Effect of PVDF, HA, and AgNO3 Annealing on β-Phase, Optical, and Mechanical Properties
Abstract
1. Introduction
2. Characterization of PVDF-Based Piezoelectric Materials
2.1. PVDF β-Phase Effects for Sensors Application
2.2. Composites Based on PVDF
3. Methods and Preparation
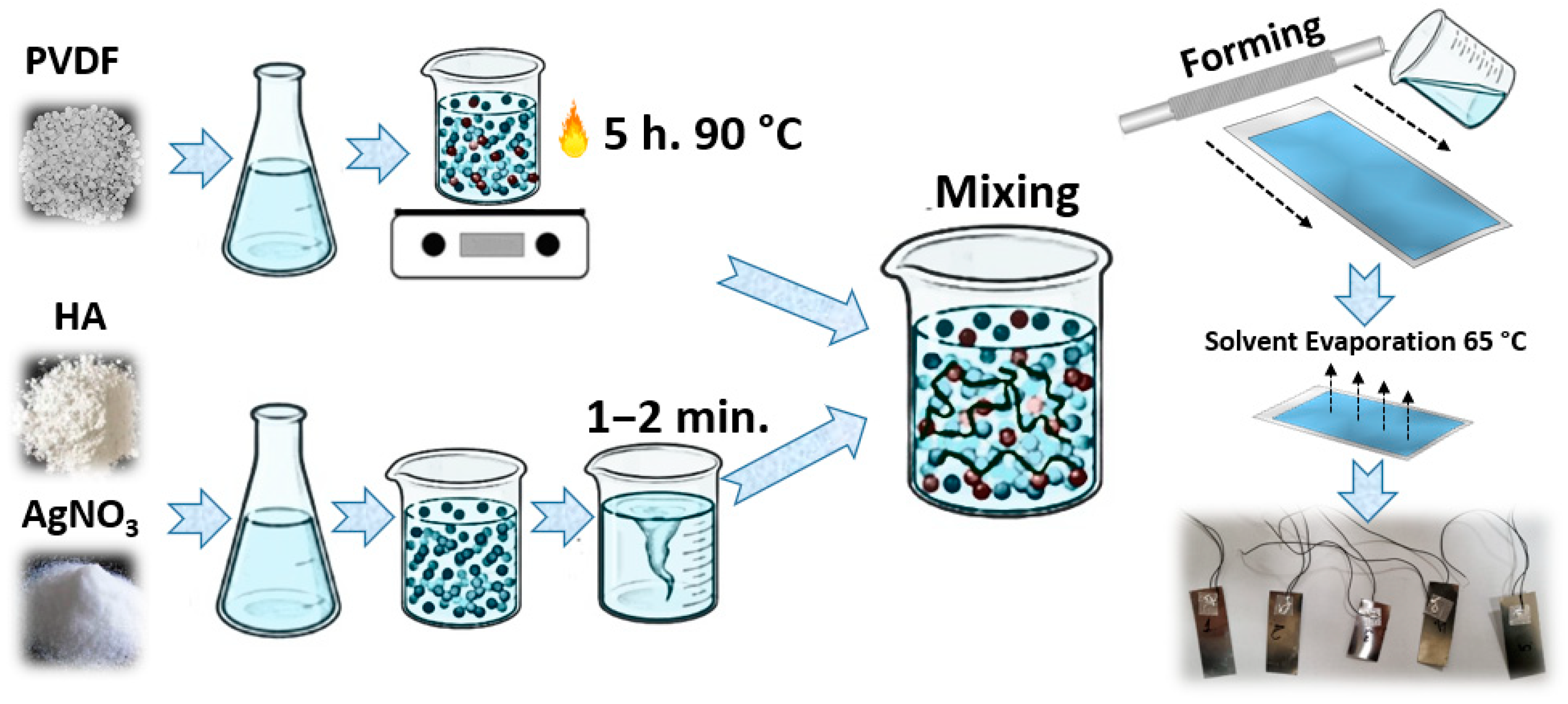
3.1. Preparation Process
3.2. Surface Roughness
3.3. Multifractal Spectra’s Method
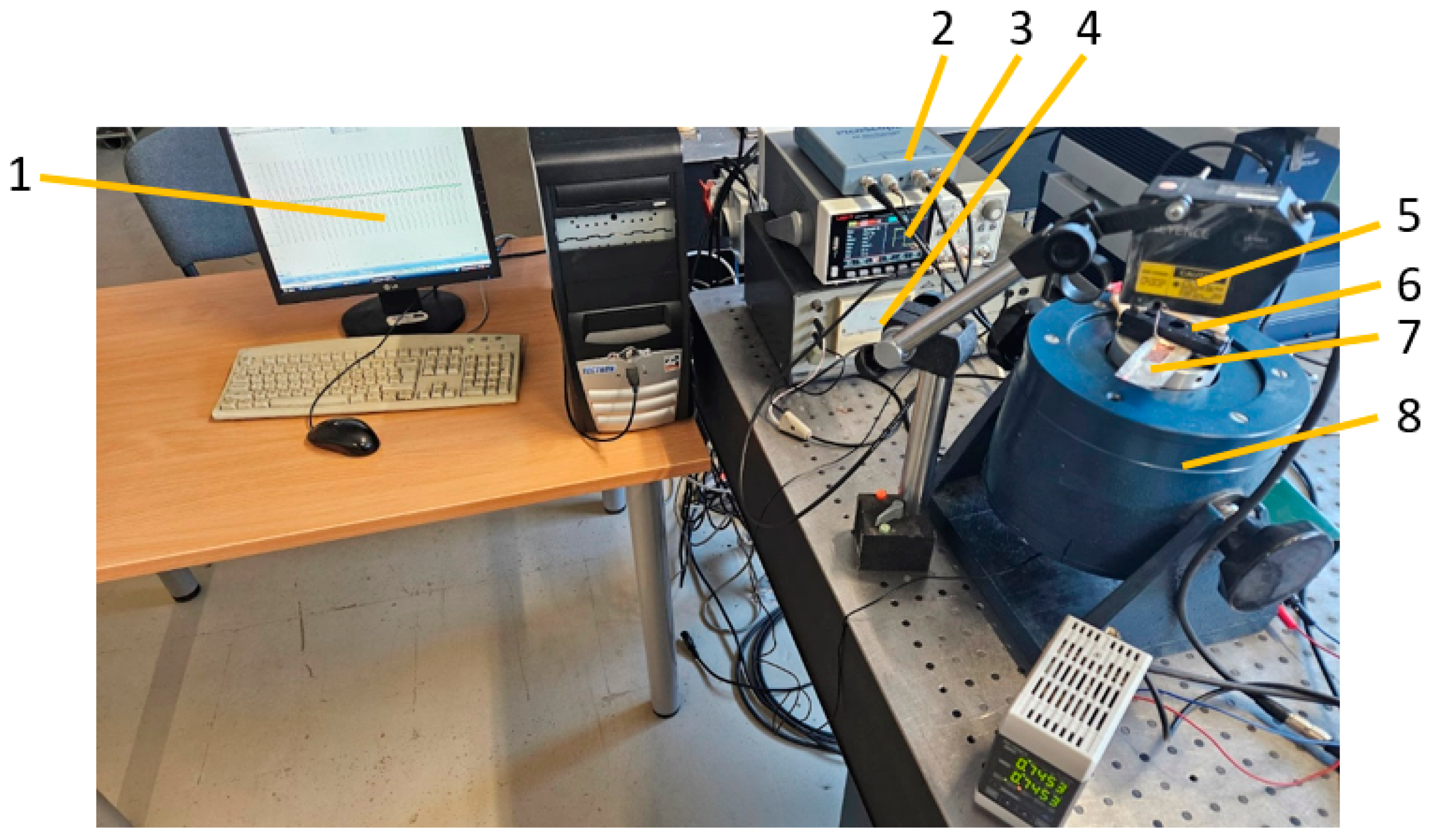
3.4. Vibration Parameters
3.5. Hydrophilicity Parameters
4. Results
4.1. Surface Roughness Results
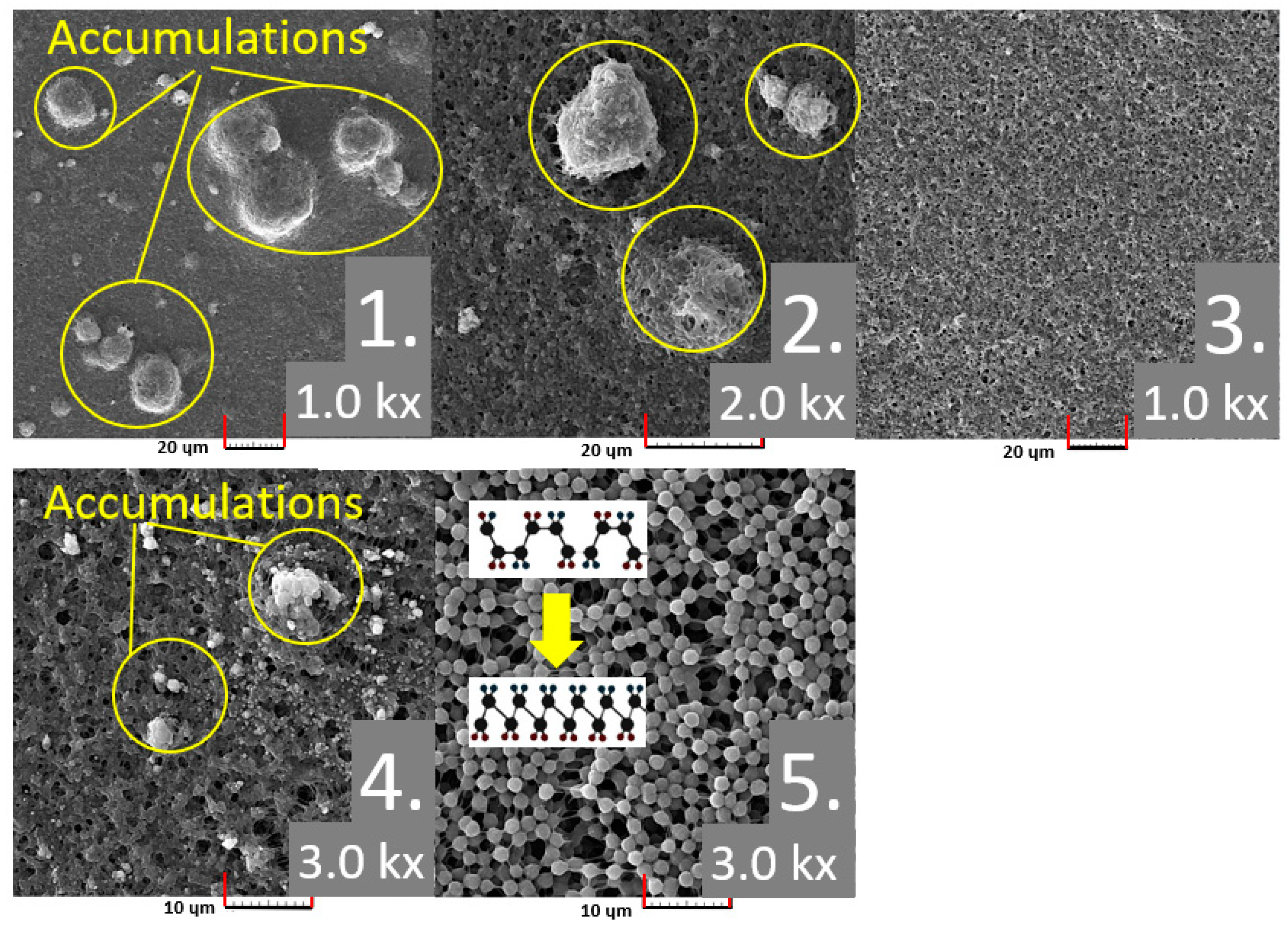
4.2. SEM Analysis and Multifractal Spectra
4.3. Vibration Results
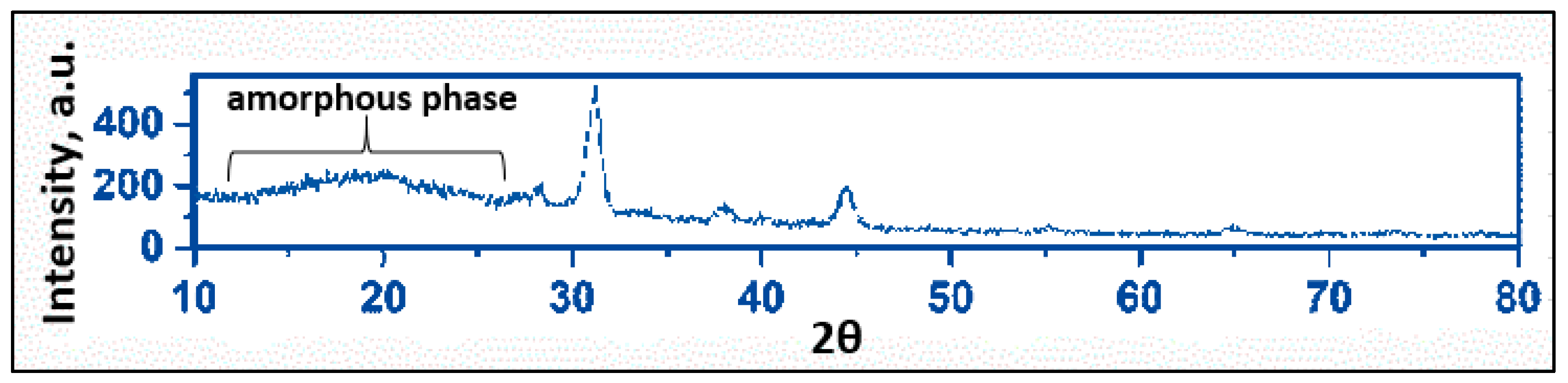
4.4. EDX, XDS and Mapping Analysis
4.5. Hydrophilicity Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, G.; Wang, Y.; Tan, D.Q. A review of surface roughness impact on dielectric film properties. IET Nanodielectr. 2021, 5, 1–23. [Google Scholar] [CrossRef]
- Al Rashid, A.; Khan, S.A.; Al-Ghamdi, S.G.; Koç, M. Additive manufacturing of polymer nanocomposites: Needs and challenges in materials, processes, and applications. J. Mater. Res. Technol. 2021, 14, 910–941. [Google Scholar] [CrossRef]
- Smirnov, A.; Chugunov, S.; Kholodkova, A.; Isachenkov, M.; Vasin, A.; Shishkovsky, I. Progress and challenges of 3D-printing technologies in the manufacturing of piezoceramics. Ceram. Int. 2021, 47, 10478–10511. [Google Scholar] [CrossRef]
- Ribeiro, A.; Vaz, L.; Guastaldi, A.; Campos, J. Adhesion strength characterization of PVDF/HA coating on cp Ti surface modified by laser beam irradiation. Appl. Surf. Sci. 2012, 258, 10110–10114. [Google Scholar] [CrossRef]
- Lu, L.; Ding, W.; Liu, J.; Yang, B. Flexible PVDF based piezoelectric nanogenerators. Nano Energy 2020, 78, 105251. [Google Scholar] [CrossRef]
- Dashtizad, S.; Alizadeh, P.; Yourdkhani, A. Improving piezoelectric properties of PVDF fibers by compositing with BaTiO3-Ag particles prepared by sol-gel method and photochemical reaction. J. Alloy. Compd. 2021, 883, 160810. [Google Scholar] [CrossRef]
- Ambrožová, N.; Zálešák, B.; Ulrichová, J.; Čížková, K.; Galandáková, A. Low concentrations of silver nanoparticles have a beneficial effect on wound healing in vitro. J. Nanopart. Res. 2017, 19, 108. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Pokhel, S. Hydroxyapatite: Preparation, Properties and Its Biomedical Applications. Adv. Chem. Eng. Sci. 2018, 8, 87542. [Google Scholar] [CrossRef]
- Roopaa, T.; Murthy, H.N.; Kumar, V.P.; Krishna, M. Development and Characterization of PVDF Thin Films for pressure sensors. Mater. Today Proc. 2018, 5, 21082–21090. [Google Scholar] [CrossRef]
- Tandon, B.; Kamble, P.; Olsson, R.T.; Blaker, J.J.; Cartmell, S.H. Fabrication and Characterisation of Stimuli Responsive Piezoelectric PVDF and Hydroxyapatite-Filled PVDF Fibrous Membranes. Molecules 2019, 24, 1903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, D.; Ma, J. Dielectric Properties of PVDF/Ag/BaTiO3Composites. Ferroelectrics 2013, 455, 77–82. [Google Scholar] [CrossRef]
- Malherbi, M.S.; Dias, L.C.; Lima, M.S.; Ribeiro, L.G.; Freitas, V.F.; Bonadio, T.G.; Silva, L.M.; Souza, G.B.; Volnistem, E.A.; Rosso, J.M.; et al. Electrically stimulated bioactivity in hydroxyapatite/β-tricalcium phosphate/polyvinylidene fluoride biocomposites. J. Mater. Res. Technol. 2022, 20, 169–179. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; He, T.; Hu, Q.; Yang, Y. Preparation of PVDF flexible piezoelectric film with high β-phase content by matching solvent dipole moment and crystallization temperature. J. Mater. Sci. Mater. Electron. 2019, 30, 20174–20180. [Google Scholar] [CrossRef]
- Satapathy, S.; Pawar, S.; Gupta, P.K.; Varma, K.B.R. Effect of annealing on phase transition in poly(vinylidene fluoride) films prepared using polar solvent. J. Indian Acad. Sci. 2011, 34, 727–733. [Google Scholar] [CrossRef]
- Parida, A.; Swain, S.; Sahu, R.; Negi, R.R. Phase formation and electrical properties study of PVDF thick films synthesized by solution casting method. Int. J. Mater. Res. 2023, 114, 344–350. [Google Scholar] [CrossRef]
- Hari, M.A.; Rajan, L.; Subash, C.; Varghese, S. Effect of nanoparticle size on the piezoelectric properties of PVDF based nanocomposite thin films. Mater. Today Proc. 2021, 46, 5781–5784. [Google Scholar] [CrossRef]
- Xie, L.; Wang, G.; Jiang, C.; Yu, F. Properties and Applications of Flexible Poly(Vinylidene Fluoride)-Based Piezoelectric Materials. Crystals 2021, 11, 644. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, Z.; Zhu, H. Dielectric properties of Ag@C/PVDF composites. J. Appl. Polym. Sci. 2013, 129, 3411–3416. [Google Scholar] [CrossRef]
- Su, Y.; Gu, Y.; Li, H.; Geng, F. Ag-NBCTO-PVDF composites with enhanced dielectric properties. Mater. Lett. 2016, 185, 208–210. [Google Scholar] [CrossRef]
- Choi, J.; Lee, M.; Kim, T.; Sim, J.H.; Lee, W.B.; Kim, Y.; Kang, C.P.Y. High β-phase Poly(vinylidene fluoride) Using a Thermally Decomposable Molecular Splint. Adv. Electron. Mater. 2022, 9, 2200279. [Google Scholar] [CrossRef]
- Fritz, S.E.; Kelley, T.W.; Frisbie, C.D. Effect of Dielectric Roughness on Performance of Pentacene TFTs and Restoration of Performance with a Polymeric Smoothing Layer. J. Phys. Chem. B 2005, 109, 10574–10577. [Google Scholar] [CrossRef]
- Zhengkun, Y.; Yilei, Z. Recognizing tactile surface roughness with a biomimetic fingertip: A soft neuromorphic approach. Neurocomputing 2017, 244, 102–111. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, Y.; Peters, J. Bioinspired tactile sensor for surface roughness discrimination. Sens. Actuators A Phys. 2017, 255, 46–53. [Google Scholar] [CrossRef]
- Suh, A.Y.; Polycarpou, A. Effect of Molecularly Thin Lubricant on Roughness and Adhesion of Magnetic Disks Intended for Extremely High-Density Recording. Tribol. Lett. 2003, 15, 365–376. [Google Scholar] [CrossRef]
- Xie, J.; Qiao, Y.; Wang, Z.; Qi, Y.; Xu, Q.; Shemtov-Yona, K.; Chen, P.; Rittel, D. Application of the Taguchi method to areal roughness-based surface topography control by waterjet treatments. Appl. Surf. Sci. Adv. 2024, 19, 100548. [Google Scholar] [CrossRef]
- Krupiński, M.; Wawrzaszek, A.; Drzewiecki, W.; Jenerowicz, M.; Aleksandrowicz, S. What Can Multifractal Analysis Tell Us about Hyperspectral Imagery. Remote Sens. 2020, 12, 4077. [Google Scholar] [CrossRef]
- Krzyszczak, J.; Baranowski, P.; Zubik, M.; Kazandjiev, V.; Georgieva, V.; Sławiński, C.; Siwek, K.; Kozyra, J.; Nieróbca, A. Multifractal characterization and comparison of meteorological time series from two climatic zones. Theor. Appl. Clim. 2019, 137, 1811–1824. [Google Scholar] [CrossRef]
- Xiao, B.; Rutherford, G.N.; Sharma, A.P.; Pradhan, S.K.; Bonner, C.E.; Bahoura, M.J. Surface Modification and Charge Injection in a Nanocomposite of Metal Nanoparticles and Semiconductor Oxide Nanostructures. Sci. Rep. 2020, 10, 4743. [Google Scholar] [CrossRef] [PubMed]
- Alencherry, T.; Naveen, A.R.; Ghosh, S.; Daniel, J.; Venkataraghavan, R. Effect of increasing electrical conductivity and hydrophilicity on the electrosorption capacity of activated carbon electrodes for capacitive deionization. Desalination 2017, 415, 14–19. [Google Scholar] [CrossRef]
- Meaney, P.M.; Fox, C.J.; Geimer, S.D.; Paulsen, K.D. Electrical Characterization of Glycerin: Water Mixtures: Implications for Use as a Coupling Medium in Microwave Tomography. IEEE Trans. Microw. Theory Tech. 2017, 65, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Imani, F.; Sakpal, A.; Reutzel, E.W.; Yang, H. Multifractal analysis of image profiles for the characterization and detection of defects in additive manufacturing. J. Manuf. Sci. Eng. 2017, 140, 031014. [Google Scholar] [CrossRef]
- Imani, F.; Yao, B.; Chen, R.; Rao, P.; Yang, H. Joint Multifractal and Lacunarity Analysis of Image Profiles for Manufacturing Quality Control. J. Manuf. Sci. Eng. 2019, 141, 044501. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H. Numerical simulation and pattern characterization of nonlinear spatiotemporal dynamics on fractal surfaces for the whole-heart modeling applications. Eur. Phys. J. B 2016, 89, 181. [Google Scholar] [CrossRef]
- Augustyniak, J.; Zgłobicka, I.; Kurzydłowski, K.; Misiak, P.; Wilczewska, A.Z.; Gluch, J.; Liao, Z.; Perkowski, D.M. Characterization of nanofluids using multifractal analysis of a liquid droplet trace. Sci. Rep. 2022, 12, 11111. [Google Scholar] [CrossRef]

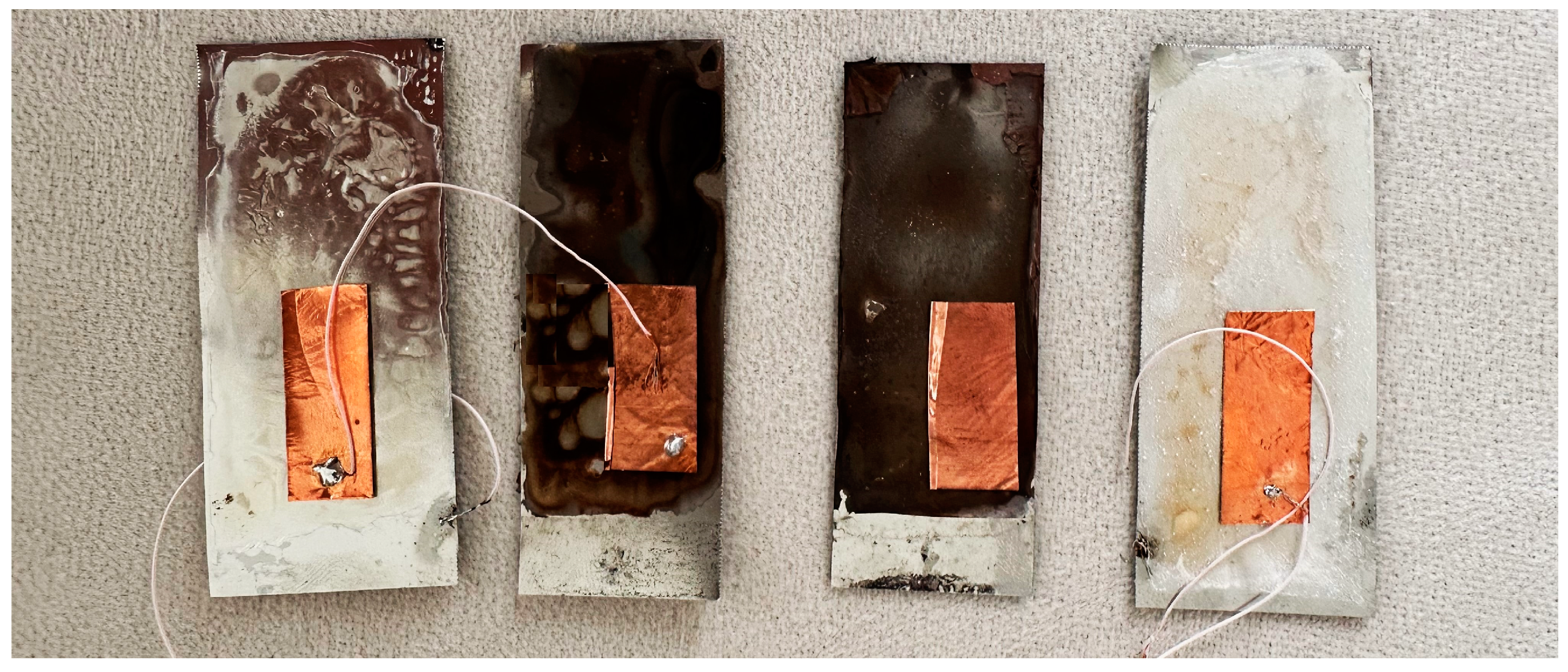










| Specimen Number | PVDF in DMSO | HA in DMSO | AgNO3 in DMSO |
|---|---|---|---|
| (1) | 0.5 g/2 mL | 0.05 g/1 mL | - |
| (2) | 0.5 g/2 mL | 0.1 g/1 mL | - |
| (3) | 0.5 g/2 mL | - | 0.2 g/1 mL |
| (4) | 0.5 g/2 mL | 0.05 g/1 mL | 0.2 g/1 mL |
| (5) | 0.5 g/2 mL | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markuniene, I.; Palevicius, A.; Vezys, J.; Augustyniak, J.; Perkowski, D.; Urbaite, S.; Janusas, G. Effect of PVDF, HA, and AgNO3 Annealing on β-Phase, Optical, and Mechanical Properties. J. Compos. Sci. 2024, 8, 240. https://doi.org/10.3390/jcs8070240
Markuniene I, Palevicius A, Vezys J, Augustyniak J, Perkowski D, Urbaite S, Janusas G. Effect of PVDF, HA, and AgNO3 Annealing on β-Phase, Optical, and Mechanical Properties. Journal of Composites Science. 2024; 8(7):240. https://doi.org/10.3390/jcs8070240
Chicago/Turabian StyleMarkuniene, Ieva, Arvydas Palevicius, Joris Vezys, Jakub Augustyniak, Dariusz Perkowski, Sigita Urbaite, and Giedrius Janusas. 2024. "Effect of PVDF, HA, and AgNO3 Annealing on β-Phase, Optical, and Mechanical Properties" Journal of Composites Science 8, no. 7: 240. https://doi.org/10.3390/jcs8070240
APA StyleMarkuniene, I., Palevicius, A., Vezys, J., Augustyniak, J., Perkowski, D., Urbaite, S., & Janusas, G. (2024). Effect of PVDF, HA, and AgNO3 Annealing on β-Phase, Optical, and Mechanical Properties. Journal of Composites Science, 8(7), 240. https://doi.org/10.3390/jcs8070240







