Water–Gas Shift Activity over Ni/Al2O3 Composites
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Catalysts Preparation
2.2. Catalyst Characterization
2.3. Water–Gas Shift Activity
3. Results and Discussion
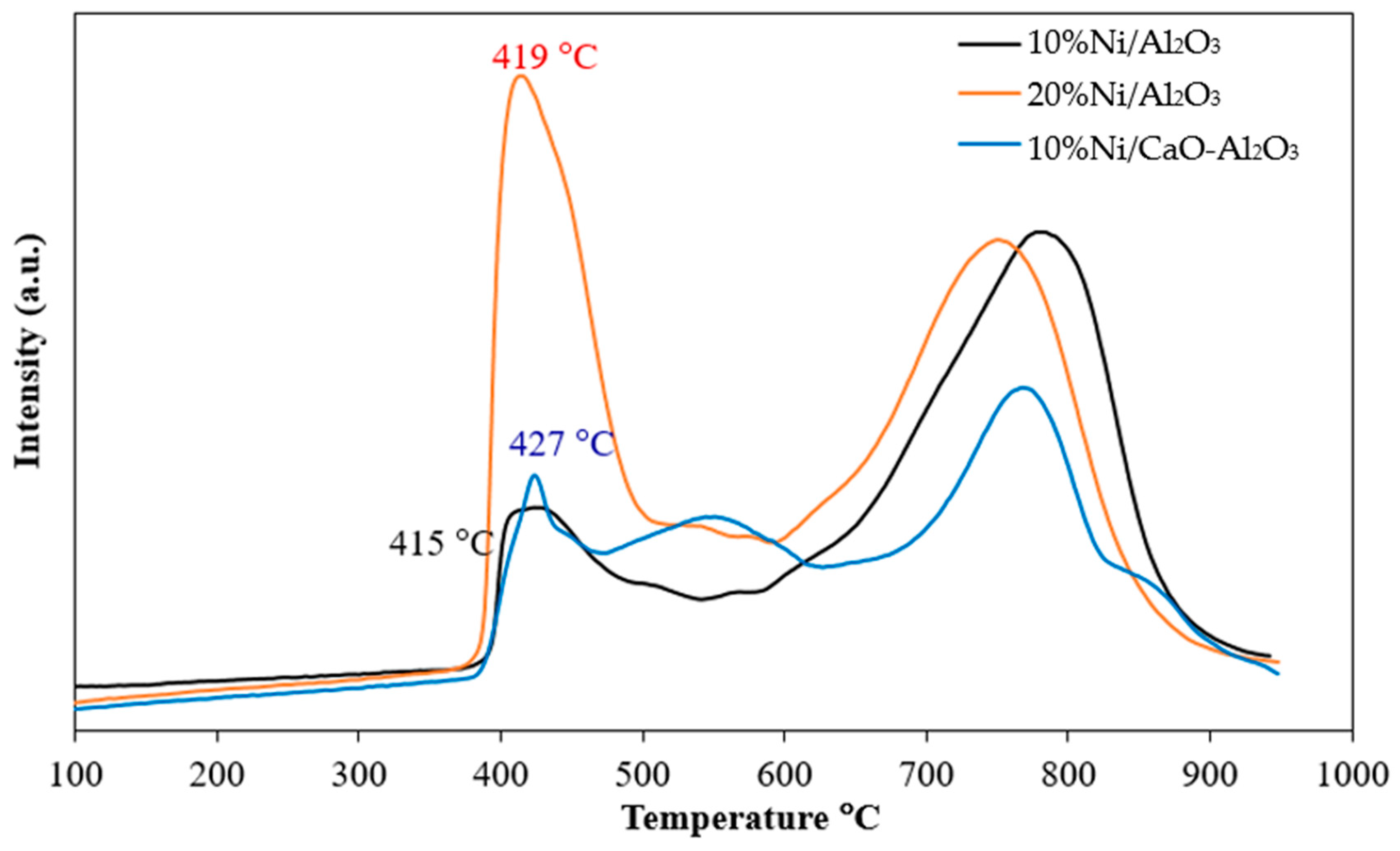
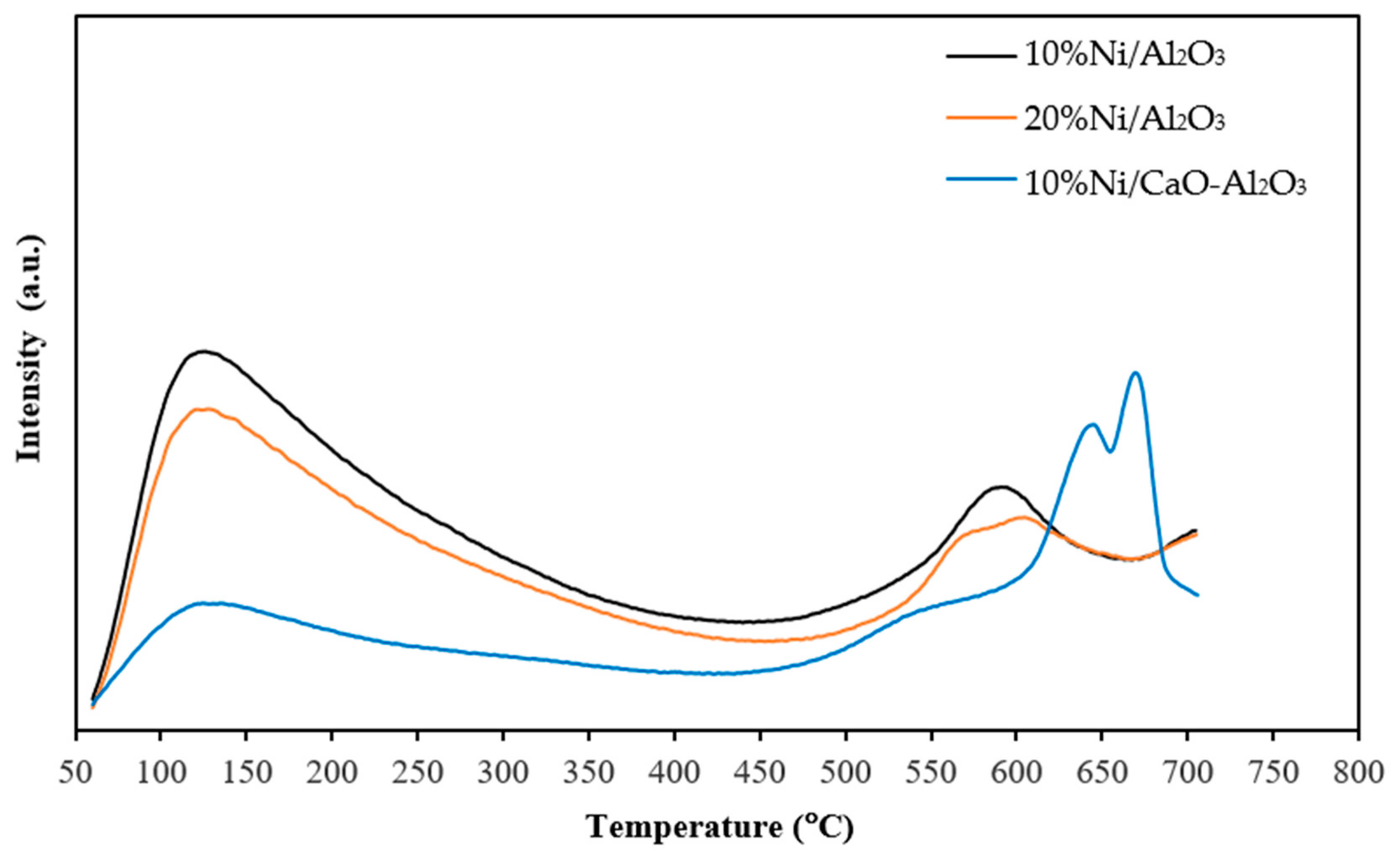
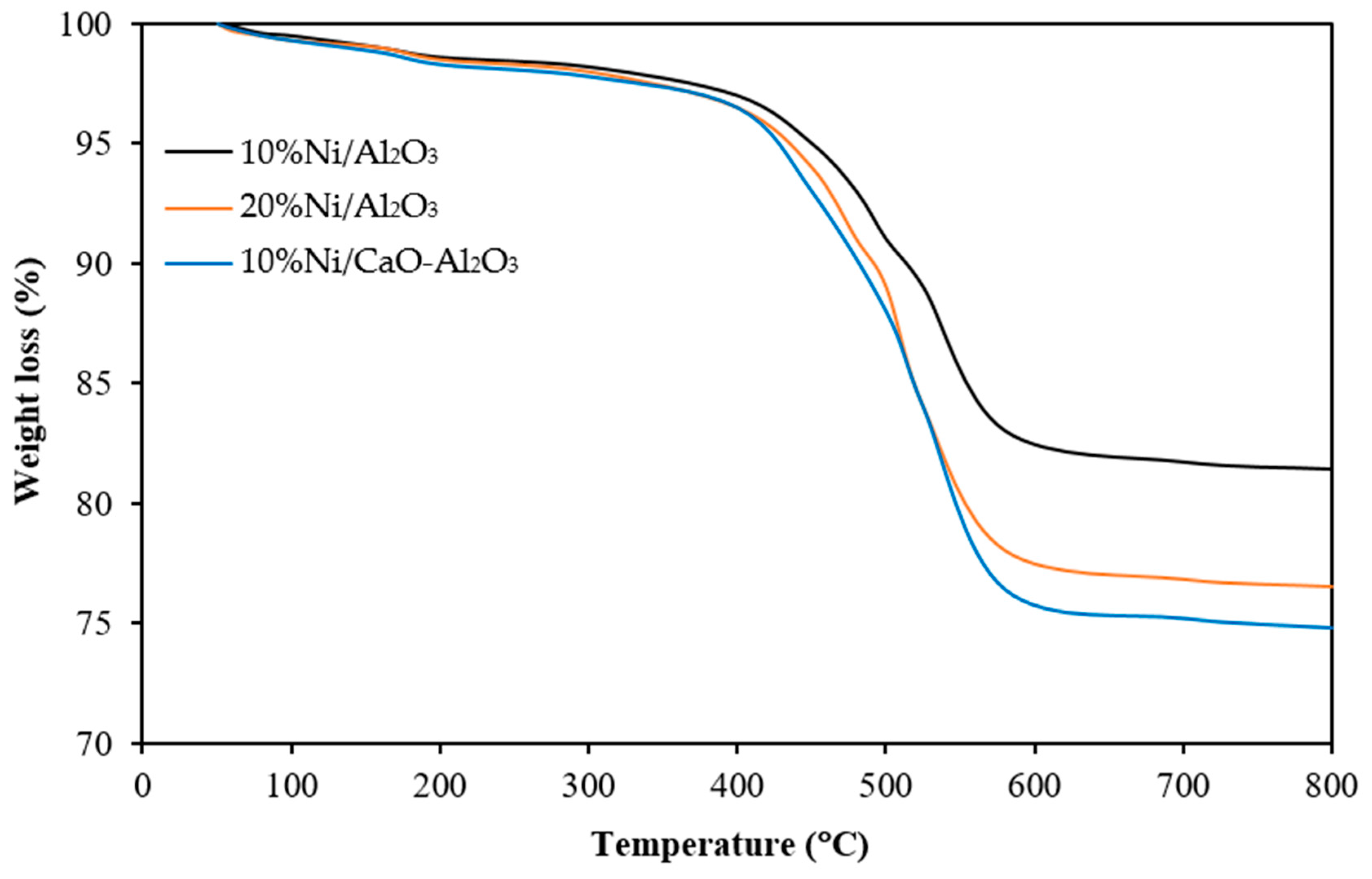
3.1. Catalysts Characterization
3.2. Water–Gas Shift Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palma, V.; Castaldo, F.; Ciambelli, P.; Iaquaniello, G. CeO2-supported Pt/Ni catalyst for the renewable and clean H2 production via ethanol steam reforming. Appl. Catal. B Environ. 2014, 145, 73–84. [Google Scholar] [CrossRef]
- Kok, E.; Scott, J.; Cant, N.; Trimm, D. The impact of ruthenium, lanthanum and activation conditions on the methanation activity of alumina-supported cobalt catalysts. Catal. Today 2011, 164, 297–301. [Google Scholar] [CrossRef]
- Tojira, O.; Lomonaco, J.G.; Sesuk, T.; Charojrochkul, S.; Tepamatr, P. Enhancement of hydrogen production using Ni catalysts supported by Gd-doped ceria. Heliyon 2021, 7, e08202. [Google Scholar] [CrossRef] [PubMed]
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukewicz, W.; Czylkowska, A.; Maniecki, T. Steam reforming of ethanol for hydrogen production: Influence of catalyst composition (Ni/Al2O3, Ni/Al2O3-CeO2, Ni/Al2O3-ZnO) and process conditions. React. Kinet. Mech. Catal. 2021, 132, 907–919. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Pant, K.K.; Mohanty, P.; Agarwal, S.; Dalai, A.K. Steam reforming of acetic acid for hydrogen production over bifunctional Ni-Co catalysts. Catal. Today 2013, 207, 36–43. [Google Scholar] [CrossRef]
- Matras, J.; Niewiadomski, M.; Ruppert, A.; Grams, J. Activity of Ni catalysts for hydrogen production via biomass pyrolysis. Kinet. Catal. 2012, 53, 565–569. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Menegazzo, F.; Ghedini, E.; Innocenti, G.; Di Michele, A.; Cruciani, G.; Cavani, F.; Signoretto, M. Increase of ceria redox ability by lanthanum addition on Ni based catalysts for hydrogen production. ACS Sustain. Chem. Eng. 2018, 6, 13867–13876. [Google Scholar] [CrossRef]
- Fuentes, E.M.; Ruiz, J.R.; Arroyo, E.C.; Rangel, M.C.; Fuentes, E.E. Study of NiO/Al2O3 and NiO/Zn-Al2O3 catalysts for water gas shift reaction. Univ. Sci. 2023, 28, 316–335. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.D.; To, S.D.F.; Steele, P.H.; Pordesimo, L.; Adhikari, S. High Temperature Water Gas Shift Reaction over Nickel Catalysts for Hydrogen Production: Effect of Supports, GHSV, Metal Loading, and Dopant Materials. J. Thermodyn. Catal. 2011, 2, 106. [Google Scholar] [CrossRef]
- Ranjbar, A.; Irankhah, A.; Aghamiri, S.F. Catalytic activity of rare earth and alkali metal promoted (Ce, La, Mg, K) Ni/Al2O3 nanocatalysts in reverse water gas shift reaction. Res. Chem. Intermediat. 2019, 45, 5125–5141. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, P.; Zhang, S.; Gu, J.; Wang, D. Effects of Metal-support interactions and interfaces on catalytic performance over M2O3-(M = La, Al-) supported Ni catalysts. Int. J. Energy Res. 2023, 2023, 6504914. [Google Scholar] [CrossRef]
- Lomonaco, J.G.; Sesuk, T.; Charojrochkul, S.; Tepamatr, P. Effect of Re addition on the water–gas shift activity of Ni catalyst supported by mixed oxide materials for H2 production. Catalysts 2023, 13, 959. [Google Scholar] [CrossRef]
- Tojira, O.; Tepamatr, P. Catalytic activity of Ni based materials prepared by different methods for hydrogen production via the water gas shift reaction. Catalysts 2023, 13, 176. [Google Scholar] [CrossRef]
- Liu, Z.; Chu, B.; Zhai, X.; Jin, Y.; Cheng, Y. Total methanation of syngas to synthetic natural gas over Ni catalyst in a micro-channel reactor. Fuel 2012, 95, 599–605. [Google Scholar] [CrossRef]
- Cai, M.D.; Wen, J.; Chu, W.; Cheng, X.Q.; Li, Z.J. Methanation of carbon dioxide on Ni/ZrO2-Al2O3 catalysts: Effects of ZrO2 promoter and preparation method of novel ZrO2-Al2O3 carrier. J. Nat. Gas Chem. 2011, 20, 318–324. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Song, I.K. Effect of SiO2-ZrO2 supports prepared by a grafting method on hydrogen production by steam reforming of liquefied natural gas over Ni/SiO2-ZrO2 catalysts. J. Mol. Catal. A 2007, 168, 251–257. [Google Scholar] [CrossRef]
- Sangrama, K.S.; Siddharth, S.R.; Singh, I.D. Structural characterization of coke on spent hydroprocessing catalysts used for processing of vacuum gas oils. Appl. Catal. A Gen. 2004, 278, 83–91. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, Y.L.; Na, H.S.; Ahn, S.Y.; Shim, J.O.; Jeon, B.H.; Roh, H.S. Efficient waste to energy conversion based on Co-CeO2 catalyzed water-gas shift reaction. Catalysts 2020, 10, 420. [Google Scholar] [CrossRef]

| Catalysts | BET Surface Area a (m2/g) | Total Pore Volume a (cm3/g) | Average Pore Diameter a (nm) | % Ni Dispersion b | Average Particle Size b (nm) |
|---|---|---|---|---|---|
| 10%Ni/Al2O3 | 98 | 0.1123 | 3.8928 | 6.74 | 8.7 |
| 10%Ni/CaO-Al2O3 | 22 | 0.0515 | 8.2550 | 4.01 | 18.6 |
| 20%Ni/Al2O3 | 84 | 0.0993 | 3.9071 | 4.38 | 14.2 |
| Samples | Weak Acidity (50–400 °C) (μmol/g) | Strong Acidity (500–700 °C) (μmol/g) | Total Acidity (μmol/g) |
|---|---|---|---|
| 10%Ni/Al2O3 | 178.2 | 25.7 | 203.9 |
| 10%Ni/CaO-Al2O3 | 50.7 | 39.5 | 90.2 |
| 20%Ni/Al2O3 | 150.7 | 20.6 | 171.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepamatr, P.; Charojrochkul, S.; Laosiripojana, N. Water–Gas Shift Activity over Ni/Al2O3 Composites. J. Compos. Sci. 2024, 8, 239. https://doi.org/10.3390/jcs8070239
Tepamatr P, Charojrochkul S, Laosiripojana N. Water–Gas Shift Activity over Ni/Al2O3 Composites. Journal of Composites Science. 2024; 8(7):239. https://doi.org/10.3390/jcs8070239
Chicago/Turabian StyleTepamatr, Pannipa, Sumittra Charojrochkul, and Navadol Laosiripojana. 2024. "Water–Gas Shift Activity over Ni/Al2O3 Composites" Journal of Composites Science 8, no. 7: 239. https://doi.org/10.3390/jcs8070239
APA StyleTepamatr, P., Charojrochkul, S., & Laosiripojana, N. (2024). Water–Gas Shift Activity over Ni/Al2O3 Composites. Journal of Composites Science, 8(7), 239. https://doi.org/10.3390/jcs8070239





