Modifying the Characteristics of the Electrical Arc Generated during Hot Switching by Reinforcing Silver and Copper Matrices with Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composite Production & Materials Characterization
2.2. Hot Switching Tests
2.3. Heterogeneity Analysis
3. Results and Discussions
3.1. ECR Prior to Arcing
| Sample | ECR/mΩ | Hardness/MPa |
|---|---|---|
| Ag Rod | 1.36 ± 0.32 | 847 ± 61 |
| Ag 0% | 1.65 ± 0.36 | 470 ± 49 |
| Ag 1% | 1.79 ± 0.53 | 505 ± 37 |
| Ag 2% | 1.94 ± 0.27 | 369 ± 54 |
| Ag 3% | 2.13 ± 0.73 | 408 ± 61 |
| Cu Rod | 6.86 ± 2.79 | 1335 ± 82 |
| Cu 0% | 3.59 ± 0.86 | 650 ± 70 |
| Cu 1% | 2.66 ± 0.06 | 619 ± 88 |
| Cu 2% | 2.73 ± 0.55 | 555 ± 81 |
| Cu 3% | 6.80 ± 0.28 | 512 ± 87 |
| Ag/Ni 90/10 | 5.75 ± 1.14 | 615 ± 89 |
| Ag/SnO2 92/8 | 2.93 ± 0.47 | 728 ± 42 |
| Ag/SnO2 90/10 | 2.63 ± 0.66 | 757 ± 58 |
| Ag/SnO2 88/12 | 4.57 ± 0.92 | 820 ± 46 |
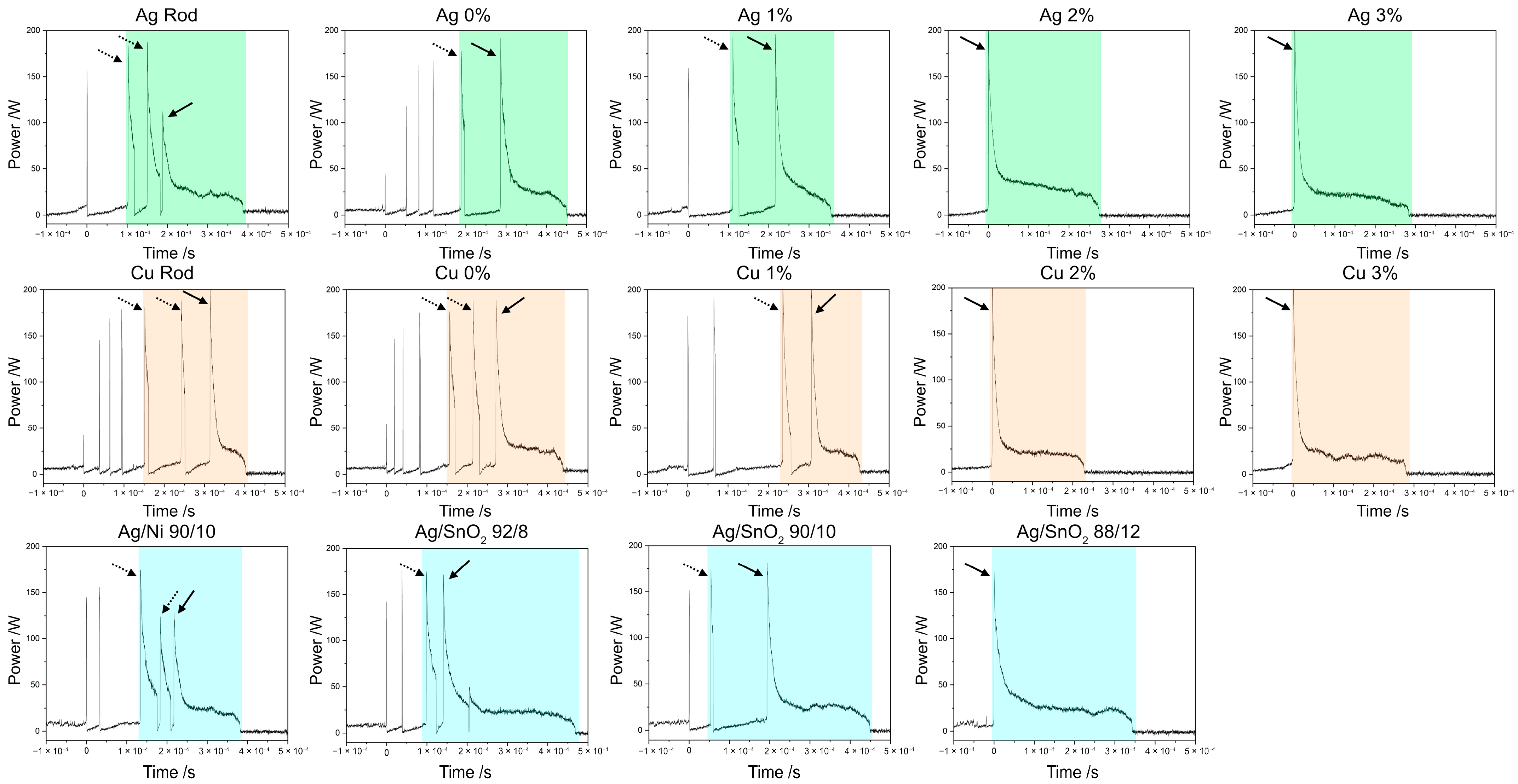
3.2. Arc Characterization
3.3. High-Speed Footage
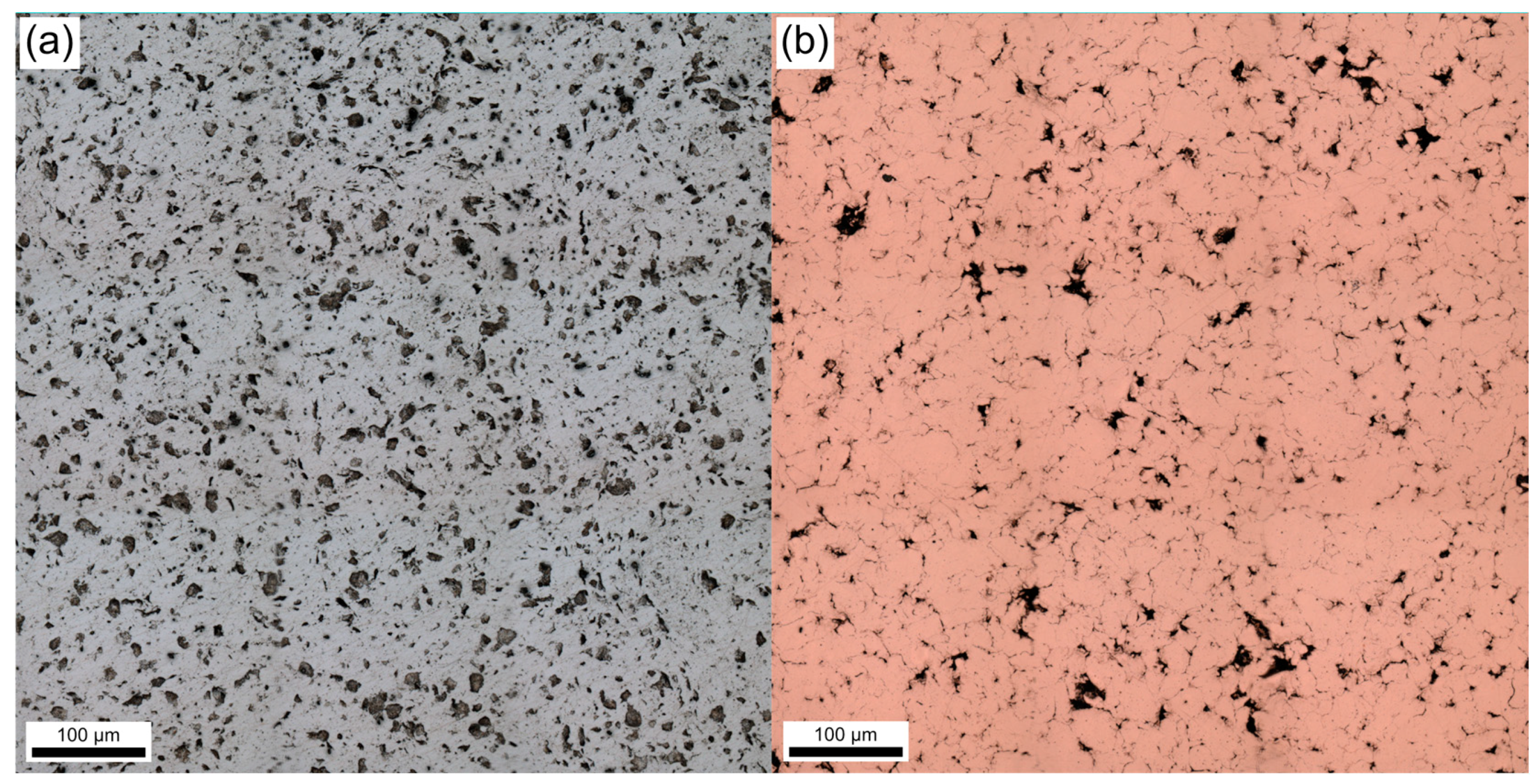
3.4. Reinforcement Phase Distribution
4. Conclusions
- All composite materials proposed performed similarly to the reference materials. The copper MMCs have the shortest arc duration and lowest arc energy among the materials herein evaluated.
- 2 wt.% CNT content is the lower limit to avoid unstable arcing. These MMCs showed unstable arcing one-third of the time, whereas unstable arcing was not observed for samples with 3 wt.% CNT.
- Increased CNT content confines the arcs, thus reducing its mobility. This effect is more significant in silver MMCs due to a more homogeneous distribution of the CNTs. Heterogeneity on the copper MMCs’ surfaces—produced by large CNT clusters—favors arc wandering.
- The results shown highlight that although copper MMCs present higher resistance values, their arcing performance is comparable to silver-based materials. Therefore, in applications where low ECR is not crucial and atmospheric conditions allow it, copper MMCs are a low-cost option for switching contact materials, thereby reducing the demand for precious metals.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Holm, R. Electric Contacts; Springer: Berlin/Heidelberg, Germany, 1967. [Google Scholar]
- Slade, P.G. Electrical Contacts: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Braunovic, M.; Konchits, V.V.; Myshkin, N.K. Electrical Contacts: Fundamentals, Applications and Technology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xie, M.; Yang, Y.; Zhang, J.; Wang, S.; Chen, Y.; Zhou, W. Effect of CNTs content on the mechanical and arc-erosion performance of Ag-CNTs composites. Diam. Relat. Mater. 2022, 128, 109211. [Google Scholar] [CrossRef]
- Selzner, C.; Mücklich, F. New microstructure investigations of arc damaged silver/tinoxide electrodes by means of FIB-technique. In Proceedings of the 27th International Conference on Electrical Contacts, Dresden, Germany, 22–26 June 2014; pp. 1–5. [Google Scholar]
- Rohberg, J.; Honig, T.; Witulski, N.; Finkbeiner, M.; Behrens, V. Performance of Different Silver/Tin Oxide Contact Materials for Applications in Low Voltage Circuit Breakers. In Proceedings of the 2009 55th IEEE Holm Conference on Electrical Contacts, Vancouver, BC, Canada, 14–16 September 2009; pp. 189–196. [Google Scholar] [CrossRef]
- Behrens, V.; Honig, T.; Kraus, A.; Mahle, E.; Michal, R.; Saeger, K.E. Test results of different silver/graphite contact materials in regard to applications in circuit breakers. In Electrical Contacts—1995, Proceedings of the Forty-First IEEE Holm Conference on Electrical Contacts, Montreal, QC, Canada, 2–4 October 1995; IEEE: Piscataway, NJ, USA, 1995; pp. 393–397. [Google Scholar] [CrossRef]
- Michal, R.; Saegar, K.E. Metallurgical aspects of silver-based contact materials for air-break switching devices for power engineering. IEEE Trans. Compon. Hybrids Manuf. Technol. 1989, 12, 71–81. [Google Scholar] [CrossRef]
- Wingert, P.; Bevington, R.; Horn, G. The effect of graphite additions on the performance of silver-nickel contacts. In Proceedings of the Thirty-Sixth IEEE Conference on Electrical Contacts, and the Fifteenth International Conference on Electrical Contacts, Montreal, QC, Canada, 20–24 August 1990; pp. 524–529. [Google Scholar] [CrossRef]
- Shobert, E. Carbon, Graphite, and Contacts. IEEE Trans. Parts Hybrids Packaging 1976, 12, 62–74. [Google Scholar] [CrossRef]
- Jiang, P.; Li, F.; Wang, Y. Effect of different types of carbon on microstructure and arcing behavior of Ag/C contact materials. IEEE Trans. Compon. Packag. Technol. 2006, 29, 420–423. [Google Scholar] [CrossRef]
- Wingert, P.C. The effects of interrupting elevated currents on the erosion and structure of silver-graphite. In Electrical Contacts—1996, Proceedings of the Forty-Second IEEE Holm Conference on Electrical Contacts, Joint with the 18th International Conference on Electrical Contacts, Chicago, IL, USA, 16–20 September 1996; IEEE: Piscataway, NJ, USA, 1996; pp. 60–69. [Google Scholar] [CrossRef]
- Xie, W.; Wu, G.; Yang, Z.; She, P.; Wang, H.; Zuo, H.; Wei, W.; Gao, G.; Tu, C. Study on the erosion characteristics of copper-carbon electrode pairs by DC air arc. High Volt. 2021, 6, 674–683. [Google Scholar] [CrossRef]
- Sawa, K.; Ueno, T.; Nakano, K. Evaluation of Arc Erosion of Cu-graphite Brush Used in Small DC Motors with and without Quenching Device. In Proceedings of the 2020 IEEE 66th Holm Conference on Electrical Contacts and Intensive Course (HLM), San Antonio, TX, USA, 30 September–7 October 2020; pp. 170–175. [Google Scholar] [CrossRef]
- Shao, G.; Liu, P.; Li, W.; Chen, X.; Ma, F.; Liu, X.; Zhou, H.; Zhang, K. Effects of graphene nanoplates on arc erosion resistance and wear behavior under electric current of copper matrix composites. J. Alloys Compd. 2020, 829, 154356. [Google Scholar] [CrossRef]
- Suarez, S.; Alderete, B.; Puyol, R.; Mücklich, F. Load-dependent electrical contact resistance of carbon nanotube-reinforced metal matrix composites. In Proceedings of the 2022 IEEE 67th Holm Conference on Electrical Contacts (HLM), Tampa, FL, USA, 23–26 October 2022; pp. 1–6. [Google Scholar] [CrossRef]
- García, D.; Suárez, S.; Aristizábal, K.; Mücklich, F. Powder-Metallurgical Fabrication and Electrical Contact Resistance Characterization of Copper–Nickel Composites Reinforced by Multiwalled Carbon Nanotubes. Adv. Eng. Mater. 2022, 24, 2100755. [Google Scholar] [CrossRef]
- Suarez, S.; Puyol, R.; Schafer, C.; Mucklich, F. Carbon Nanotube-reinforced Metal Matrix Composites as Novel Electrodes for Low-voltage Switching Applications: A Surface Degradation Analysis. In Proceedings of the 2019 IEEE Holm Conference on Electrical Contacts, Milwaukee, WI, USA, 14–18 September 2019; pp. 135–141. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R. Physics of carbon nanotubes. Carbon 1995, 33, 883–891. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Carbon Nanotubes. Annu. Rev. Mater. Sci. 1994, 24, 235–264. [Google Scholar] [CrossRef]
- Maiti, A.; Svizhenko, A.; Anantram, M.P. Electronic Transport through Carbon Nanotubes: Effects of Structural Deformation and Tube Chirality. Phys. Rev. Lett. 2002, 88, 126805. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.M.; Martín, N. π–π interactions in carbon nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Klinovaja, J.; Schmidt, M.J.; Braunecker, B.; Loss, D. Carbon nanotubes in electric and magnetic fields. Phys. Rev. B 2011, 84, 085452. [Google Scholar] [CrossRef]
- Ben Jemaa, N.; Morin, L.; Benhenda, S.; Nedelec, L. Anodic to cathodic arc transition according to break arc lengthening. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 2005, 21, 599–603. [Google Scholar] [CrossRef]
- Ben Jemaa, N.; Nedelec, L.; Benhenda, S. Break arc duration and contact erosion in automotive application. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1996, 19, 82–86. [Google Scholar] [CrossRef]
- Jemaa, N.; Nedelec, L.; Benhenda, S.; Neveu, J. Anodic and cathodic erosion of Ag, Ag alloys and Ag-MeO contact materials in energy range below 10 joules. In Electrical Contacts—1996, Forty-Second IEEE Holm Conference on Electrical Contacts, Joint with the 18th International Conference on Electrical Contacts, Chicago, IL, USA, 16–20 September 1996; IEEE: Piscataway, NJ, USA, 1996; pp. 70–74. [Google Scholar]
- Swingler, J.; McBride, J. The net zero erosion phenomena on opening switching contacts with AC loading. In Electrical Contacts—1997, Forty-Third IEEE Holm Conference on Electrical Contacts, Philadelphia, PA, USA, 20–22 October 1997; IEEE: Piscataway, NJ, USA, 1997; pp. 238–245. [Google Scholar] [CrossRef]
- Braunovic, M. Effect of connection design on the contact resistance of high power overlapping bolted joints. IEEE Trans. Compon. Packag. Technol. 2002, 25, 642–650. [Google Scholar] [CrossRef]
- Feng, Y.; Yuan, H.L.; Zhang, M. Fabrication and properties of silver-matrix composites reinforced by carbon nanotubes. Mater. Charact. 2005, 55, 211–218. [Google Scholar] [CrossRef]
- Silvestre, N. State-of-the-art Review on Carbon Nanotube Reinforced Metal Matrix Composites. Int. J. Compos. Mater. 2013, 3, 28–44. [Google Scholar] [CrossRef]
- Suarez, S.; Lasserre, F.; Prat, O.; Mücklich, F. Processing and interfacial reaction evaluation in MWCNT/Ni composites. Phys. Status Solidi (a) 2014, 211, 1555–1561. [Google Scholar] [CrossRef]
- Singh, D.K.; Iyer, P.K.; Giri, P.K. Diameter dependence of oxidative stability in multiwalled carbon nanotubes: Role of defects and effect of vacuum annealing. J. Appl. Phys. 2010, 108, 084313. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Luo, G.; Wei, F. 99.9% purity multi-walled carbon nanotubes by vacuum high-temperature annealing. Carbon 2003, 41, 2585–2590. [Google Scholar] [CrossRef]
- Alderete, B.; Suarez, S.; Mücklich, F. On the Production & Tribo-Electrical Characterization of Carbon Nanotube-Reinforced Ag & Cu Metal Matrix Composites. In Proceedings of the 2023 IEEE 68th Holm Conference on Electrical Contacts (HOLM), Seattle, WA, USA, 4–11 October 2023; pp. 1–8. [Google Scholar] [CrossRef]
- Alderete, B.; Mücklich, F.; Suarez, S. Electrical Characterization of Carbon Nanotube Reinforced Silver and Copper Composites for Switching Contacts. J. Compos. Sci. 2023, 7, 284. [Google Scholar] [CrossRef]
- Puyol, R.; Suarez, S. A contact resistance measurement setup for the study of novel contacts. In Proceedings of the 2017 IEEE URUCON, Montevideo, Uruguay, 23–25 October 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Nayak, U.P.; Müller, M.; Britz, D.; Guitar, M.A.; Mücklich, F. Image Processing using Open Source Tools and their Implementation in the Analysis of Complex Microstructures. Pract. Metallogr. 2021, 58, 484–506. [Google Scholar] [CrossRef]
- Limpert, E.; Stahel, W.A.; Abbt, M. Log-normal Distributions across the Sciences: Keys and Clues: On the charms of statistics, and how mechanical models resembling gambling machines offer a link to a handy way to characterize log-normal distributions, which can provide deeper insight into variability and probability—Normal or log-normal: That is the question. BioScience 2001, 51, 341–352. [Google Scholar] [CrossRef]
- Yang, N.; Boselli, J.; Sinclair, I. Simulation and quantitative assessment of homogeneous and inhomogeneous particle distributions in particulate metal matrix composites. J. Microsc. 2001, 201, 189–200. [Google Scholar] [CrossRef]
- Nayak, U.P.; Mücklich, F.; Guitar, M.A. Time-Dependant Microstructural Evolution and Tribological Behaviour of a 26 wt% Cr White Cast Iron Subjected to a Destabilization Heat Treatment. Met. Mater. Int. 2023, 29, 934–947. [Google Scholar] [CrossRef]
- Seki, K.; Kubo, T.; Ye, N.; Shimizu, T. Quantifying the spreading resistance of an anisotropic thin film conductor. Sci. Rep. 2020, 10, 10633. [Google Scholar] [CrossRef]
- Henger, U.; Korn, D. Electrical resistivity of thin films of AuCo solid solutions. J. Phys. F Met. Phys. 1981, 11, 2575–2584. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Y.; Song, K.; Shaolin, L.; Jiang, F.; Wang, X. Arc erosion resistance of hybrid copper matrix composites reinforced with CNTs and micro-TiB2 particles. J. Mater. Res. Technol. 2021, 11, 1469–1479. [Google Scholar] [CrossRef]
- Shiraishi, M.; Ata, M. Work function of carbon nanotubes. Carbon 2001, 39, 1913–1917. [Google Scholar] [CrossRef]
- Reinert, L.; Zeiger, M.; Suárez, S.; Presser, V.; Mücklich, F. Dispersion analysis of carbon nanotubes, carbon onions, and nanodiamonds for their application as reinforcement phase in nickel metal matrix composites. RSC Adv. 2015, 5, 95149–95159. [Google Scholar] [CrossRef]
- Hilding, J.; Grulke, E.A.; Zhang, Z.G.; Lockwood, F. Dispersion of Carbon Nanotubes in Liquids. J. Dispers. Sci. Technol. 2003, 24, 1–41. [Google Scholar] [CrossRef]
- Alderete, B.; Nayak, U.P.; Mücklich, F.; Suarez, S. Influence of topography on electrical contact resistance of copper-based materials. Surf. Topogr. Metrol. Prop. 2023, 11, 025027. [Google Scholar] [CrossRef]
- Alderete, B.; Mücklich, F.; Suarez, S. Characterization and electrical analysis of carbon-based solid lubricant coatings. Carbon Trends 2022, 7, 100156. [Google Scholar] [CrossRef]
- Alderete, B.; Mücklich, F.; Suarez, S. Evaluating the effect of unidirectional loading on the piezoresistive characteristics of carbon nanoparticles. Sci. Rep. 2024, 14, 9247. [Google Scholar] [CrossRef]




| Matrix/vol.% | Reinforcement/vol.% | Reinforcement/wt.% | Nomenclature |
|---|---|---|---|
| 100 | 0.00 | 0 | Ag 0% |
| 94.77 | 5.23 | 1 | Ag 1% |
| 89.97 | 10.03 | 2 | Ag 2% |
| 85.54 | 14.46 | 3 | Ag 3% |
| 100 | 0.00 | 0 | Cu 0% |
| 95.50 | 4.50 | 1 | Cu 1% |
| 91.30 | 8.70 | 2 | Cu 2% |
| 87.39 | 12.61 | 3 | Cu 3% |
| Sample | Resistivity/Ωm | Holm Radius/µm | Spreading Resistance/mΩ |
|---|---|---|---|
| Ag Rod | 2.6 × 10−8 | 9.70 | 0.68 |
| Ag 0% | 4.3 × 10−8 | 13.02 | 0.83 |
| Ag 1% | 4.5 × 10−8 | 12.56 | 0.90 |
| Ag 2% | 5.7 × 10−8 | 14.69 | 0.97 |
| Ag 3% | 6.0 × 10−8 | 13.97 | 1.07 |
| Cu Rod | 1.1 × 10−7 | 7.72 | 3.43 |
| Cu 0% | 7.9 × 10−8 | 11.07 | 1.80 |
| Cu 1% | 6.0 × 10−8 | 11.34 | 1.33 |
| Cu 2% | 6.5 × 10−8 | 11.98 | 1.37 |
| Cu 3% | 1.7 × 10−7 | 12.47 | 3.40 |
| Ag/Ni 90/10 | 1.3 × 10−7 | 11.38 | 2.88 |
| Ag/SnO2 92/8 | 6.1 × 10−8 | 10.46 | 1.47 |
| Ag/SnO2 90/10 | 5.4 × 10−8 | 10.26 | 1.32 |
| Ag/SnO2 88/12 | 9.0 × 10−8 | 9.85 | 2.29 |
| Sample | Arc Duration/ms | Arc Energy/mJ |
|---|---|---|
| Ag Rod | 0.29 ± 0.01 | 10.25 ± 0.36 |
| Ag 0% | 0.30 ± 0.02 | 9.86 ± 2.11 |
| Ag 1% | 0.29 ± 0.04 | 8.79 ± 1.64 |
| Ag 2% | 0.37 ± 0.08 | 12.62 ± 2.45 |
| Ag 3% | 0.36 ± 0.05 | 10.71 ± 2.52 |
| Cu Rod | 0.30 ± 0.01 | 8.28 ± 1.30 |
| Cu 0% | 0.34 ± 0.04 | 10.87 ± 0.82 |
| Cu 1% | 0.20 ± 0.01 | 6.38 ± 0.25 |
| Cu 2% | 0.23 ± 0.06 | 6.30 ± 0.76 |
| Cu 3% | 0.23 ± 0.05 | 6.19 ± 0.95 |
| Ag/Ni 90/10 | 0.27 ± 0.09 | 8.34 ± 1.68 |
| Ag/SnO2 92/8 | 0.40 ± 0.02 | 11.49 ± 0.78 |
| Ag/SnO2 90/10 | 0.36 ± 0.03 | 9.56 ± 1.11 |
| Ag/SnO2 88/12 | 0.37 ± 0.03 | 11.71 ± 1.13 |
| Sample | Count | Size/µm2 | Area/% | COV |
|---|---|---|---|---|
| Ag 1% | 1374 | 16.48 | 5.05 | 0.49 |
| Ag 2% | 1977 | 20.64 | 9.10 | 0.44 |
| Ag 3% | 3018 | 24.32 | 16.36 | 0.41 |
| Cu 1% | 1106 | 9.86 | 2.43 | 0.53 |
| Cu 2% | 1124 | 14.72 | 3.69 | 0.50 |
| Cu 3% | 1367 | 18.05 | 5.51 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alderete, B.; Schäfer, C.; Nayak, U.P.; Mücklich, F.; Suarez, S. Modifying the Characteristics of the Electrical Arc Generated during Hot Switching by Reinforcing Silver and Copper Matrices with Carbon Nanotubes. J. Compos. Sci. 2024, 8, 285. https://doi.org/10.3390/jcs8070285
Alderete B, Schäfer C, Nayak UP, Mücklich F, Suarez S. Modifying the Characteristics of the Electrical Arc Generated during Hot Switching by Reinforcing Silver and Copper Matrices with Carbon Nanotubes. Journal of Composites Science. 2024; 8(7):285. https://doi.org/10.3390/jcs8070285
Chicago/Turabian StyleAlderete, Bruno, Christian Schäfer, U. Pranav Nayak, Frank Mücklich, and Sebastian Suarez. 2024. "Modifying the Characteristics of the Electrical Arc Generated during Hot Switching by Reinforcing Silver and Copper Matrices with Carbon Nanotubes" Journal of Composites Science 8, no. 7: 285. https://doi.org/10.3390/jcs8070285







