Facile Synthesis of PVP-Coated Silver Nanoparticles and Evaluation of Their Physicochemical, Antimicrobial and Toxic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Silver Nanoparticles (AgNPs)
2.2. Physicochemical Characterization
2.3. Evaluation of Antimicrobial Activity
2.3.1. Definition of Inhibition Halo
2.3.2. Minimum Inhibitory Concentration (MIC)
2.3.3. Cytotoxicity of AgNPs
2.3.4. Statistical Analysis
3. Results and Discussions
3.1. Mechanism of Formation of AgNPs
3.2. Physicochemical Properties of AgNPs
3.3. Antimicrobial Properties of AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Gao, J.; Na, H.; Zhong, R.; Yuan, M.; Guo, J.; Zhao, L.; Wang, Y.; Wang, L.; Zhang, F. One step synthesis of antimicrobial peptide protected silver nanoparticles: The core-shell mutual enhancement of antibacterial activity. Colloids Surf. B Biointerfaces 2020, 186, 110704. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Tormena, R.P.I.; Rosa, E.V.; Mota, B.d.F.O.; Chaker, J.A.; Fagg, C.W.; Freire, D.O.; Martins, P.M.; Silva, I.C.R.; Sousa, M.H. Evaluation of the Antimicrobial Activity of Silver Nanoparticles Obtained by Microwave-Assisted Green Synthesis Using Handroanthus impetiginosus (Mart. ex DC.) Mattos Underbark Extract. RSC Adv. 2020, 10, 20676–20781. [Google Scholar] [CrossRef] [PubMed]
- Ban, D.K.; Paul, S. Protein corona over silver nanoparticles triggers conformational change of proteins and drop in bactericidal potential of nanoparticles: Polyethylene glycol capping as preventive strategy. Colloid Surf. B 2016, 146, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Ferrag, C.; Li, S.; Jeon, K.; Andoy, N.M.; Sullan, R.M.A.; Mikhaylichenko, S.; Kerman, K. Polyacrylamide hydrogels doped with different shapes of silver nanoparticles: Antibacterial and mechanical properties. Colloid Surf. B 2021, 197, 111397–111405. [Google Scholar] [CrossRef]
- Gheitaran, R.; Afkhami, A.; Madrakian, T. Effect of light at different wavelengths on polyol synthesis of silver nanocubes. Sci. Rep. 2022, 12, 19202–19213. [Google Scholar] [CrossRef] [PubMed]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of Metal Nanoparticles and Their Pharmacological Applications: Present Status and Application Prospects. J. Nanostruct. Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.-l.; Gu, Y.; Huang, H.; Zhang, G.-W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799–816. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Zheng, Y.; Wang, Z.; Ma, Z.; Yang, Q.; Yao, B.; Zhao, Y.; Zhang, H. A green approach for synthesizing silver nanoparticles, and their antibacterial and cytotoxic activities. New J. Chem. 2018, 42, 2882–2888. [Google Scholar] [CrossRef]
- Yan, K.; Xu, F.; Wei, W.; Yang, C.; Wang, D.; Shi, X. Electrochemical Synthesis of Chitosan/Silver Nanoparticles Multilayer Hydrogel Coating with pH-Dependent Controlled Release Capability and Antibacterial Property. Colloids Surf. B Biointerfaces 2021, 202, 111711–111719. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An Overview of application of Silver Nanoparticles for Biomaterials in Dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Shan, Y.; Shen, Q.; Li, Y.; Jiang, J.; Liu, Y.; Liu, X. Potential impact of organic ligands on the antibacterial activity of silver nanoparticles. New J. Chem. 2019, 43, 2870–2874. [Google Scholar] [CrossRef]
- Mandal, A.; Meda, V.; Zhang, W.J.; Farhan, K.M.; Gnanamani, A. Synthesis, Characterization and Comparison of Antimicrobial Activity of PEG/TritonX-100 Capped Silver Nanoparticles on Collagen Scaffold. Colloids Surf. B Biointerfaces 2012, 90, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and cytotoxicity of chitosan-based hydrogels modified with silver nanoparticles. Colloids Surf. B 2017, 160, 325–330. [Google Scholar] [CrossRef]
- Sofia, N.G.; Nikos, B.; Elias, S.; Eleni, E.K. Synthesis of biocompatible silver nanoparticles by a modified polyol method for theranostic applications: Studies on red blood cells, internalization ability and antibacterial activity. J. Inorg. Biochem. 2020, 211, 111177–111187. [Google Scholar]
- Marambio-Jones, C.; Hoek, E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Szymczak, M.; Maciejewska, M.; Laskowski, Ł.; Laskowska, M.; Ostaszewski, R.; Skiba, G.; Franiak-Pietryga, I. All That Glitters Is Not Silver-ANew Look at Microbiological and Medical Applications of Silver Nanoparticles. Int. J. Mol. Sci. 2021, 22, 854. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Bryam, A.M.; Yoshimura, I.; Santos, L.P.; Moura, C.C.; Santos, C.C.; Silva, V.L.; Lovaglio, R.B.; Costa Marques, R.F.; Jafelicci Junior, M.; Contiero, J. Silver Nanoparticles Stabilized by Ramnolipids: Effect of pH. Colloids Surf. B Biointerfaces 2021, 205, 111883–111889. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ji, X.; Wu, H.; Zhao, L.; Li, J.; Yang, W. Shape control of silver nanoparticles by stepwise citrate reduction. J. Phys. Chem. C 2009, 113, 6573–6576. [Google Scholar] [CrossRef]
- Mavaei, M.; Chahardoli, A.; Shokoohinia, Y.; Khoshroo, A.; Fattahi, A. One-step Synthesized Silver Nanoparticles Using Isoimperatorin: Evaluation of Photocatalytic, and Electrochemical Activities. Sci. Rep. 2020, 10, 1762–1773. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green Synthesis of Silver Nanoparticles Using Plant Extracts and Their Antimicrobial Activities: A Review of Recent Literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Riaz, M.; Mutreja, V.; Sareen, S.; Ahmad, B.; Faheem, M.; Zahid, N.; Jabbour, G.; Park, J. Exceptional Antibacterial and Cytotoxic Potency of Monodisperse Greener AgNPs Prepared under Optimized pH and Temperature. Sci. Rep. 2021, 11, 2866–2876. [Google Scholar] [CrossRef] [PubMed]
- Muscia, G.C.; Carnevale, J.P.; Luczywo, A.; Peláez, M.V.; O’Toole, A.R.; Buldain, G.Y.; Casal, J.J.; Asís, S.E. Synthesis, Anti-Tuberculosis Activity and QSAR Study of 2,4-Diarylquinolines and Analogous Polycyclic Derivatives. Arab. J. Chem. 2019, 12, 932–945. [Google Scholar] [CrossRef]
- Polte, J. Fundamental Growth Principles of Colloidal Metal Nanoparticles—A New Perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Fiorati, A.; Bellingeri, A.; Punta, C.; Corsi, I.; Venditti, I. Silver Nanoparticles forWater Pollution Monitoring and Treatments: Ecosafety Challenge and Cellulose-Based Hybrids Solution. Polymers 2020, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Torras, M.; Roig, A. From Silver Plates to Spherical Nanoparticles: Snapshots of Microwave-Assisted Polyol Synthesis. ACS Omega 2020, 5, 5731–5738. [Google Scholar] [CrossRef]
- Turkevic, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- La Spina, R.; Mehn, D.; Fumagalli, F.; Holland, M.; Reniero, F.; Rossi, F.; Gilliland, D. Synthesis of Citrate-Stabilized Silver Nanoparticles Modified by Thermal and pH Preconditioned Tannic Acid. Nanomaterials 2020, 10, 2031. [Google Scholar] [CrossRef] [PubMed]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in New Robes: Key Questions Answered for the Most Common Gold Nanoparticle Synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef]
- Kim, J.-S. Antibacterial Activity of Ag+ Ion-Containing Silver Nanoparticles Prepared Using the Alcohol Reduction Method. J. Ind. Eng. Chem. 2007, 13, 718–722. [Google Scholar]
- Ali, I.; Akl, M.R.; Meligi, G.A.; Saleh, T.A. Silver Nanoparticles Embedded in Polystyrene-Polyvinyl Pyrrolidone Nanocomposites Using γ-Ray Irradiation: Physico-Chemical Properties. Results Phys. 2017, 7, 1319–1328. [Google Scholar] [CrossRef]
- Cao, L.; Huang, Q.; Cui, J.; Lin, H.; Li, W.; Lin, Z.; Zhang, P. Rapid and Facile Synthesis of High-Performance Silver Nanowires by a Halide-Mediated, Modified Polyol Method for Transparent Conductive Films. Nanomaterials 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M.; Lado-Touriño, I. Reduction and Stabilization of Silver Nanoparticles in Ethanol by Nonionic Surfactants. Langmuir 1996, 12, 3585–3589. [Google Scholar] [CrossRef]
- Hah, H.J.; Koo, S.M. Preparation of Silver Nanoparticles through Alcohol Reduction with Organoalkoxysilanes. J. Sol-Gel Sci. Technol. 2003, 26, 467–471. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, Y.; Tang, Z.; Hu, J.; Su, R.; Lin, J.; Zhou, T.; Guo, H.; Wang, N.; Xu, R. A size-controlled green synthesis of silver nanoparticles by using the berry extract of Sea Buckthorn and their biological activities. New J. Chem. 2020, 44, 9304–9312. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.u.; Ao, Q. Role of capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Peralta, L.C.F.; Almeida, N.L.M.; Pontes, F.M.L.; Rinaldo, D.; Carneiro, C.A.; Neppelenbroek, K.H.; Lara, V.S.; Porto, V.C. Silver nanoparticles in denture adhesive: An antimicrobial approach against Candida albicans. J. Dent. 2023, 131, 104445. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, H.; Jia, J.; Wei, Y. Facile synthesis of high-concentration, stable aqueous dispersions of uniform silver nanoparticles using aniline as a reductant. Langmuir 2011, 27, 5047–5053. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Oh, S.-G. Ostwald Ripening and Control of Ag Ion Reduction Degree by Ammonium Hydroxide in Alcohol Reduction Process. J. Ind. Eng. Chem. 2015, 21, 768–771. [Google Scholar] [CrossRef]
- Hosida, T.Y.; Cavazana, T.P.; Henriques, M.; Pessan, J.P.; Delbem, A.C.B.; Monteiro, D.R. Interactions between Candida albicans and Candida glabrata in Biofilms: Influence of the Strain Type, Culture Medium and Glucose Supplementation. Mycoses 2018, 61, 270–278. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Silva, S.; Negri, M.; Gorup, L.F.; Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver Nanoparticles: Influence of Stabilizing Agent and Diameter on Antifungal Activity Against Candida albicans and Candida glabrata Biofilm. Lett. Appl. Microbiol. 2012, 54, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Gouvêa, C.C.; do Amaral, J.G.; Fernandes, R.A.; Fernandes, G.L.; Gorup, L.F.; Camargo, E.R. Sodium trimetaphosphate and hexametaphosphate impregnated with silver nanoparticles: Characteristics and antimicrobial efficacy. Biofouling 2018, 34, 299–308. [Google Scholar] [CrossRef]
- Baber, R.; Mazzei, L.; Thanh, N.T.K.; Gavriilidis, A. An Engineering Approach to Synthesis of Gold and Silver Nanoparticles by Controlling Hydrodynamics and Mixing Based on a Coaxial Flow Reactor. Nanoscale 2017, 9, 14149–14161. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of Silver Nanoparticles with Different Shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Tatarchuk, V.; Sergievskaya, A.; Korda, T.; Druzhinina, I.; Zaikovsky, V. Kinetic Factors in the Synthesis of Silver Nanoparticles by Reduction of Ag+ with Hydrazine in Reverse Micelles of Triton N-42. Chem. Mater. 2013, 25, 3570–3579. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed]
- Murshid, N.; Kitaev, V. Role of Poly(vinylpyrrolidone) (PVP) and Other Sterically Protecting Polymers in Selective Stabilization of {111} and {100} Facets in Pentagonally Twinned Silver Nanoparticles. Chem. Commun. 2014, 50, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Hassan, M.L.; Ward, A.A. Novel Nanofibrillated Cellulose/Polyvinylpyrrolidone/Silver Nanoparticles Films with Electrical Conductivity Properties. Carbohydr. Polym. 2017, 157, 503–511. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the Preparation of Silver Nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Vlahovic, B. Preparation of Silver Nanoparticles in Poly(N-vinylpyrrolidone)/Ethanol Solutions. Int. J. Nanosci. 2017, 16, 1750008–1750012. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, A.K.; Reddy, S.; Jeon, H.-J.; Ahn, C.W. Role of Capping Agents in Controlling Silver Nanoparticles Size, Antibacterial Activity and Potential Application as Optical Hydrogen Peroxide Sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Gharibshahi, L.; Saion, E.; Gharibshahi, E.; Shaari, A.H.; Matori, K.A. Influence of Poly(vinylpyrrolidone) Concentration on Properties of Silver Nanoparticles Manufactured by Modified Thermal Treatment Method. PLoS ONE 2017, 12, e0186094–e0186110. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Characterization and Optical Studies of PVP-Capped Silver Nanoparticles. J. Nanostruct. Chem. 2017, 7, 37–46. [Google Scholar] [CrossRef]
- Song, M.; Wang, D.; Peana, S.; Choudhury, S.; Nyga, P.; Kudyshev, Z.A.; Yu, H.; Boltasseva, A.; Shalaev, V.M.; Kildishev, A.V. Colors with Plasmonic Nanostructures: A Full-Spectrum Review. Appl. Phys. Rev. 2019, 6, 041308. [Google Scholar] [CrossRef]
- Ider, M.; Abderrafi, K.; Eddahbi, A.; Ouaskit, S.; Kassiba, A. Silver Metallic Nanoparticles with Surface Plasmon Resonance: Synthesis and Characterizations. J. Clust. Sci. 2017, 28, 1051–1069. [Google Scholar] [CrossRef]
- Lee, K.-C.; Lin, S.-J.; Lin, C.-H.; Tsai, C.-S.; Lu, Y.-J. Size Effect of Ag Nanoparticles on Surface Plasmon Resonance. Surf. Coat. Technol. 2008, 202, 5339–5342. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Dmitruk, I.M.; Alexeenko, A.A.; Kotko, A.V.; Verdal, J.; Pinchuk, A.O. Size and Temperature Effects on the Surface Plasmon Resonance in Silver Nanoparticles. Plasmonics 2012, 7, 685–694. [Google Scholar] [CrossRef]
- Barman, T.; Hussain, A.A.; Sharma, B.; Pal, A.R. Plasmonic Hot Hole Generation by Interband Transition in Gold-Polyaniline. Sci. Rep. 2015, 5, 18276. [Google Scholar] [CrossRef]
- Najafi, A.; Khoeini, M.; Khalaj, G.; Sahebgharan, A. Synthesis of silver nanoparticles from electronic scrap by chemical reduction. Mater. Res. Express 2021, 8, 125009–125017. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Duzagac, F.; Canzonieri, V.; Rizzolio, F. Self-Therapeutic Nanomaterials for Cancer Therapy: A Review. ACS Appl. Nano Mater. 2020, 3, 4962–4971. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; Mendoza, E.G.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green Synthesis of Silver Nanoparticles using Lysiloma acapulcensis Exhibit High-Antimicrobial Activity. Sci. Rep. 2020, 10, 12805–12814. [Google Scholar] [CrossRef]
- Atta, A.M.; Moustafa, Y.M.; Ezzat, A.O.; Hashem, A.I. Novel Magnetic Silica-Ionic Liquid Nanocomposites for Wastewater Treatment. Nanomaterials 2019, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Baek, D.R.; Kim, J.S.; Joo, S.-W.; Lim, J.K. Green Synthesis of Silver Nanoparticles with Size Distribution Depending on Reducing Species in Glycerol at Ambient pH and Temperatures. ACS Omega 2020, 5, 16246–16254. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Oh, S.-G. Counter-ion effects of silver salt on the production yield of silver nanoparticles in alcohol reduction process. Colloid Surf. A 2014, 459, 172–176. [Google Scholar] [CrossRef]
- Wasilewska, A.; Klekotka, U.; Zambrzycka, M.; Zambrowski, G.; Święcicka, I.; Kalska-Szostko, B. Physico-chemical properties and antimicrobial activity of silver nanoparticles fabricated by green synthesis. Food Chem. 2023, 400, 133960. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Al-Homaidan, A.A.; Al-Sabri, A.; Almansob, A.; AlNadhari, S. Anti-oxidant, anti-fungal and cytotoxic effects of silver nanoparticles synthesized using marine fungus Cladosporium halotolerans. Appl. Nanosci. 2023, 13, 623–631. [Google Scholar] [CrossRef]
- Majumdar, R.; Kar, P.K. Biosynthesis, characterization and anthelmintic activity of silver nanoparticles of Clerodendrum infortunatum isolate. Sci. Rep. 2023, 13, 7415–7425. [Google Scholar] [CrossRef]
- Palomares, C.V.V.; Barreto, Y.B.; Bexiga, N.M.; Toma, S.H.; Julival dos Santos, J.; Araki, K.; Alencar, A.M.; Bloise, A.C. Metabolic profiling of murine macrophages exposed to silver nanoparticles at dose and time dependencies. Part. Part. Syst. Charact. 2023, 40, 2200191–2200202. [Google Scholar] [CrossRef]
- Washio, I.; Xiong, Y.; Yin, Y.; Xia, Y. Reduction by the End Groups of Poly(vinyl pyrrolidone): A New and Versatile Route to the Kinetically Controlled Synthesis of Ag Triangular Nanoplates. Adv. Mater. 2006, 18, 1745–1749. [Google Scholar] [CrossRef]
- Chou, K.-S.; Lai, Y.-S. Effect of Polyvinyl Pyrrolidone Molecular Weights on the Formation of Nanosized Silver Colloids. Mater. Chem. Phys. 2004, 83, 82–88. [Google Scholar] [CrossRef]
- Du, B.D.; Phu, D.V.; Duy, N.N.; Lan, N.T.K.; Lang, V.T.K.; Thanh, N.V.K.; Phong, N.T.P.; Hien, N.Q. Preparation of Colloidal Silver Nanoparticles in Poly(N-vinylpyrrolidone) by g-Irradiation. J. Exp. Nanosci. 2008, 3, 207–213. [Google Scholar] [CrossRef]
- Song, Y.-J.; Wang, M.; Zhang, X.-Y.; Wu, J.-Y.; Zhang, T. Investigation on the Role of the Molecular Weight of Polyvinyl Pyrrolidone in the Shape Control of High-Yield Silver Nanospheres and Nanowires. Nanoscale Res. Lett. 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Malina, D.; Sobczak-Kupiec, A.; Wzorek, Z.; Kowalski, Z. Silver Nanoparticles Synthesis with Different Concentrations of Polyvinylpyrrolidone. Dig. J. Nanomater. Biostructures 2012, 7, 1527–1534. [Google Scholar]
- Nishimoto, M.; Abeb, S.; Yonezawa, T. Preparation of Ag Nanoparticles Using Hydrogen Peroxide as a Reducing Agent. New J. Chem. 2018, 42, 14493–14501. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F.; Catalano, M.; Taurino, A.; Licciulli, A.; Maffezzoli, A.; Sannino, A. Antibacterial Coatings on Haemodialysis Catheters by Photochemical Deposition of Silver Nanoparticles. J. Mater. Sci. Mater. Med. 2011, 22, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Davies, J.K.; Sundqvist, G.; Figdor, D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int. Endod. J. 2002, 35, 221–228. [Google Scholar] [CrossRef]
- McIntosh, J.; James, W.W.; Lazarus-Barlow, P. An investigation into the etiology of dental caries. I: The nature of the destructive agent and the production of artificial caries. Br. J. Exp. Pathol. 1922, 3, 138–145. [Google Scholar]
- Rodriguez, F. Studies in the specific bacteriology of dental caries. Mil. DJ 1922, 5, 199. [Google Scholar]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Chen, W.; Zhu, C.; Liang, J. Bacterial flora and extraradicular biofilm associated with the apical segment of teeth with post-treatment apical periodontitis. J. Endod. 2012, 38, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R.; Brundin, M.; Sundqvist, G.; Sjögren, U. Building biofilms in vital host tissues: A survival strategy of Actinomyces radicidentis. Oral Surg. Oral Med. Oral Path. Oral Radiol. Endod. 2008, 106, 595–603. [Google Scholar] [CrossRef][Green Version]
- Tennert, C.; Fuhrmann, M.; Wittmer, A.; Karygianni, L.; Altenburger, M.J.; Pelz, K.; Hellwig, E.; Al-Ahmad, A. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J. Endod. 2014, 40, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Signoretti, F.G.C.; Endo, M.S.; Gomes, B.P.F.A.; Montagner, F.; Tosello, F.B.; Jacinto, R.C. Persistent extraradicular infection in root-filled asymptomatic human tooth: Scanning electron microscopic analysis and microbial investigation after apical microsurgery. J. Endod. 2011, 37, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Signoretti, F.G.C.; Gomes, B.P.F.A.; Montagner, F.; Jacinto, R.C. Investigation of cultivable bacteria isolated from longstanding retreatment-resistant lesions of teeth with apical periodontitis. J. Endod. 2013, 39, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Lara-Carrillo, E.; Toral-Rizo, V.H.; López-Castañares, R.; Morales-Luckie, R.A. Synthesis and Characterization of Silver Nanoparticles on Orthodontic Brackets: A New Alternative in the Prevention of White Spots. Coatings 2019, 9, 480. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Pazos-Ortiz, E.; Roque-Ruiz, J.H.; Hinojos-Márquez, E.A.; López-Esparza, J.; Donohué-Cornejo, A.; Cuevas-González, J.C.; Espinosa-Cristóbal, L.F.; Reyes-López, S.Y. Dose-Dependent Antimicrobial Activity of Silver Nanoparticles on Polycaprolactone Fibers against Gram-Positive and Gram-Negative Bacteria. J. Nanomater. 2017, 2017, 4752314. [Google Scholar] [CrossRef]
- De Moura, M.R.; Mattoso, L.H.C.; Zucolotto, V. Development of cellulose-Based Bactericidal Nanocomposites Containing Silver Nanoparticles and their Use as Active Food Packaging. J. Food Eng. 2012, 109, 520–524. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial Nanomaterials for Water Disinfection and Microbial Control: Potential Applications and Implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of Enhanced Antibacterial Effects of Novel Silver Nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Hartemann, P. Silver as an Antimicrobial: Facts and Gaps in Knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Gram-Positive Bacterial Cell Envelopes: The Impact on the Activity of Antimicrobial Peptides. Biochim. Biophys. Acta 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Mandal, D.; Dash, S.K.; Das, B.; Chattopadhyay, S.; Ghosh, T.; Das, D.; Roy, S. Bio-Fabricated Silver Nanoparticles Preferentially Targets Gram Positive Depending on Cell Surface Charge. Biomed. Pharmacother. 2016, 83, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver Colloidal Nanoparticles: Antifungal Effect against Adhered Cells and Biofilms of Candida albicans and Candida glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef]
- Wady, A.F.; Machado, A.L.; Zucolotto, V.; Zamperini, C.A.; Berni, E.; Vergani, C.E. Evaluation of Candida albicans Adhesion and Biofilm Formation on a Denture Base Acrylic Resin Containing Silver Nanoparticles. J. Appl. Microbiol. 2012, 112, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal Activity and Mode of Action of Silver Nano-Particles on Candida albicans. Biometals 2009, 22, 235–242. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 16, 53–60. [Google Scholar] [CrossRef]
- Martínez-Robles, Á.M.; Loyola-Rodríguez, J.P.; Zavala-Alonso, N.V.; Martinez-Martinez, R.E.; Ruiz, F.; Lara-Castro, R.H.; Donohué-Cornejo, A.; Reyes-López, S.Y.; Espinosa-Cristóbal, L.F. Antimicrobial Properties of Biofunctionalized Silver Nanoparticles on Clinical Isolates of Streptococcus mutans and Its Serotypes. Nanomaterials 2016, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-Dependent Antibacterial Activities of Silver Nanoparticles against Oral Anaerobic Pathogenic Bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of Protein Modification on the Protein Corona on Nanoparticles and Nanoparticle—Cell Interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Wady, A.F.; Machado, A.L.; Foggi, C.C.; Zamperini, C.A.; Zucolotto, V.; Moffa, E.B.; Vergani, C.E. Effect of a Silver Nanoparticles Solution on Staphylococcus aureus and Candida spp. J. Nanomater. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Elorza, M.V.; Valentín, E.; Sentandreu, R. Molecular Organization of the Cell Wall of Relation to Pathogenicity. FEMS Yeast Res. 2006, 6, 14–29. [Google Scholar] [CrossRef]
- Klis, F.M.; de Koster, C.G.; Brul, S. Cell Wall-Related Bionumbers and Bioestimates of Saccharomyces cerevisiae and Candida albicans. Eukaryot. Cell 2014, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Nomura, R.; Nakagawa, I.; Hamada, S.; Ooshima, T. Demonstration of Streptococcus mutans with a Cell Wall Polysaccharide Specific to a New Serotype, k, in the Human Oral Cavity. J. Clin. Microbiol. 2004, 42, 198–202. [Google Scholar] [CrossRef]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopfe, L.; Green, D.W. The Nanotipped Hairs of Gecko Skin and Biotemplated Replicas Impair and/or Kill Pathogenic Bacteria with High Efficiency. Nanoscale 2016, 8, 18860–18870. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Stern, S.T.; McNeil, S.E. Nanotechnology Safety Concerns Revisited. Toxicol. Sci. 2008, 101, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of Surfactants and Polymers on Stability and Antibacterial Activity of Silver Nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, Z.; Yang, J.; Song, C.; Zhang, R.; Cui, Y. Polyvinylpyrrolidone- (PVP-) coated silver aggregates for high performance surface-enhanced Raman scattering in living cells. Nanotechnology 2009, 20, 445102. [Google Scholar] [CrossRef]
- Mei, L.; Lu, Z.; Zhang, X.; Li, C.; Jia, Y. Polymer-Ag Nanocomposites with Enhanced Antimicrobial Activity against Bacterial Infection. ACS Appl. Mater. Interfaces 2014, 6, 15813–15821. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yu, L.; Liu, A.; Alahmadi, T.A.; Almoallim, H.S.; Durairaj, K. Ononin mitigates streptozotocin-induced diabetic nephropathy in rats via alleviating oxidative stress and inflammatory markers. J. King Saud Univ. Sci. 2022, 34, 102029–102036. [Google Scholar] [CrossRef]

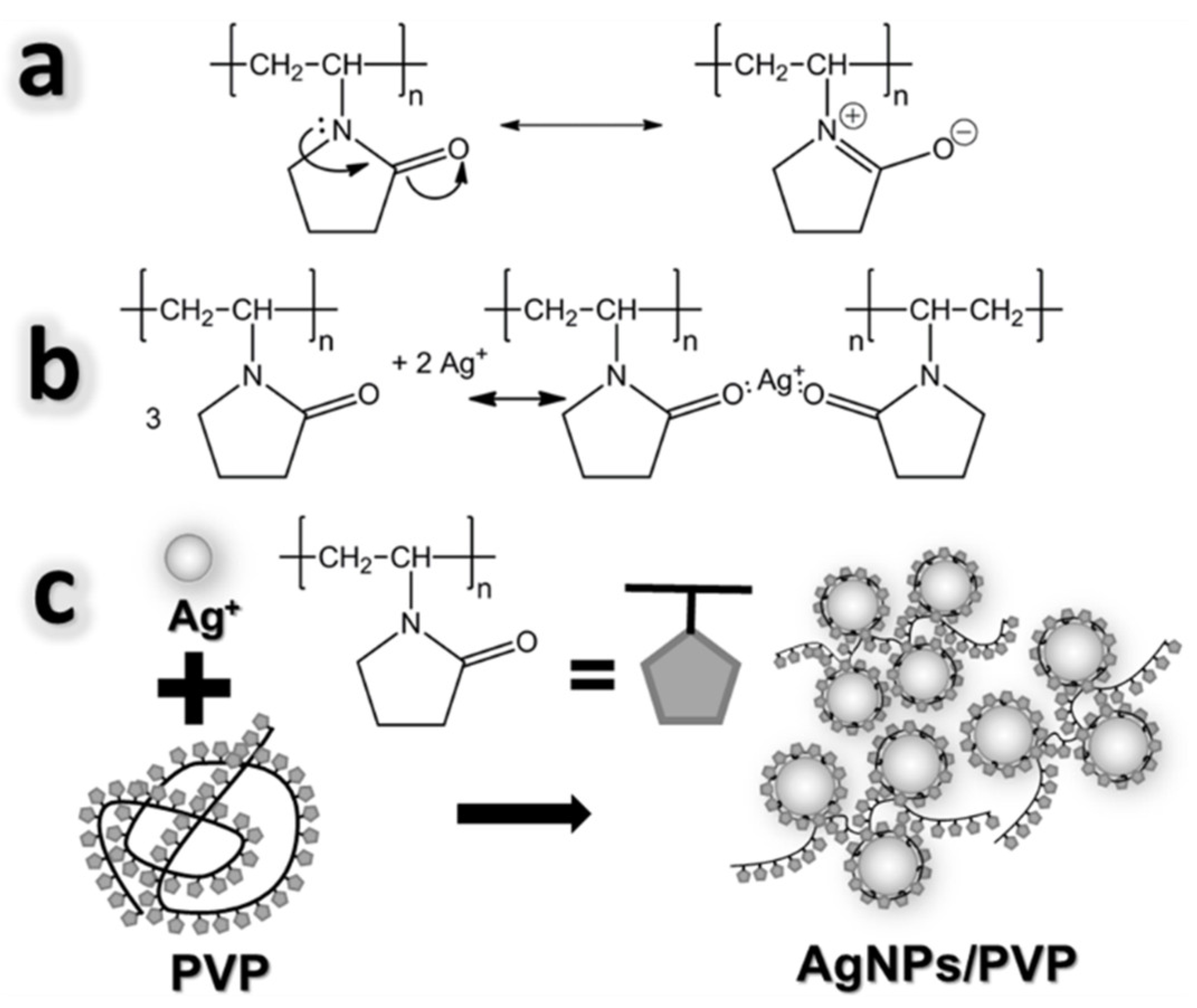

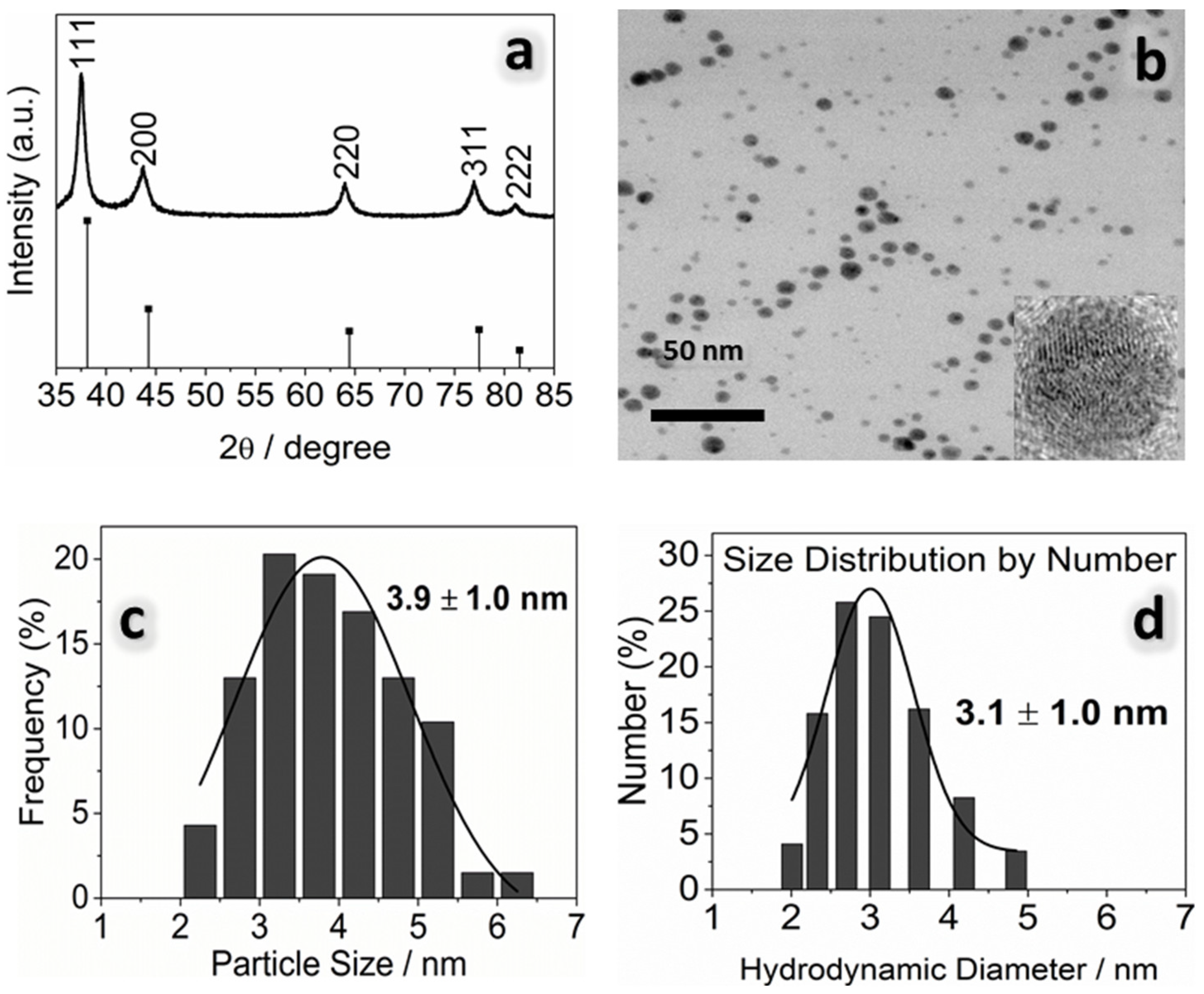

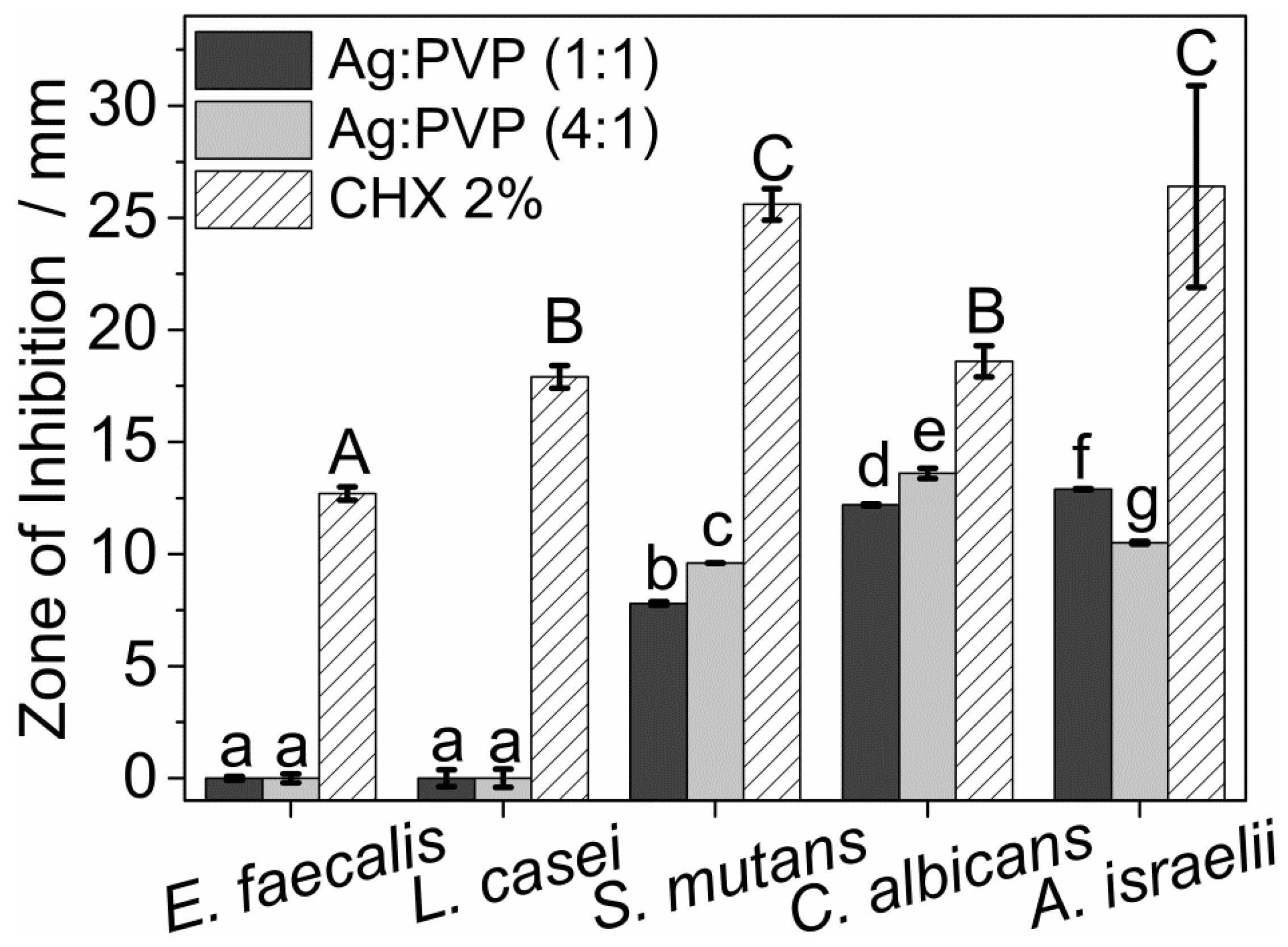

| Method | Size Particle/nm | Morphology | Solution Concentration | Reference |
|---|---|---|---|---|
| Alcoholic | 3.9 ± 1.0 | Spherical | 45 mM | This study |
| Alcoholic | 651 ± 91 | Spherical | 180 mM | This study |
| Polyol | 48.8 ± 3.3 | Nanocubes | 16 mM | [6] |
| Alcoholic | 4.6 ± 0.8 | Spherical | 40 mM | [73] |
| Alcoholic | 10–15 | Spherical | 1 mM | [66] |
| Turkevich | 30 ± 3.9 | Spherical | 7 mM | [23] |
| Polyol | 15.6 ± 8.30 | Spherical | 6 mM | [17] |
| Turkevich | 21 | Spherical | 4 mM | [42] |
| Green synthesis | 25 ± 2.0 | Spherical | 0.3 mM | [74] |
| Green synthesis | 20 | Spherical | 1 mM | [75] |
| Green synthesis | 27.8 ± 3.13 | Spherical | 1 mM | [76] |
| Turkevich | 40 | Spherical | 0.25 mM | [77] |
| Species | Ag:PVP (1:1) | Ag:PVP (4:1) | ||
|---|---|---|---|---|
| MIC (mg mL−1) | MFC/MBC (mg mL−1) | MIC (mg mL−1) | MFC/MBC (mg mL−1) | |
| C. albicans | 9.400 | 300.9 | 9.400 | 9.400 |
| S. mutans | 601.9 | 300.9 | 75.23 | 75.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, F.N.S.; Morais, L.A.; Gorup, L.F.; Ribeiro, L.S.; Martins, T.J.; Hosida, T.Y.; Francatto, P.; Barbosa, D.B.; Camargo, E.R.; Delbem, A.C.B. Facile Synthesis of PVP-Coated Silver Nanoparticles and Evaluation of Their Physicochemical, Antimicrobial and Toxic Activity. Colloids Interfaces 2023, 7, 66. https://doi.org/10.3390/colloids7040066
Neto FNS, Morais LA, Gorup LF, Ribeiro LS, Martins TJ, Hosida TY, Francatto P, Barbosa DB, Camargo ER, Delbem ACB. Facile Synthesis of PVP-Coated Silver Nanoparticles and Evaluation of Their Physicochemical, Antimicrobial and Toxic Activity. Colloids and Interfaces. 2023; 7(4):66. https://doi.org/10.3390/colloids7040066
Chicago/Turabian StyleNeto, Francisco N. Souza, Leonardo A. Morais, Luiz F. Gorup, Lucas S. Ribeiro, Tassia J. Martins, Thayse Y. Hosida, Patricia Francatto, Debora B. Barbosa, Emerson R. Camargo, and Alberto C. B. Delbem. 2023. "Facile Synthesis of PVP-Coated Silver Nanoparticles and Evaluation of Their Physicochemical, Antimicrobial and Toxic Activity" Colloids and Interfaces 7, no. 4: 66. https://doi.org/10.3390/colloids7040066
APA StyleNeto, F. N. S., Morais, L. A., Gorup, L. F., Ribeiro, L. S., Martins, T. J., Hosida, T. Y., Francatto, P., Barbosa, D. B., Camargo, E. R., & Delbem, A. C. B. (2023). Facile Synthesis of PVP-Coated Silver Nanoparticles and Evaluation of Their Physicochemical, Antimicrobial and Toxic Activity. Colloids and Interfaces, 7(4), 66. https://doi.org/10.3390/colloids7040066









