High-Temperature Synthesis of Superconducting MgB2 Materials in a Centrifuge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
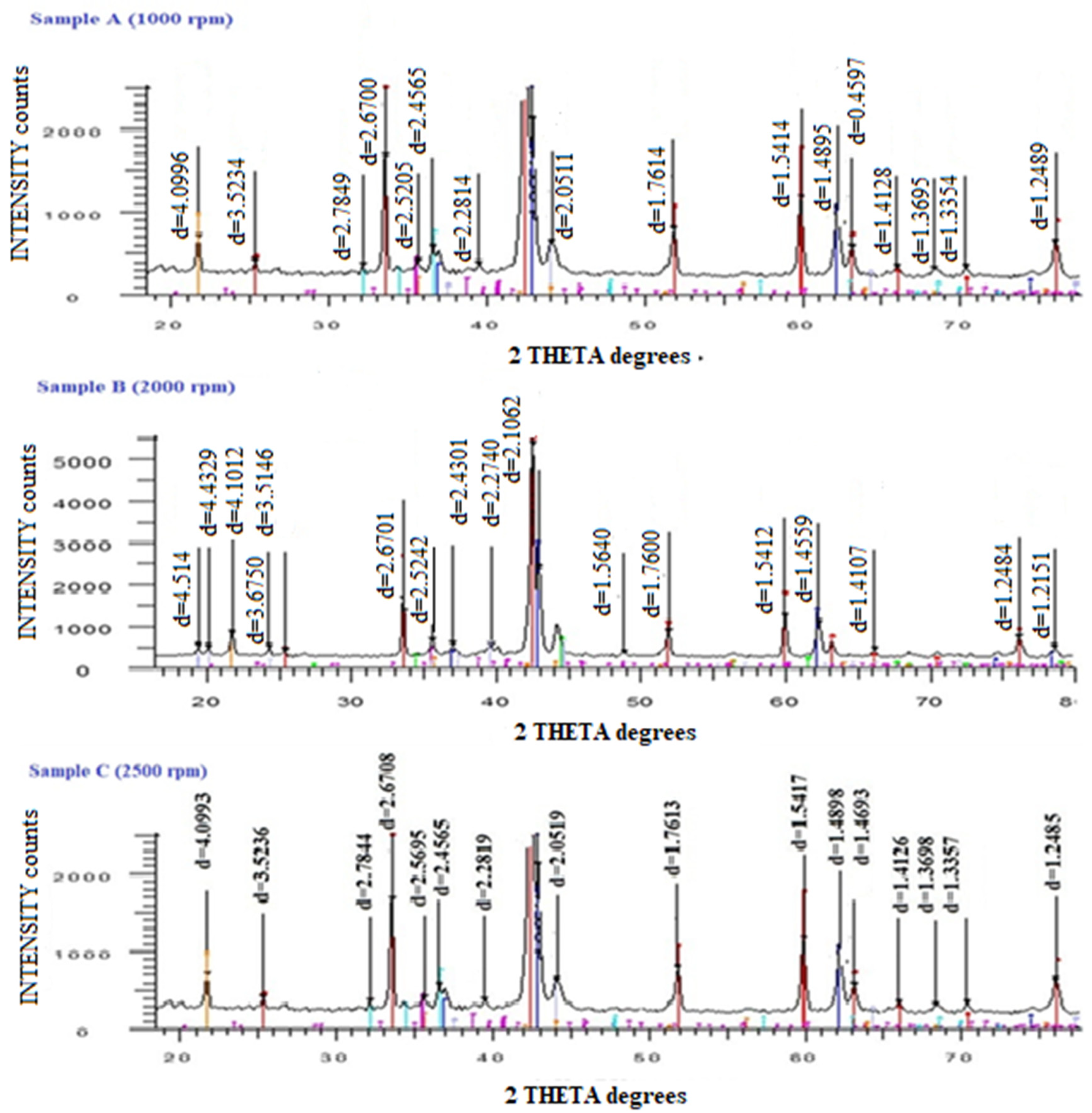
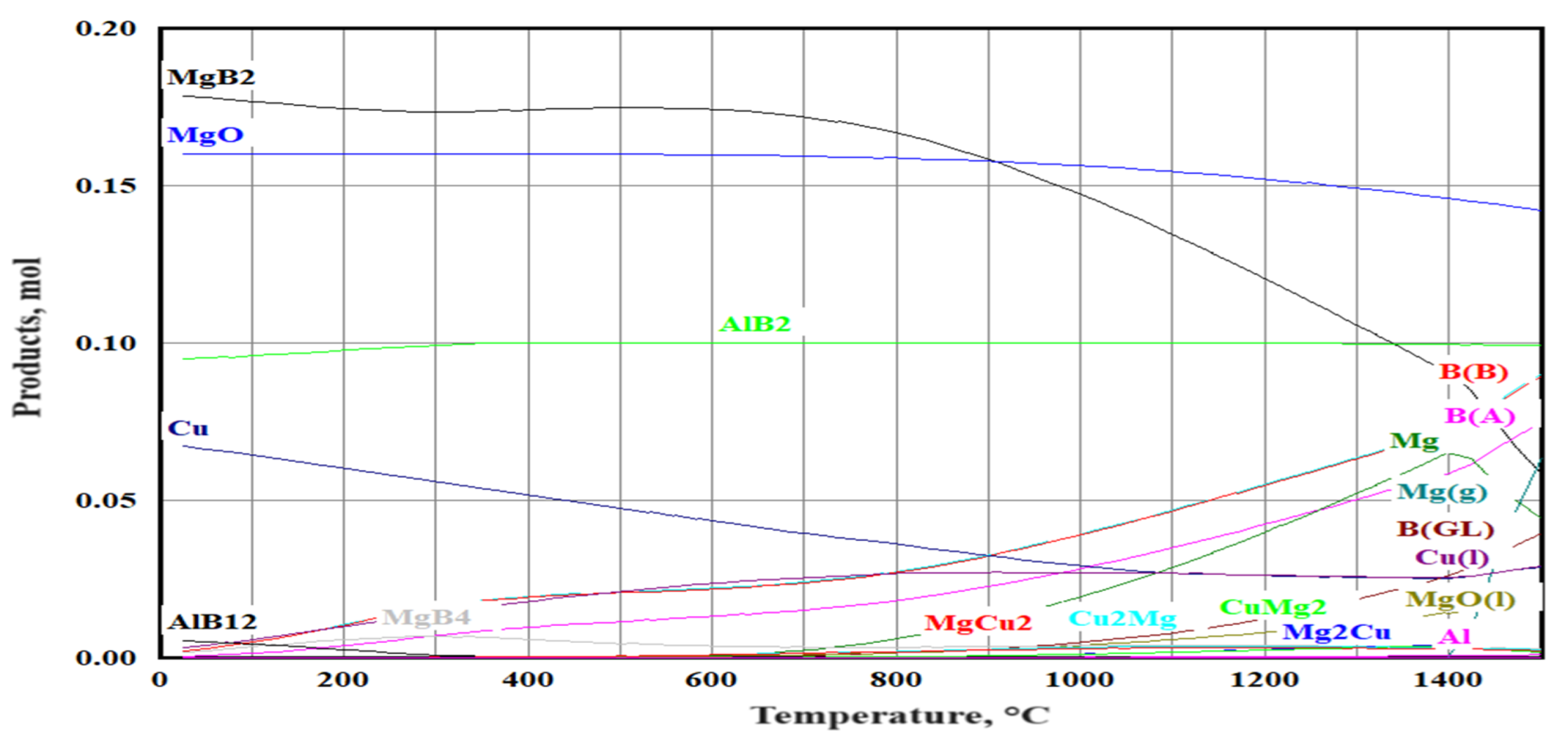
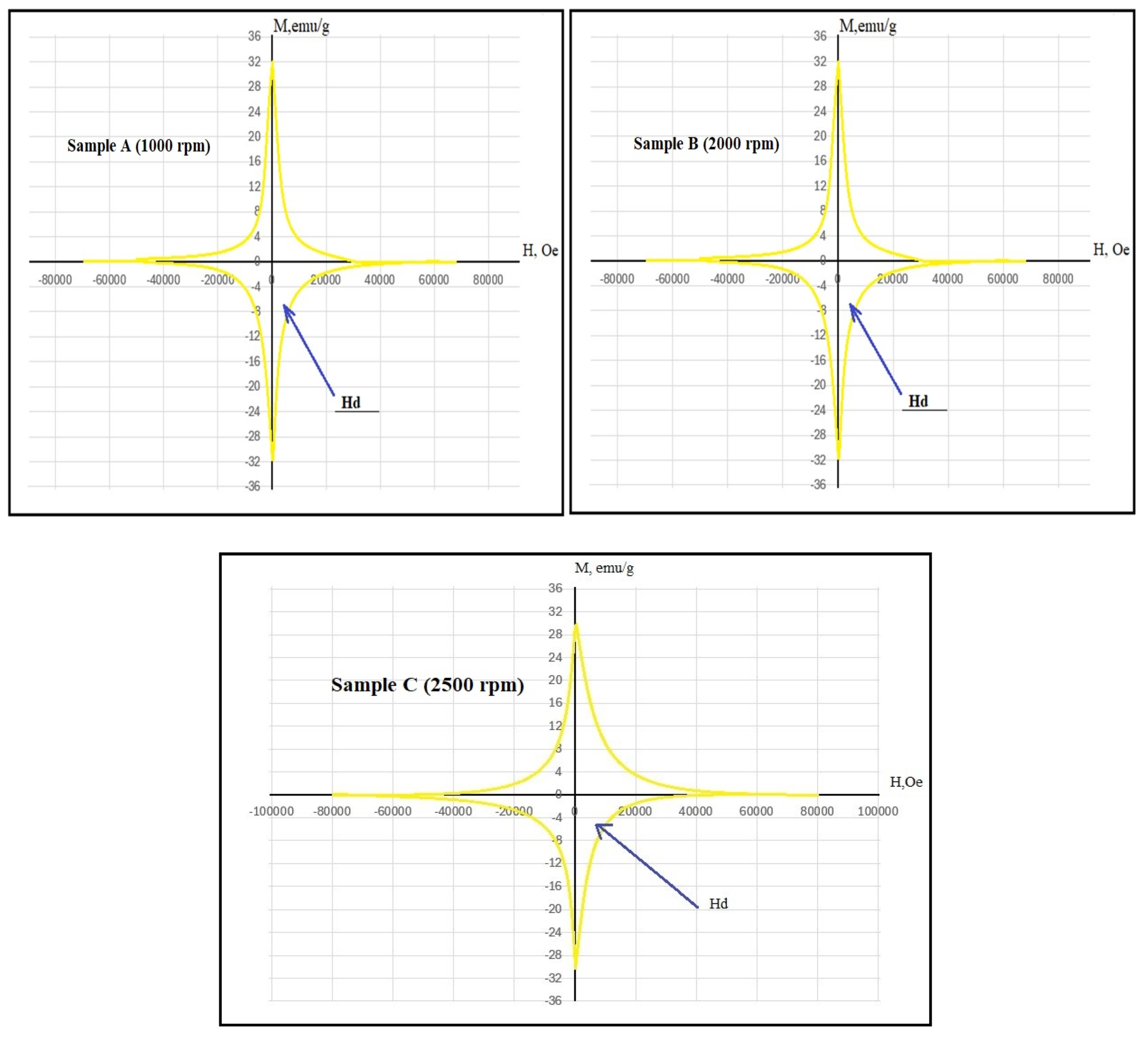
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nagamatsu, J.; Nakagawa, N.; Muranaka, T.; Zenitani, Y.; Akimitsu, J. Superconductivity at 39 K in magnesium diboride. Nature 2001, 410, 4. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C. Physics of High-Tc Superconductors; Academic Press: Boston, MA, USA, 1989; Volume 5, p. 393. [Google Scholar]
- Shackelford, J.F. Introduction to Material Science for Engineers; Prentice Hall: Upper Saddle River, NJ, USA, 2000; Volume 4, p. 877. [Google Scholar]
- de Castro, S.; Fabiano, C. Review on the state-of-the-art and challenges in the MgB2 component manufacturing for superconducting applications. Superconductivity 2023, 9, 100083. [Google Scholar]
- Zlotnikov, I.; Gotman, I.; Gutmanas, E.Y. Processing of dense bulk MgB2 superconductor via pressure-assisted thermal explosion mode of SHS. J. Eur. Ceram. Soc. 2005, 25, 3517–3522. [Google Scholar] [CrossRef]
- Mamalisa, A.G.; Hristoforoua, E.; Manolakosa, D.E.; Svec, P.; Prikhna, T.; Theodorakopoulos, J.D.; Kouzilos, G. Explosive compaction and synthesis of MgB2 superconductor using the powder in tube technique. J. Optoelectron. Adv. Mater. 2008, 10, 1000–1004. [Google Scholar]
- Cheng, S.H.; Zhang, Y.; Wang, H.; Li, Y.; Yang, C.; Wang, Y. Fabrication, and characterization of superconducting MgB2 thin film on graphene. AIP Adv. 2018, 8, 075015. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Wang, Y.; Wang, Y.; Feng, Q.; Gan, Z.; Jang, J. A sol–gel method for growing superconducting MgB2 films. Supercond. Sci. Technol. 2011, 24, 015002. [Google Scholar] [CrossRef]
- Naito, M.; Yamamoto, A.; Ueda, S.; Nishiyuki, K. Simple Route to Grow High-Quality MgB2 Thin Films by Pyrolysis of Decaborane in Mg Vapor. Appl. Phys. Express 2011, 4, 073101. [Google Scholar] [CrossRef]
- Zhang, Z.; MacManus-Driscoll, J.; Suo, H.; Wang, Q. Review of synthesis of high volumetric density, low gravimetric density MgB2 bulk for potential magnetic field applications. Superconductivity 2022, 3, 100015. [Google Scholar] [CrossRef]
- Shapovalov, A.P. High pressure synthesis of nanostructured superconducting materials based on magnesium diboride. Fiz. Tekh. Vysok. Davleniy 2013, 23, 35–47. [Google Scholar]
- Tolendiuly, S.; Fomenko, S.M.; Mansurov, Z.A.; Dannangoda, G.; Martirosyan, K.S. Self-propagating high temperature synthesis of MgB2 superconductor in high-pressure of argon condition. Eurasian Chem. Technol. J. 2017, 19, 177–181. [Google Scholar] [CrossRef]
- Chen, X.; Xia, T.; Wang, M.; Zhao, W.; Liu, T. Microstructural transformation during combustion synthesis of MgB2 superconductor. Physica C 2007, 454, 38–42. [Google Scholar] [CrossRef]
- Tolendiuly, S.; Fomenko, S.; Abdulkarimova, R.; Akishev, A. Synthesis, and superconducting properties of the MgB2@BaO composites. Inorg. Nano-Met. Chem. 2020, 50, 349–353. [Google Scholar] [CrossRef]
- Tolendiuly, S.; Fomenko, S.M.; Abdulkarimova, R.G.; Mansurov, Z.A.; Dannangoda, G.C.; Martirosyan, K.S. The effect of MWCNT addition on superconducting properties of MgB2. Int. J. Self-Propag. High-Temp. Synth. 2016, 25, 97–101. [Google Scholar] [CrossRef]
- Prikhna, T.A. Modern superconductive materials for electrical machines and devices working on the principle of levitation. Fiz. Nizk. Temp. 2006, 32, 661–676. [Google Scholar] [CrossRef]
- Ksandopulo, G.I. The efficiency of the Centrifuge is force action on the propagation mechanism of SHS–wave. Eurasian Chem. Technol. J. 2014, 16, 35–39. [Google Scholar] [CrossRef]
- Ksandopulo, G.I.; Shevchenko, V.N.; Baideldinova, A.N. High-Temperature Centrifuge. RoK Patent No 68317, 26 May 2010. [Google Scholar]
- Ksandopulo, G.I. Non–chain autoacceleration of the self–propagating high temperature synthesis (SHS) wave at rotation conditions. Int. J. Self-Propagating High-Temp. Synth. 2015, 24, 8–13. [Google Scholar] [CrossRef]
- Ksandopulo, G.I.; Baideldinova, A.N.; Omarova, K.I.; Ainabayev, A.M. Initiating Potential of Centrifugally Accelerated Metal Particles in the Inorganic Synthesis Reactions. Eurasian Chem. Technol. J. 2014, 16, 49–53. [Google Scholar] [CrossRef]
- Bean, C.P. Magnetization of High-Field Superconductors. Rev. Mod. Phys. 1964, 36, 31–39. [Google Scholar] [CrossRef]



| The Layer’s Composition (Sandwich Location) | The Layer Mass, gr | The Layer Height, mm |
|---|---|---|
| An initiating mixture (55%CuO + 12%Al + 33% Al2O3) | 10 | ~45 |
| Studied Sample (Mg + B powders) | 15 | ~10 |
| Quartzite grains | 11 | ~30 |
| Name | The Content, wt.% | |||||
|---|---|---|---|---|---|---|
| MgB2 | MgO | CuMg2 | MgB4 | Mg | AlB2 | |
| Sample A (1000 rpm) | 63.7 | 23.1 | 2.1 | 6.9 | - | 4.2 |
| Sample B (2000 rpm) | 70.8 | 21.7 | 1.8 | 3.8 | 1.9 | - |
| Sample C (2500 rpm) | 63.2 | 15.4 | 3.7 | 10.4 | - | 7.3 |
| Name | Critical Transition Temperature, K | The Critical Current Density, A/cm2 |
|---|---|---|
| Sample A (1000 rpm) | 37.5 | 0.9 × 106 |
| Sample B (2000 rpm) | 38.0 | 1.4 × 106 |
| Sample C (2500 rpm) | 36.2 | 0.8 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolendiuly, S.; Nur-Akasyah, J.; Fomenko, S.; Turan, A.; Assylkhan, S.; Abisheva, A. High-Temperature Synthesis of Superconducting MgB2 Materials in a Centrifuge. J. Compos. Sci. 2024, 8, 267. https://doi.org/10.3390/jcs8070267
Tolendiuly S, Nur-Akasyah J, Fomenko S, Turan A, Assylkhan S, Abisheva A. High-Temperature Synthesis of Superconducting MgB2 Materials in a Centrifuge. Journal of Composites Science. 2024; 8(7):267. https://doi.org/10.3390/jcs8070267
Chicago/Turabian StyleTolendiuly, Sanat, Jaafar Nur-Akasyah, Sergey Fomenko, Ahmet Turan, Sharafhan Assylkhan, and Aigul Abisheva. 2024. "High-Temperature Synthesis of Superconducting MgB2 Materials in a Centrifuge" Journal of Composites Science 8, no. 7: 267. https://doi.org/10.3390/jcs8070267
APA StyleTolendiuly, S., Nur-Akasyah, J., Fomenko, S., Turan, A., Assylkhan, S., & Abisheva, A. (2024). High-Temperature Synthesis of Superconducting MgB2 Materials in a Centrifuge. Journal of Composites Science, 8(7), 267. https://doi.org/10.3390/jcs8070267








