Toward Eco-Friendly Rubber: Utilizing Paper Waste-Derived Calcium Carbonate to Replace Carbon Black in Natural Rubber Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compounding and Mixing
2.3. Filler Characterization
2.3.1. Brunauer–Emmett–Teller (BET) Surface Area Measurement
2.3.2. Thermogravimetric Analysis (TGA)
2.4. In-Rubber Tests
2.4.1. Mooney Viscosity
2.4.2. Cure Behavior
2.4.3. Payne Effect
2.4.4. Macro-Dispersion
2.4.5. Tensile Behavior
3. Results and Discussion
3.1. BET Analysis
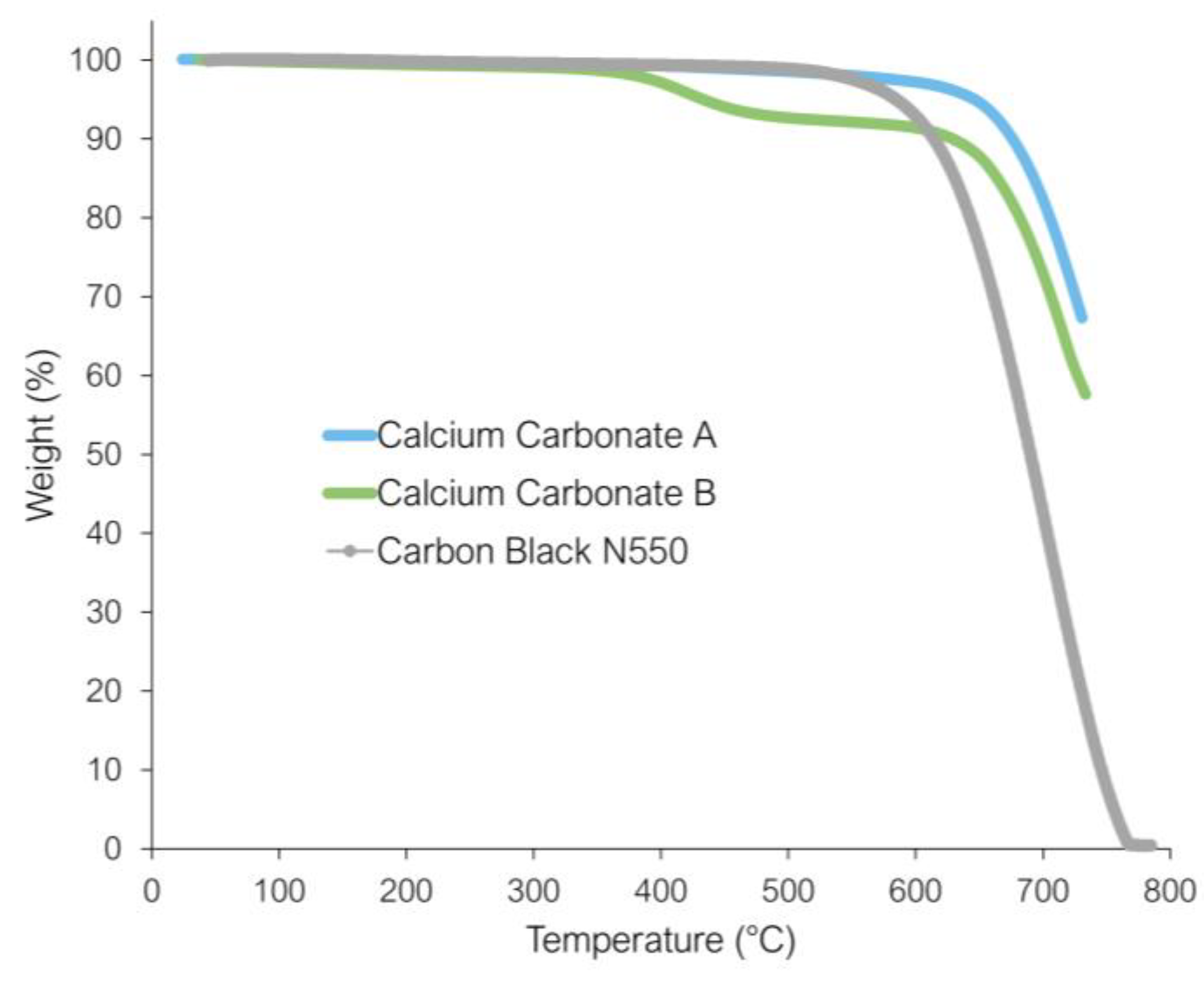
3.2. Thermogravimetric Analysis (TGA)
3.3. Mooney Viscosity
3.4. Cure Behavior
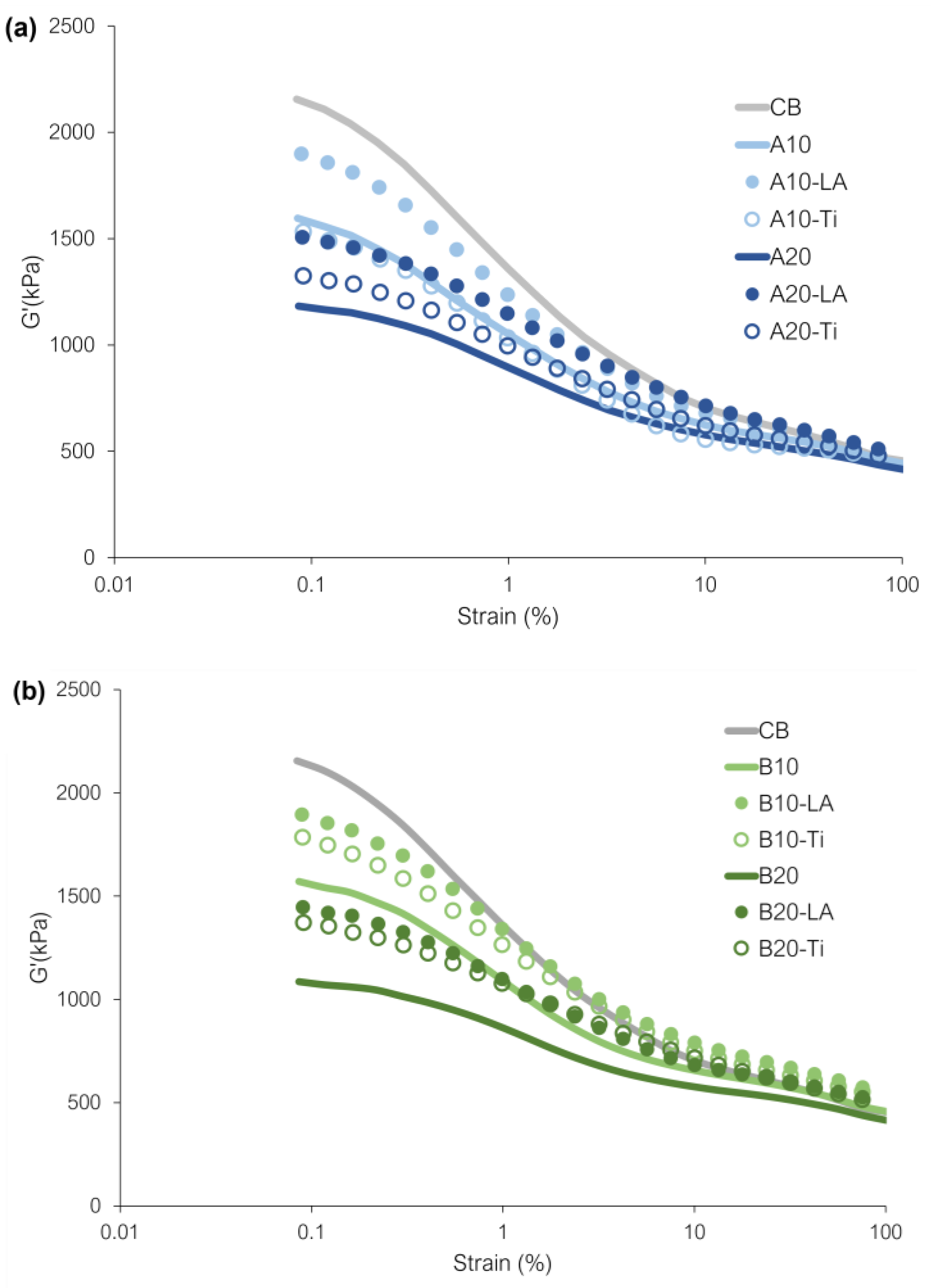
3.5. Payne Effect
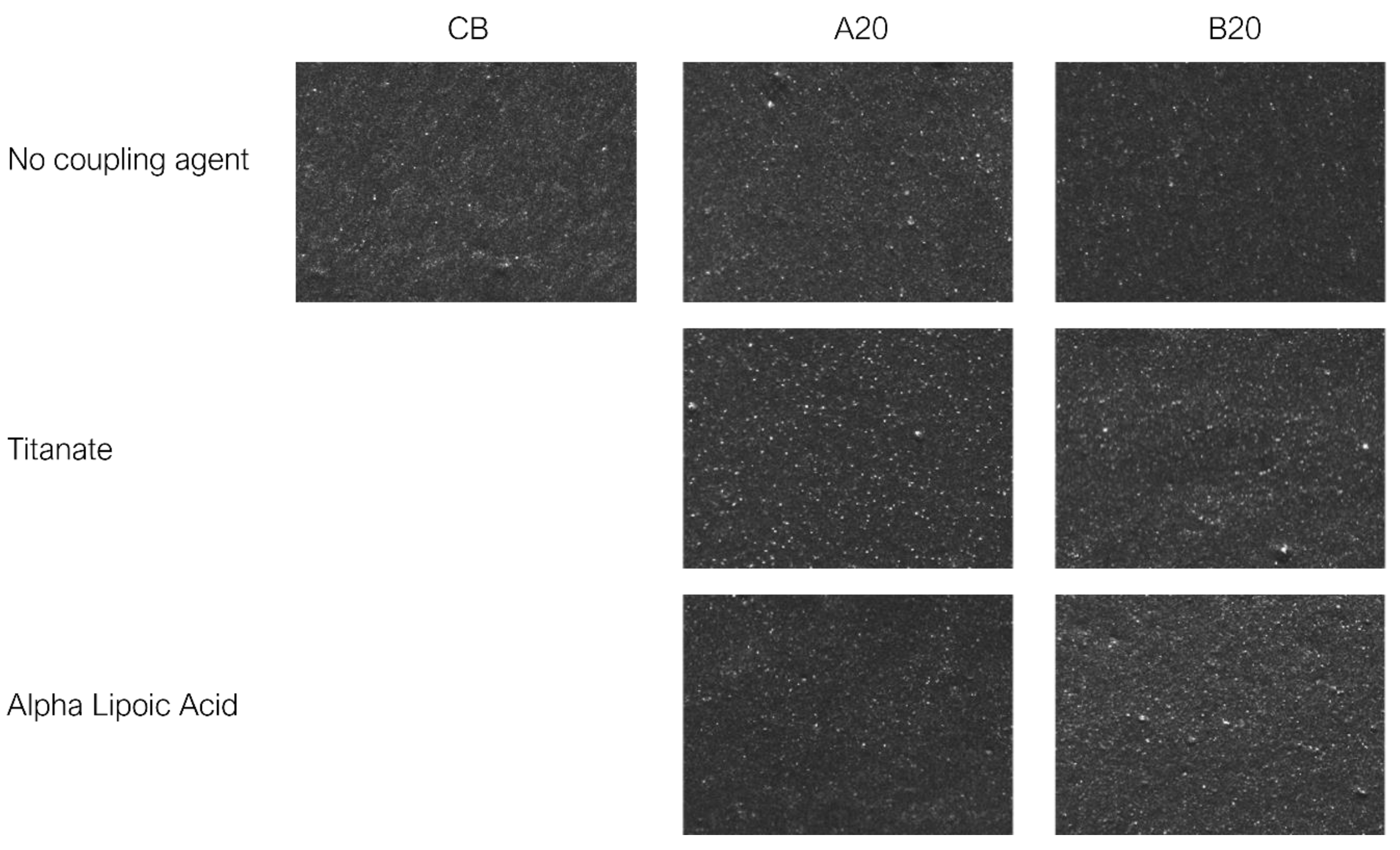
3.6. Macro-Dispersion
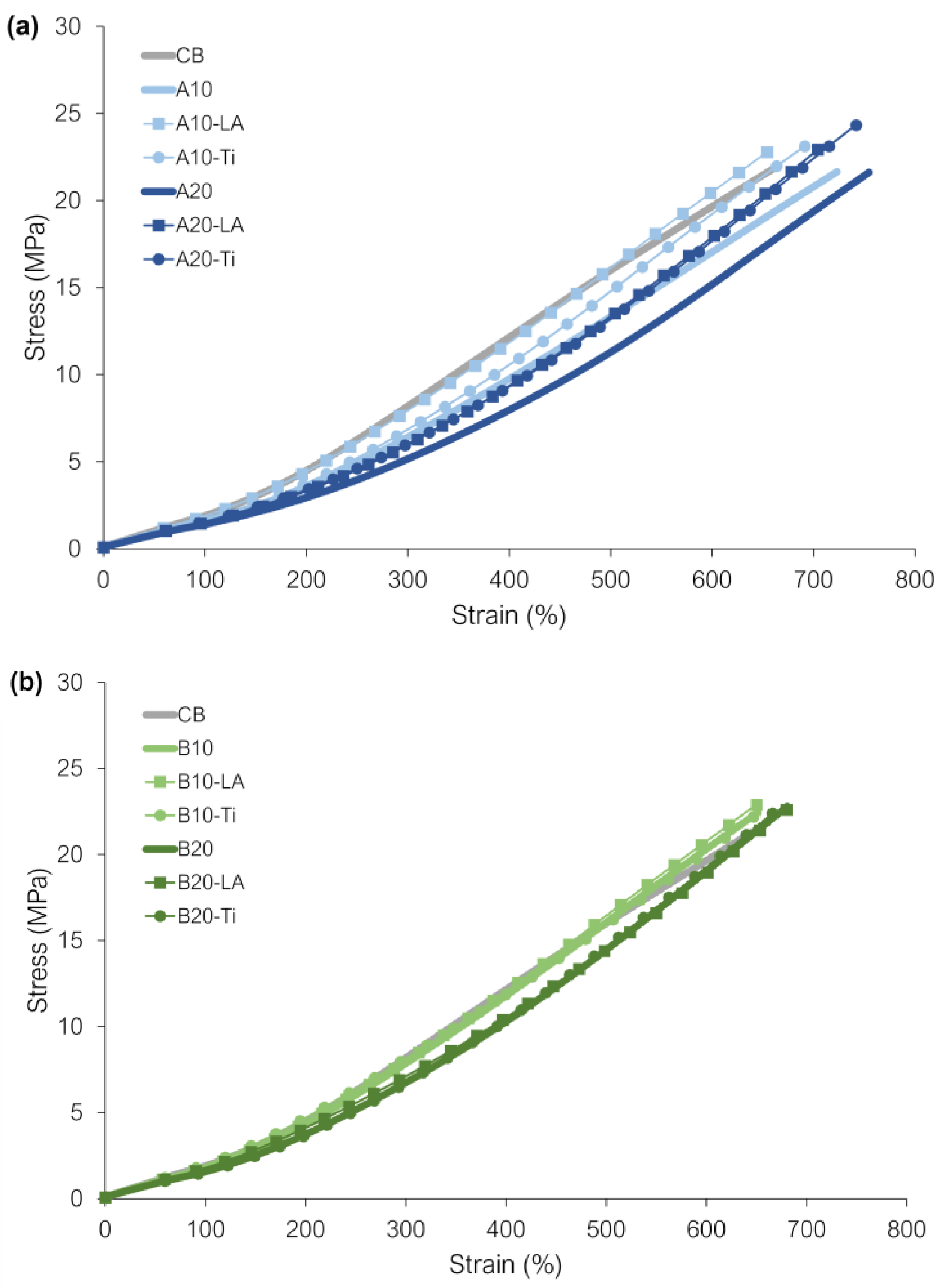
3.7. Tensile Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Xu, Y.; Wang, Y. How to improve sustainability for industrial sectors: Optimizing production scales based on performance-oriented resource reallocation. Energy Econ. 2023, 119, 106525. [Google Scholar] [CrossRef]
- Fan, Y.; Fowler, G.D.; Zhao, M. The Past, Present and Future of Carbon Black as A Rubber Reinforcing Filler—A Review. J. Clean. Prod. 2019, 247, 119115. [Google Scholar] [CrossRef]
- Chang, B.P.P.; Gupta, A.; Muthuraj, R.; Mekonnen, T.H. Bioresourced fillers for rubber composite sustainability: Current development and future opportunities. Green Chem. 2021, 23, 5337–5378. [Google Scholar] [CrossRef]
- Mohamed, N.R.; Othman, N.; Shuib, R.K.; Hayeemasae, N. Perspective on opportunities of bio-based processing oil to rubber industry: A short review. Iran. Polym. J. 2023, 32, 1455–1475. [Google Scholar] [CrossRef]
- Azhar, N.N.H.; Ang, D.T.-C.; Abdullah, R.; Harikrishna, J.A.; Cheng, A. Bio-Based Materials Riding the Wave of Sustainability: Common Misconceptions, Opportunities, Challenges and the Way Forward. Sustainability 2022, 14, 5032. [Google Scholar] [CrossRef]
- Sae-Oui, P.; Sirisinha, C.; Thaptong, P. Utilization of limestone dust waste as filler in natural rubber. J. Mater. Cycles Waste Manag. 2009, 11, 166–171. [Google Scholar] [CrossRef]
- Prochoń, M.; Sawicki, J.; Reda, M.; Sirek, P.; Suwalska, M.; Werstak, B. Shot blasting dust as a filler in elastomer composites. J. Elastomers Plast. 2021, 53, 1105–1127. [Google Scholar] [CrossRef]
- Hussain, A.I.; El-Sabbagh, S.H.; Abadir, I.F. Cement Dust as Filler in NBR Vulcanizates. J. Elastomers Plast. 2003, 35, 161–179. [Google Scholar] [CrossRef]
- Delgado, E.; Espitia, A.; Aperador, W. Comparative evaluation of Clusia multiflora wood flour, against mineral fillers, as reinforcement in SBR rubber composites. Iran. Polym. J. 2020, 29, 13–23. [Google Scholar] [CrossRef]
- Kaewpruk, C.; Boopasiri, S.; Poonsawat, C.; Sae-Oui, P.; Siriwong, C. Utilization of Sawdust and Wood Ash as a Filler in Natural Rubber Composites. ChemistrySelect 2021, 6, 264–272. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Ismail, H. Utilization of Tea Waste as an Alternative Filler for Natural Rubber. J. Teknol. 2020, 82, 109–115. [Google Scholar] [CrossRef]
- Juszkiewicz, A.; Maciejewska, M. Tea Grounds as a Waste Biofiller for Natural Rubber. Materials 2024, 17, 1516. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Prayitno, A.; Satoto, R. Morphology and Mechanical Properties of Palm Based Fly Ash Reinforced Dynamically Vulcanized Natural Rubber/Polypropylene Blends. Procedia Chem. 2012, 4, 146–153. [Google Scholar] [CrossRef]
- Ren, X.; Sancaktar, E. Use of fly ash as eco-friendly filler in synthetic rubber for tire applications. J. Clean. Prod. 2018, 206, 374–382. [Google Scholar] [CrossRef]
- Barrera, C.S.; Cornish, K. Characterization of Agricultural and Food Processing Residues for Potential Rubber Filler Applications. J. Compos. Sci. 2019, 3, 102. [Google Scholar] [CrossRef]
- El Mogy, S.A.; Darwish, N.A.; Awad, A. Comparative Study of the Cure Characteristics and Mechanical Properties of Natural Rubber Filled with Different Calcium Carbonate Resources. J. Vinyl Addit. Technol. 2020, 26, 309–315. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Raengthon, N.; Potiyaraj, P. Understanding the reinforcing efficiency of waste eggshell-derived nano calcium carbonate in natural rubber composites with maleated natural rubber as compatibilizer. Polym. Eng. Sci. 2019, 59, 1428–1436. [Google Scholar] [CrossRef]
- Zuiderduin, W.; Westzaan, C.; Huétink, J.; Gaymans, R. Toughening of polypropylene with calcium carbonate particles. Polymer 2003, 44, 261–275. [Google Scholar] [CrossRef]
- He, S.; Bijl, A.; Rohrbach, L.; Yuan, Q.; Santosa, D.S.; Wang, Z.; Heeres, H.J.; Brem, G. Catalytic upcycling paper sludge for the recovery of minerals and production of renewable high-grade biofuels and bio-based chemicals. Chem. Eng. J. 2021, 420, 129714. [Google Scholar] [CrossRef]
- Shi, X.; Rosa, R.; Lazzeri, A. On the Coating of Precipitated Calcium Carbonate with Stearic Acid in Aqueous Medium. Langmuir 2010, 26, 8474–8482. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, B.D.; Singh, S.P.; Bowman, C.N.; Anseth, K.S. Photodegradable, Photoadaptable Hydrogels via Radical-Mediated Disulfide Fragmentation Reaction. Macromolecules 2011, 44, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Waymouth, R.M. 1,2-Dithiolane-Derived Dynamic, Covalent Materials: Cooperative Self-Assembly and Reversible Cross-Linking. J. Am. Chem. Soc. 2017, 139, 3822–3833. [Google Scholar] [CrossRef]
- Wiita, A.P.; Ainavarapu, S.R.K.; Huang, H.H.; Fernandez, J.M. Force-dependent chemical kinetics of disulfide bond reduction observed with single-molecule techniques. Proc. Natl. Acad. Sci. USA 2006, 103, 7222–7227. [Google Scholar] [CrossRef]
- Anyszka, R.; Jia, L.; Blume, A. Tires for Mars Rovers: Reinforcing BR and BR/Vinyl-Methyl Silicone Rubber Compounds with Carbon Black, Nano-CaCO3, or Silica for Good Low-Temperature Dynamic-Mechanical Performance. Tire Sci. Technol. 2024, 52, 225–254. [Google Scholar] [CrossRef]
- Sarazyn, J. Smart Electronic Pet Collar System for Training and Tracking Health, Location, and Accurate Activity Levels of Pets. US20140331942A1, 13 November 2014. Available online: https://patents.google.com/patent/US20140331942A1/en (accessed on 13 June 2023).
- Yanti, E.D.; Pratiwi, I. Correlation between thermal behavior of clays and their chemical and mineralogical composition: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012078. [Google Scholar] [CrossRef]
- Worrall, W.E. Clays: Their Nature, Origin and General Properties; Maclaren: London, UK, 1968; Available online: https://books.google.nl/books?id=OVQSAQAAIAAJ (accessed on 13 June 2023).
- Elshereksi, N.W.; Ghazali, M.; Muchtar, A.; Azhari, C.H. Review of titanate coupling agents and their application for dental composite fabrication. Dent. Mater. J. 2017, 36, 539–552. [Google Scholar] [CrossRef] [PubMed]



| Ingredient | CB | A/B-10 | A/B-LA10 | A/B-Ti10 | A/B-20 | A/B-LA20 | A/B-Ti20 |
|---|---|---|---|---|---|---|---|
| NR—TSR10 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| TDAE 1 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Zinc oxide | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Stearic acid | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 6PPD 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Paraffin wax | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Sulfur | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| TBBS 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| TBzTD 4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Carbon Black N550 | 60 | 50 | 50 | 50 | 40 | 40 | 40 |
| Calcium Carbonate A/B | - | 10 | 10 | 10 | 20 | 20 | 20 |
| Alpha-Lipoic Acid | - | - | 0.7 | - | - | 1.4 | - |
| Titanate | - | - | - | 0.5 | - | - | 0.5 |
| Time (min) | Action |
|---|---|
| Step 1 pre-heating 70 °C—70 rpm | |
| 0.00 | Addition of rubber |
| 1.00 | Addition of 1/2 filler, processing oil |
| 2.30 | Addition of 1/2 filler, anti-degradants, and activators |
| 4.00 | Ram up, sweep for 15 s |
| 6.00 | Stop mixing |
| Step 2 pre-heating 50 °C—50 rpm | |
| 0.00 | Addition elastomer batch stage 1 |
| 1.00 | Addition curing system |
| 3.00 | Stop mixing |
| Filler Name | Code | BET SA [m2g−1] |
|---|---|---|
| Carbon Black N550 | CB | 42 |
| Calcium Carbonate A | CCA | 11.0 |
| Calcium Carbonate B | CCB | 14.9 |
| ΔG’(kPa) | G’100%(kPa) | |
|---|---|---|
| CB | 1700 | 455 |
| A10 | 1154 | 442 |
| A10-LA | 1430 | 470 |
| A10-Ti | 1070 | 462 |
| A20 | 766 | 416 |
| A20-LA | 1020 | 486 |
| A20-Ti | 867 | 458 |
| B10 | 1114 | 457 |
| B10-LA | 1350 | 544 |
| B10-Ti | 1255 | 530 |
| B20 | 671 | 414 |
| B20-LA | 944 | 503 |
| B20-Ti | 880 | 492 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schouw, C.; Bernal-Ortega, P.; Anyszka, R.; Bijl, A.; Gucho, E.; Blume, A. Toward Eco-Friendly Rubber: Utilizing Paper Waste-Derived Calcium Carbonate to Replace Carbon Black in Natural Rubber Composites. J. Compos. Sci. 2025, 9, 115. https://doi.org/10.3390/jcs9030115
Schouw C, Bernal-Ortega P, Anyszka R, Bijl A, Gucho E, Blume A. Toward Eco-Friendly Rubber: Utilizing Paper Waste-Derived Calcium Carbonate to Replace Carbon Black in Natural Rubber Composites. Journal of Composites Science. 2025; 9(3):115. https://doi.org/10.3390/jcs9030115
Chicago/Turabian StyleSchouw, Colin, Pilar Bernal-Ortega, Rafal Anyszka, Anton Bijl, Eyerusalem Gucho, and Anke Blume. 2025. "Toward Eco-Friendly Rubber: Utilizing Paper Waste-Derived Calcium Carbonate to Replace Carbon Black in Natural Rubber Composites" Journal of Composites Science 9, no. 3: 115. https://doi.org/10.3390/jcs9030115
APA StyleSchouw, C., Bernal-Ortega, P., Anyszka, R., Bijl, A., Gucho, E., & Blume, A. (2025). Toward Eco-Friendly Rubber: Utilizing Paper Waste-Derived Calcium Carbonate to Replace Carbon Black in Natural Rubber Composites. Journal of Composites Science, 9(3), 115. https://doi.org/10.3390/jcs9030115








