Composite Coatings with Liposomes of Melissa officinalis Extract for Extending Tomato Shelf Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Fabrication of Liposomes
2.3. Preparation and Application of Edible Coating
2.4. Respiration Rate, Soluble Solids, and Titratable Acidity
2.5. Determination of Lightness and Weight Loss
2.6. Malondialdehyde (MDA) Content
2.7. Hydrogen Peroxide Content
2.8. Determination of Total Phenol Content
2.9. DPPH Scavenging Ability
2.10. Statistical Analysis
3. Results and Discussion
3.1. Respiration Rate, Soluble Solids, and Titratable Acidity
3.2. Determination of Weight Loss and Luminosity
3.3. Malondialdehyde (MDA) and Hydrogen Peroxide Content
3.4. Determination of Total Phenol Content and DPPH Scavenging Ability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.M.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.; Kalia, A.; Gill, K. Influence of carboxy methylcellulose, chitosan and beeswax coatings on cold storage life and quality of Kinnow mandarin fruit. Sci. Hortic. 2020, 260, 108887. [Google Scholar] [CrossRef]
- Ahmed, L.; Martin-Diana, A.B.; Rico, D.; Barry-Ryan, C. Effect of delactosed whey permeate treatment on physico-chemical, sensorial, nutritional and microbial properties of whole tomatoes during postharvest storage. LWT Food Sci. Technol. 2013, 51, 367–374. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Desarrollo Rural de Colombia. 2019. Reporte: Área, Producción y Rendimiento Nacional por Cultivo (tomate) Agronet. Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1# (accessed on 25 July 2023).
- Mabrouki, H.; Duarte, C.M.; Akretche, D.E. Estimation of total phenolic contents and in vitro antioxidant and antimicrobial activities of various solvent extracts of Melissa officinalis L. Arab. J. Sci. Eng. 2018, 43, 3349–3357. [Google Scholar] [CrossRef]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Rassam, G.; Asili, J. Chemical composition, antibacterial activity, and cytotoxicity of essential oil from Nepeta ucrainica L. spp. kopetdaghensis. Ind. Crops Prod. 2014, 58, 315–321. [Google Scholar] [CrossRef]
- Mencherini, T.; Picerno, P.; Scesa, C.; Aquino, R. Triterpene, antioxidant, and antimicrobial compounds from Melissa officinalis. J. Nat. Prod. 2007, 70, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- González, R.E.; Restrepo, S.; Anaya, Y.; Zapateiro, L. Efecto de los recubrimientos binarios conteniendo extracto acuoso de laurel sobre la calidad pos cosecha de la fresa (Fragaria × ananassa). Inf. Tecnológica 2022, 33, 213–222. [Google Scholar] [CrossRef]
- Cazón, P.; Velázquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Michelin, M.; Marqués, M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A. Carboxymethyl cellulose-based films: Effect of organosolv lignin incorporation on physicochemical and antioxidant properties. J. Food Eng. 2020, 285, 110107. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, P.; Villanueva, F. Encapsulation of food active ingredients in liposomes. J. Nutr. Health Food Eng. 2018, 8, 238–239. [Google Scholar] [CrossRef]
- Liu, X.; Bourvellec, C.L.; Renard, C.M. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Liu, J.; Fan, Y.; Cheng, J.; Shi, Y.; Zou, J.; Zhang, X. Optimization, characterization and evaluation of liposomes from Malus hupehensis (Pamp.) Rehd. Extracts. J. Liposome Res. 2020, 30, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Shakir, M.; Ejaz, S.; Hussain, S.; Ali, S.; Sardar, H.; Azam, M.; Ullah, S.; Khaliq, G.; Saleem, M.; Nawaz, A.; et al. Synergistic effect of gum Arabic and carboxymethyl cellulose as biocomposite coating delays senescence in stored tomatoes by regulating antioxidants and cell wall degradation. Int. J. Biol. Macromol. 2022, 201, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, F.; Song, L.; Zheng, X. Alleviation of chilling injury in tomato fruit by exogenous application of oxalic acid. Food Chem. 2016, 202, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Loreto, F. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar] [CrossRef]
- Karina, R.L.; Olga, M.B.; Robert, S.F. Effect of pulsed light treatments on quality and antioxidant properties of fresh-cut strawberries. Food Chem. 2018, 264, 393–400. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.C.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Sandoval-Contreras, T.; Calderón-Santoyo, M. Sodium alginate coatings added with Meyerozyma caribbica: Postharvest biocontrol of Colletotrichum gloeosporioides in avocado (Persea americana Mill. cv. Hass). Postharvest Biol. Technol. 2020, 163, 111123. [Google Scholar] [CrossRef]
- Gurjar, P.S.; Killadi, B.; Lenka, J.; Shukla, D.K. Effect of gum arabic coatings on physico-chemical and sensory qualities of guava (Psidium guajava L) cv. Shweta. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3769–3775. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2010, 58, 42–47. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Du, M.; Tian, Y. Effect of polysaccharide derived from Osmunda japonica Thunb-incorporated carboxymethyl cellulose coatings on preservation of tomatoes. J. Food Process. Preserv. 2019, 43, 18. [Google Scholar] [CrossRef]
- Ahmed, M.; Saini, P.; Iqbal, U. Bio cellulose-based edible composite coating for shelf-life extension of tomatoes. Food Humanit. 2023, 1, 973–984. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Dastjerdi, M.; Ramezanian, A.; Ehteshami, S. Ameliorative effect of gum arabic, oleic acid and/or cinnamon essential oil on chilling injury and quality loss of guava fruit. Sci. Hortic. 2020, 266, 109255. [Google Scholar] [CrossRef]
- Jhanani, G.K.; AlSalhi, M.; Naveena, T.; Shanmuganathan, R. As assessment of shelf life increasing competence of pectin (Zucchini) based edible coating on tomatoes. Environ. Res. 2024, 119368. [Google Scholar] [CrossRef]
- Anthon, G.E.; Lestrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pratibha, N.; Neeraj, N.; Ojha, A.; Upadhyay, A.; Singh, R.; Kumar, S. Effect of active chitosan-pullulan composite edible coating enrich with pomegranate peel extract on the storage quality of green bell pepper. LWT Food Sci. Technol. 2021, 138, 110435. [Google Scholar] [CrossRef]
- Wardak, M.; Nkede, F.; Van, T.; Meng, F.; Tanaka, F.; Tanaka, F. Development of edible films and partial coating, a novel coating technique for tomato fruits, using citric acid-crosslinked starch and cellulose nanofiber. Prog. Org. Coat. 2024, 187, 108127. [Google Scholar] [CrossRef]
- Carrillo-Lomelí, D.A.; Cerqueira, M.A.; Moo-Huchin, V.; Bourbon, A.; Souza, V.; Lestido-Cardama, A.; Pastrana, L.; Ochoa-Fuentes, Y.; Hernández-Castillo, D.; Villarreal-Quintanilla, J.; et al. Influence of edible multilayer coatings with Opuntia stenopetala polysaccharides and Flourensia microphylla extract on the shelf-life of cherry tomato (Solanum lycopersicum L.). Sci. Hortic. 2024, 332, 113224. [Google Scholar] [CrossRef]
- Rives-Castillo, S.; Ventura-Aguilar, R.; Hernández-López, M.; Bautista-Baños, S. Evaluación de recubrimientos biodegradables para la conservación en fresco de jitomate Kenton. Acta Agrícola Pecu. 2018, 4, 80–91. [Google Scholar]
- da Silva, A.C.; Rodrigues Barbosa, J.; da Silva Araújo, C.; Sousa Batista, J.; Xavier Neves, E.; Pereira Cardoso, D.; Peixoto Joele, M.; Henriques Lourenço, L. A new edible coating of fish gelatin incorporated into açaí oil to increase the post-harvest shelf life of tomatoes. Food Chem. 2024, 438, 138047. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, M.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Sardar, H.; Saddiq, B. Tragacanth gum coating modulates oxidative stress and maintains quality of harvested apricot fruits. Int. J. Biol. Macromol. 2020, 163, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Chaple, S.; Vishwasrao, C.; Ananthanarayan, L. Edible composite coating of methyl cellulose for postharvest extension of shelf-life of finger hot indian pepper (Pusa jwala). J. Food Process. Preserv. 2017, 41, e12807. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Ejaz, S.; Hussain, S.; Ercisli, S.; Saleem, M.; Sardar, H. Carboxymethyl cellulose coating delays chilling injury development and maintains eating quality of ‘Kinnow’ mandarin fruits during low temperature storage. Int. J. Biol. Macromol. 2021, 168, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, G.; Mohamed, M.T.; Ghazali, H.M.; Ding, P.; Ali, A. Influence of gum arabic coating enriched with calcium chloride on physiological, biochemical and quality responses of mango (Mangifera indica L.) fruit stored under low temperature stress. Postharvest Biol. Technol. 2016, 111, 362–369. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zhihua, L.; Mahunu, G.; Xiaobo, Z.; Arslan, M.; Xiaowei, H.; Yang, Z.; Mariod, A. Effect of gum arabic edible coating incorporated with African baobab pulp extract on postharvest quality of cold stored blueberries. Food Sci. Biotechnol. 2020, 29, 217–226. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; Song, X.; Guo, M.; Wang, Z.; Geng, F.; Zhou, X.; Nie, S. Compound hydrogels derived from gelatin and gellan gum regulates the release of anthocyanins in simulated digestion. Food Hydrocoll. 2022, 127, 107487. [Google Scholar] [CrossRef]
- Ávila-Juárez, L.; Miranda-Rodríguez, H. Variations in bioactive content in different tomato trusses due to elicitor effects. J. Chem. 2018, 2736070. [Google Scholar] [CrossRef]
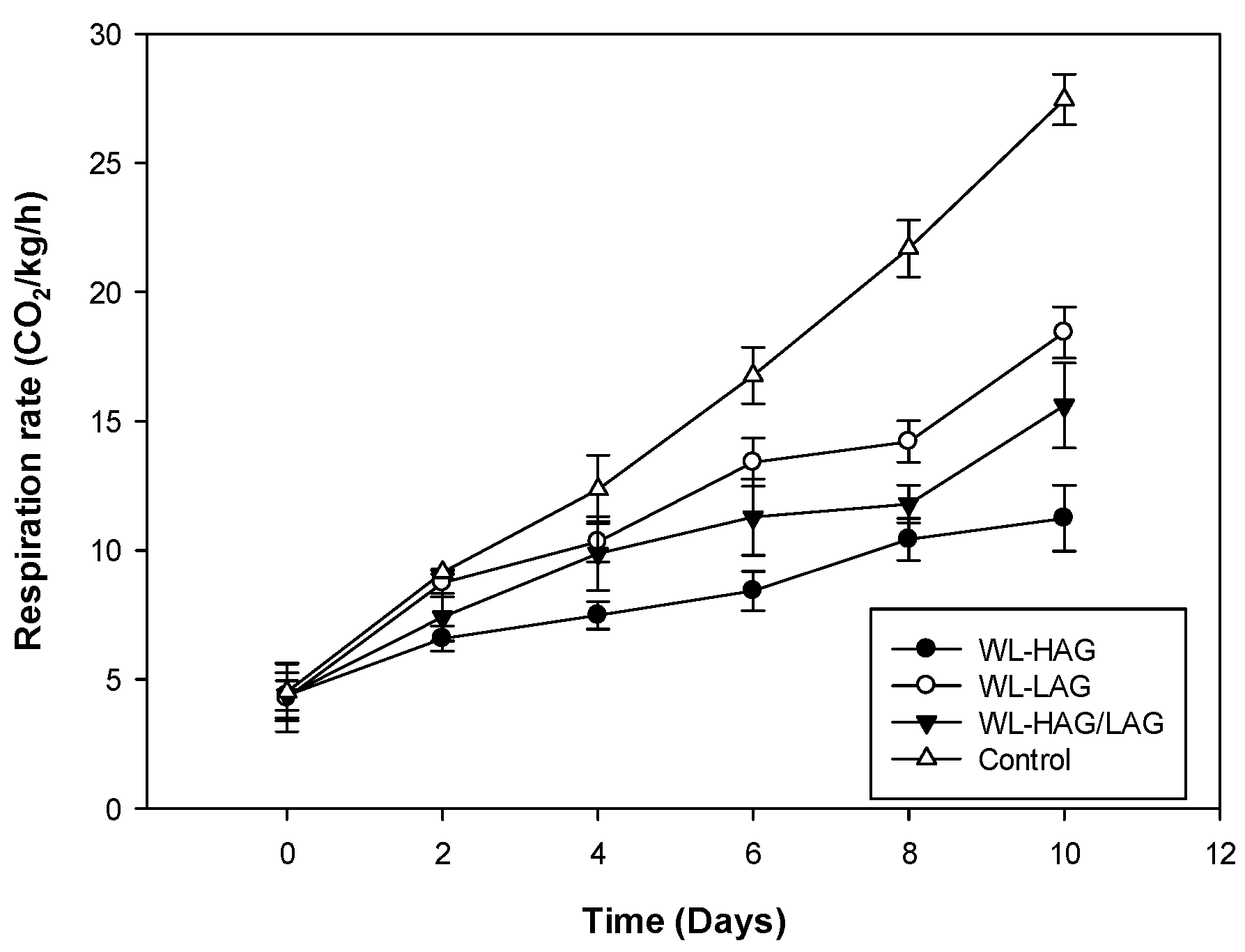


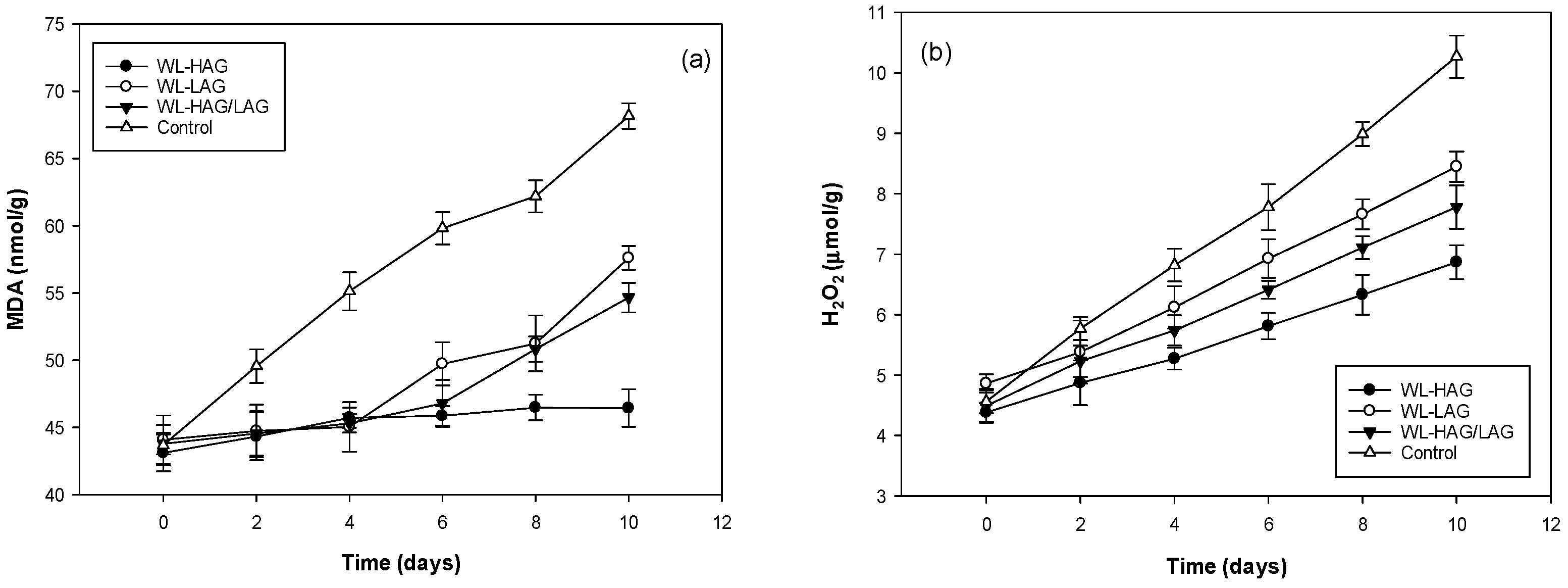
| Storage Time (Days) | Edible Coating | Soluble Solids | Titratable Acidity (%) |
|---|---|---|---|
| 0 | WL-HAG | 3.70 ± 0.12 a | 0.77 ± 0.03 a |
| WL-LAG | 3.67 ± 0.05 a | 0.76 ± 0.00 a | |
| WL-HAG/LAG | 3.72 ± 0.00 a | 0.76 ± 0.04 a | |
| Control | 3.71 ± 0.10 a | 0.75 ± 0.02 a | |
| 2 | WL-HAG | 3.75 ± 0.05 a | 0.75 ± 0.02 a |
| WL-LAG | 3.80 ± 0.00 a | 0.68 ± 0.04 b | |
| WL-HAG/LAG | 3.77 ± 0.08 a | 0.71 ± 0.12 a | |
| Control | 3.92 ± 0.19 b | 0.66 ± 0.04 b | |
| 4 | WL-HAG | 4.13 ± 0.08 b | 0.70 ± 0.12 a |
| WL-LAG | 4.21 ± 0.02 b | 0.63 ± 0.05 b | |
| WL-HAG/LAG | 4.18 ± 0.05 b | 0.67 ± 0.00 b | |
| Control | 4.57 ± 0.08 c | 0.60 ± 0.02 b | |
| 6 | WL-HAG | 4.82 ± 0.16 c | 0.64 ± 0.01 b |
| WL-LAG | 5.27 ± 0.15 d | 0.59 ± 0.02 b | |
| WL-HAG/LAG | 5.01 ± 0.05 c | 0.61 ± 0.04 b | |
| Control | 5.48 ± 0.12 e | 0.57 ± 0.10 b | |
| 8 | WL-HAG | 5.26 ± 0.05 d | 0.59 ± 0.04 b |
| WL-LAG | 5.41 ± 0.08 e | 0.52 ± 0.08 c | |
| WL-HAG/LAG | 5.32 ± 0.12 d | 0.56 ± 0.00 c | |
| Control | 5.82 ± 0.11 e | 0.49 ± 0.10 c | |
| 10 | WL-HAG | 5.60 ± 0.08 e | 0.57 ± 0.08 c |
| WL-LAG | 6.07 ± 0.04 f | 0.50 ± 0.02 c | |
| WL-HAG/LAG | 5.88 ± 0.15 e | 0.54 ± 0.00 c | |
| Control | 6.51 ± 0.05 g | 0.42 ± 0.08 d |
| Storage Time (Days) | Edible Coating | Total Phenol Content (mg Ac Gallic/100 g) | The DPPH Scavenging Ability (mmol Equivalent Trolox/100 g) |
|---|---|---|---|
| 0 | WL-HAG | 92.55 ± 1.70 a | 168.03 ± 3.15 a |
| WL-LAG | 92.43 ± 1.08 a | 167.77 ± 2.18 a | |
| WL-HAG/LAG | 92.51 ± 1.00 a | 168.12 ± 3.05 a | |
| Control | 90.48 ± 0.86 b | 153.03 ± 2.78 b | |
| 2 | WL-HAG | 100.04 ± 1.30 c | 186.11 ± 2.76 c |
| WL-LAG | 93.37 ± 1.20 a | 172.18 ± 2.55 d | |
| WL-HAG/LAG | 98.55 ± 1.10 d | 179.41 ± 1.40 e | |
| Control | 95.77 ± 1.15 e | 170.32 ± 2.35 d | |
| 4 | WL-HAG | 118.08 ± 2.10 f | 184.55 ± 3.00 c |
| WL-LAG | 107.66 ± 2.05 g | 170.33 ± 2.00 d | |
| WL-HAG/LAG | 110.07 ± 1.50 h | 176.71 ± 2.10 e | |
| Control | 106.31 ± 1.70 i | 171.06 ± 2.05 d | |
| 6 | WL-HAG | 110.15 ± 1.85 h | 184.12 ± 2.15 c |
| WL-LAG | 104.22 ± 2.05 j | 165.23 ± 2.06 f | |
| WL-HAG/LAG | 108.46 ± 2.15 g | 175.61 ± 1.88 e | |
| Control | 99.12 ± 1.80 d | 172.08 ± 2.70 d | |
| 8 | WL-HAG | 96.72 ± 1.12 e | 182.14 ± 1.70 g |
| WL-LAG | 93.23 ± 1.20 a | 156.73 ± 3.05 h | |
| WL-HAG/LAG | 97.41 ± 1.10 e | 161.55 ± 2.05 i | |
| Control | 80.44 ± 1.10 k | 146.83 ± 3.01 j | |
| 10 | WL-HAG | 86.61 ± 1.80 l | 175.47 ± 2.08 e |
| WL-LAG | 78.28 ± 1.55 m | 138.81 ± 0.00 k | |
| WL-HAG/LAG | 80.83 ± 1.40 n | 153.77 ± 2.40 l | |
| Control | 71.22 ± 1.24 o | 125.33 ± 1.80 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cuello, R.; Fuentes, L.G.; Castellanos, H.M.; Hernández-Fernández, J.; Ortega-Toro, R. Composite Coatings with Liposomes of Melissa officinalis Extract for Extending Tomato Shelf Life. J. Compos. Sci. 2024, 8, 283. https://doi.org/10.3390/jcs8070283
González-Cuello R, Fuentes LG, Castellanos HM, Hernández-Fernández J, Ortega-Toro R. Composite Coatings with Liposomes of Melissa officinalis Extract for Extending Tomato Shelf Life. Journal of Composites Science. 2024; 8(7):283. https://doi.org/10.3390/jcs8070283
Chicago/Turabian StyleGonzález-Cuello, Rafael, Luis Gabriel Fuentes, Heliana Milena Castellanos, Joaquín Hernández-Fernández, and Rodrigo Ortega-Toro. 2024. "Composite Coatings with Liposomes of Melissa officinalis Extract for Extending Tomato Shelf Life" Journal of Composites Science 8, no. 7: 283. https://doi.org/10.3390/jcs8070283
APA StyleGonzález-Cuello, R., Fuentes, L. G., Castellanos, H. M., Hernández-Fernández, J., & Ortega-Toro, R. (2024). Composite Coatings with Liposomes of Melissa officinalis Extract for Extending Tomato Shelf Life. Journal of Composites Science, 8(7), 283. https://doi.org/10.3390/jcs8070283







