science 8, no. 4: 123. https://doi.org/10.3390/jcs8040123
APA StyleRashid, F. L., Hashim, A., Dulaimi, A., Hadi, A., Ibrahim, H., Al-Obaidi, M. A., & Ameen, A. (2024). Enhancement of Polyacrylic Acid/Silicon Carbide Nanocomposites’ Optical Properties for Potential Application in Renewable Energy. Journal of Composites Science, 8(4), 123. https://doi.org/10.3390/jcs8040123









