Heat Transfer Fluids Based on Amino-Functionalized Silica Dispersed in 1,2-Propylene Glycol and in 50-50 Aqueous 1,2-Propylene Glycol
Abstract
:1. Introduction
2. Materials and Methods
- 100% PG;
- 100% PG + 75 μL of 35–38% HCl per 150 g of PG;
- 100% PG + 750 μL of glacial acetic acid per 150 g of PG;
- 50% PG + 75 μL of 35–38% HCl per 150 g of 50% PG;
- 50% PG + 750 μL of glacial acetic acid per 150 g of 50% PG.
3. Results and Discussion
3.1. IEP in Aqueous Dispersion
3.2. Dissociation of Hydrochloric and Acetic Acid in PG and 50% Aqueous PG
3.3. Visual Observation of Dispersions
3.4. Electrokinetic Potential in Aged Dispersions
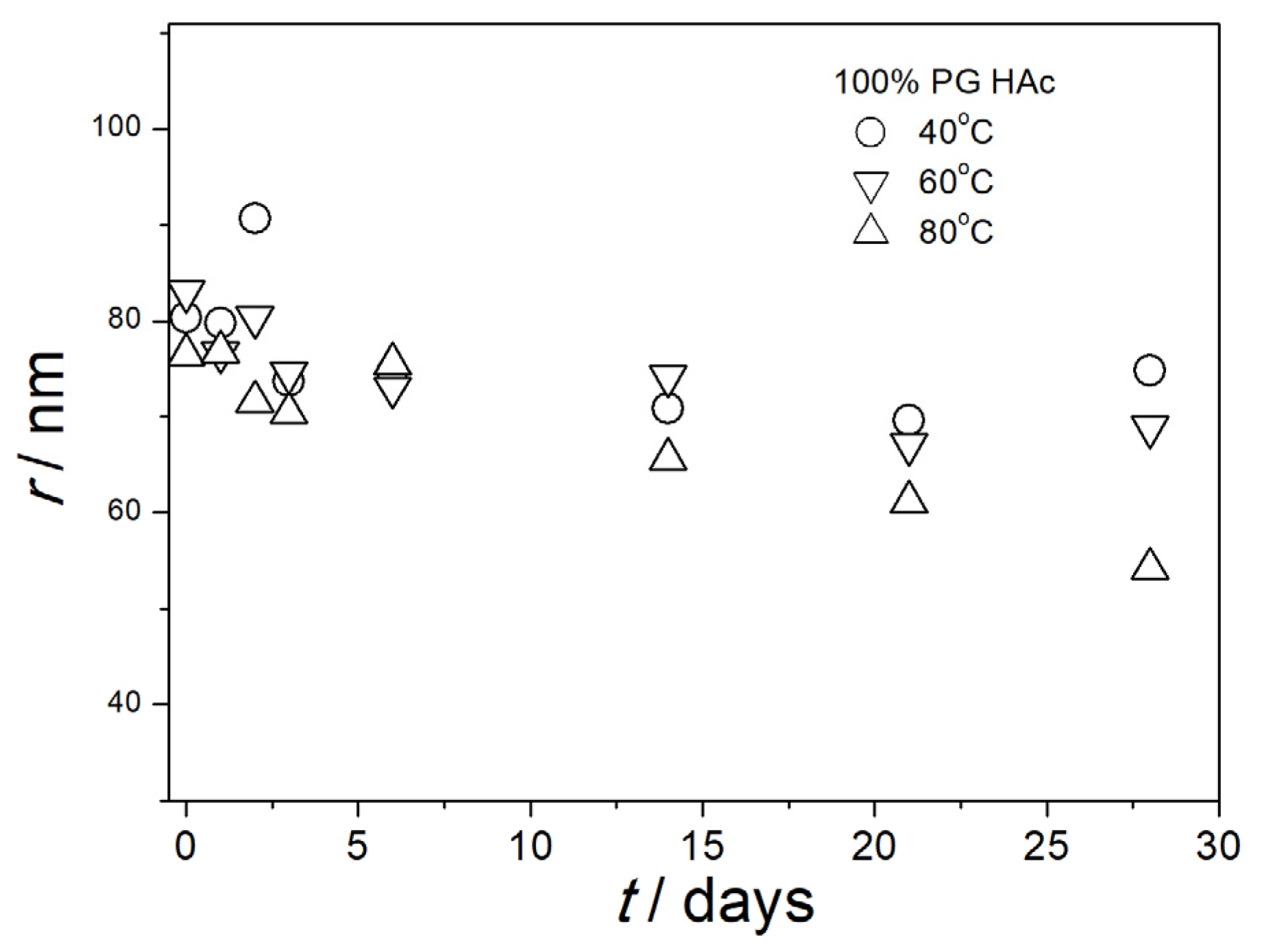
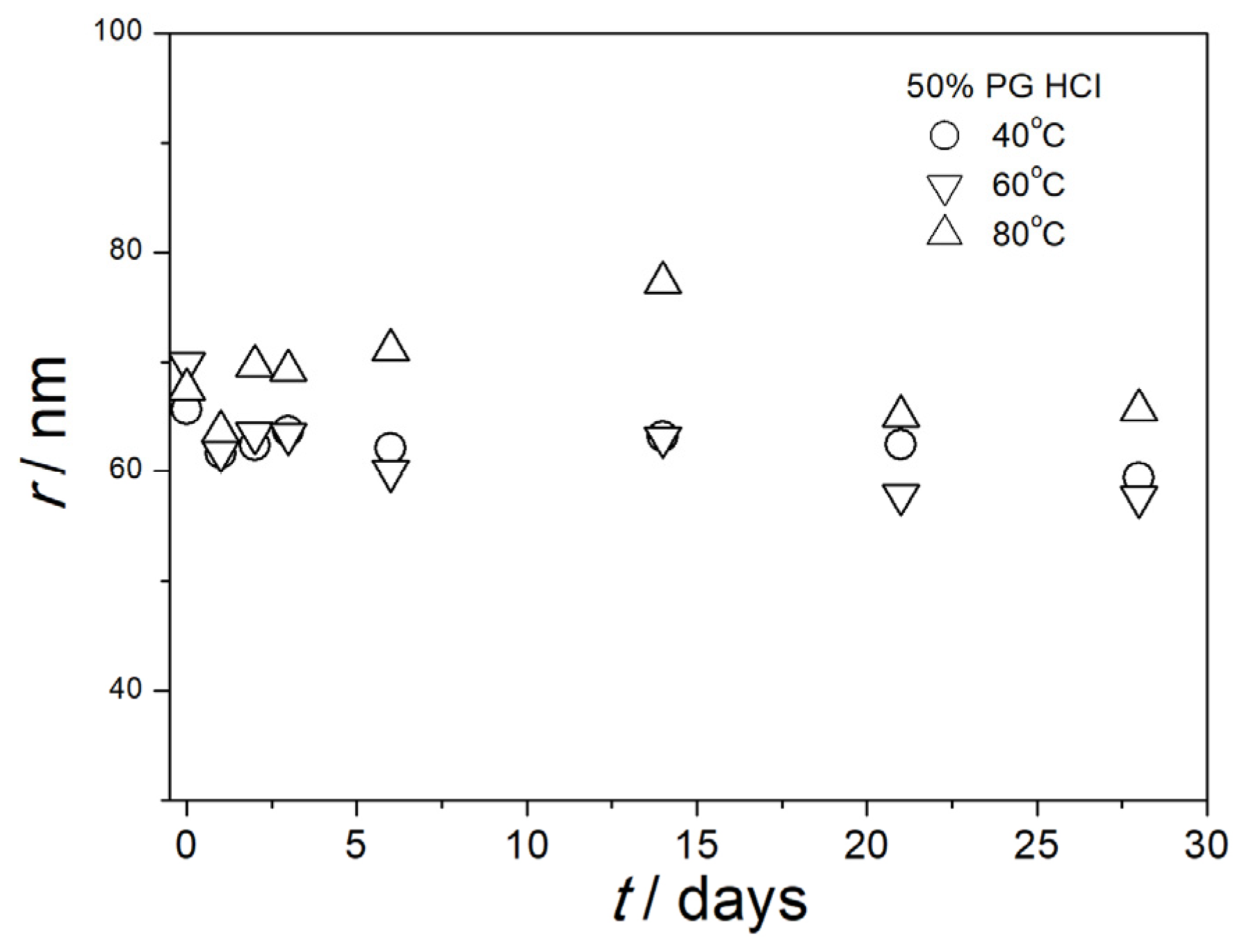
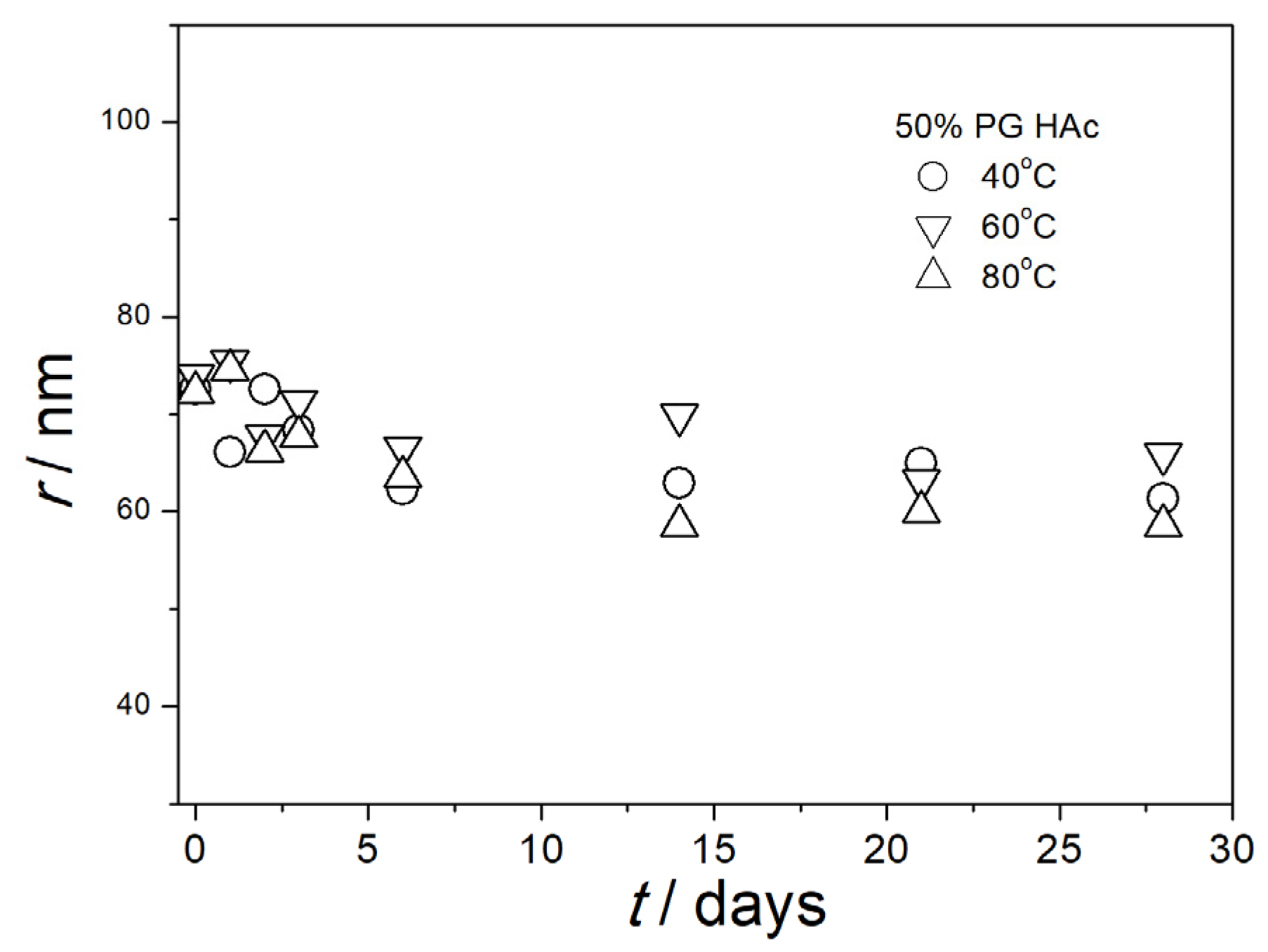
3.5. Particle Size in Aged Dispersions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atmaca, B.; Yalçın, G.; Küçükyıldırım, B.O.; Arkadumnuay, T.; Leunanonchai, W.; Manova, S.; Dalkılıç, A.S.; Wongwises, S. Determination of Dynamic Viscosity and Stability for Single and Hybrid Nanofluids of SiO2, TiO2, MWCNT and ZnO Nanoparticles. J. Therm. Anal. Calorim. 2024. [Google Scholar] [CrossRef]
- Hložek, T.; Bursová, M.; Čabala, R. Fast Determination of Ethylene Glycol, 1,2-Propylene Glycol and Glycolic Acid in Blood Serum and Urine for Emergency and Clinical Toxicology by GC-FID. Talanta 2014, 130, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Kumar, P.; Sakthivadivel, D.; Thangapandian, N.; Salman, M.; Kumar Thakur, A.; Sathyamurthy, R.; Chul Kim, S. Effects of Ultasonication and Surfactant on the Thermal and Electrical Conductivity of Water—Solar Glycol Mixture Based Al2O3 Nanofluids for Solar-Thermal Applications. Sustain. Energy Technol. Assess. 2021, 47, 101371. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Zheng, K.; Huang, H. Comparison of Heat Transfer Performance of ZnO-PG, α-Al2O3-PG, and γ-Al2O3-PG Nanofluids in Car Radiator. Nanomater. Nanotechnol. 2019, 9, 1847980419876465. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Chaudhary, R.; Mehrotra, S.C. Dielectric Behaviour of Propylene Glycol-Water Mixtures Studied by Time Domain Reflectometry. Mol. Phys. 2001, 99, 1805–1812. [Google Scholar] [CrossRef]
- George, J.; Sastry, N.V. Densities, Dynamic Viscosities, Speeds of Sound, and Relative Permittivities for Water + Alkanediols (Propane-1,2- and -1,3-Diol and Butane-1,2-, -1,3-, -1,4-, and -2,3-Diol) at Different Temperatures. J. Chem. Eng. Data 2003, 48, 1529–1539. [Google Scholar] [CrossRef]
- MacBeth, G.; Thompson, A.R. Densities and Refractive Indexes for Propylene Glycol-Water Solutions. ACS Publ. 1951, 23, 618–619. [Google Scholar] [CrossRef]
- Kalbarczyk, M.; Skupiński, S.; Kosmulski, M. Amino-Functionalized Silica as a Component of Heat-Transfer Fluids. J. Mol. Liq. 2024, 397, 124195. [Google Scholar] [CrossRef]
- Sekrani, G.; Poncet, S. Ethylene- and Propylene-Glycol Based Nanofluids: A Litterature Review on Their Thermophysical Properties and Thermal Performances. Appl. Sci. 2018, 8, 2311. [Google Scholar] [CrossRef]
- Kosmulski, M.; Kalbarczyk, M. Zeta Potential of Nanosilica in 50% Aqueous Ethylene Glycol and in 50% Aqueous Propylene Glycol. Molecules 2023, 28, 1335. [Google Scholar] [CrossRef]
- Kosmulski, M.; Mączka, E. Zeta Potential in Dispersions of Titania Nanoparticles in Moderately Polar Solvents Stabilized with Anionic Surfactants. J. Mol. Liq. 2022, 355, 118972. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Wilfred, C.D.; Saidur, R.; Saha, B.B. Comparative Evaluation on the Thermal Properties and Stability of MWCNT Nanofluid with Conventional Surfactants and Ionic Liquid. J. Therm. Anal. Calorim. 2022, 147, 393–408. [Google Scholar] [CrossRef]
- Zainon, S.N.M.; Azmi, W.H. Stability and Thermo-Physical Properties of Green Bio-Glycol Based TiO2-SiO2 Nanofluids. Int. Commun. Heat Mass Transf. 2021, 126, 105402. [Google Scholar] [CrossRef]
- Prabhakaran, M.; Manikandan, S.; Suganthi, K.S.; Leela Vinodhan, V.; Rajan, K.S. Development and Assessment of Ceria–Propylene Glycol Nanofluid as an Alternative to Propylene Glycol for Cooling Applications. Appl. Therm. Eng. 2016, 102, 329–335. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Febrero-Garrido, L.; Cacabelos, A.; González-Gil, A.; Lugo, L. Influence of Crystal Structure on the Thermophysical Properties and Figures-of-Merit of Propylene Glycol: Water-Based SiC Nanofluids. Powder Technol. 2024, 433, 119299. [Google Scholar] [CrossRef]
- Sekhar, G.C.; Thimothy, P.; Surakasi, R.; Khan, N.A.; Zahmatkesh, S. Graphene Nanopowder and Propylene Glycol Solutions: Thermal and Physical Properties. Arab. J. Sci. Eng. 2023, 48, 16039–16050. [Google Scholar] [CrossRef]
- Kosmulski, M.; Eriksson, P.; Rosenholm, J.B. Application of Zetametry To Determine Concentrations of Acidic and Basic Impurities in Analytical Reagents. Anal. Chem. 1999, 71, 2518–2522. [Google Scholar] [CrossRef] [PubMed]
- Radtke, V.; Stoica, D.; Leito, I.; Camões, F.; Krossing, I.; Anes, B.; Roziková, M.; Deleebeeck, L.; Veltzé, S.; Näykki, T.; et al. A Unified pH Scale for All Solvents: Part I—Intention and Reasoning (IUPAC Technical Report). Pure Appl. Chem. 2021, 93, 1049–1060. [Google Scholar] [CrossRef]
- Kalbarczyk, M.; Skupiński, S.; Kosmulski, M. Thermal Stability of Dispersions of Amino-Functionalized Silica in Glycol and in 50–50 Aqueous Glycol. Molecules 2024, 29, 2686. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Lively, R.P.; Labreche, Y.; Chen, G.; Fan, Y.; Koros, W.J.; Jones, C.W. Aminosilane-Grafted Polymer/Silica Hollow Fiber Adsorbents for CO2 Capture from Flue Gas. ACS Appl. Mater. Interfaces 2013, 5, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M.; Hartikainen, J.; Ma̧czka, E.; Janusz, W.; Rosenholm, J.B. Multiinstrument Study of the Electrophoretic Mobility of Fumed Silica. Anal. Chem. 2002, 74, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. Surface Charging and Points of Zero Charge; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-09339-5. [Google Scholar]
- Rosenholm, J.M.; Lindén, M. Wet-Chemical Analysis of Surface Concentration of Accessible Groups on Different Amino-Functionalized Mesoporous SBA-15 Silicas. Chem. Mater. 2007, 19, 5023–5034. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Lindén, M. Towards Establishing Structure–Activity Relationships for Mesoporous Silica in Drug Delivery Applications. J. Control. Release 2008, 128, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Saita, S.; Kawasaki, H. Origin of the Fluorescence in Silica-Based Nanoparticles Synthesized from Aminosilane Coupling Agents. J. Lumin. 2021, 232, 117849. [Google Scholar] [CrossRef]
- Raj, A.F.P.A.M.; Krajnc, S.; Bauman, M.; Lakić, M.; Gutmaher, A.; Lobnik, A.; Košak, A. Removal of Pb2+, Cr3+ and Hg2+ Ions from Aqueous Solutions Using SiO2 and Amino-Functionalized SiO2 Particles. J. Sol-Gel Sci. Technol. 2022, 103, 290–308. [Google Scholar] [CrossRef]
- Barak, M.; Aghdam, A.S.; Ozbulut, E.B.S.; Unal, S.; Cebeci, F.Ç. Layer-by-Layer Assembly of Nanofilms from Colloidally Stable Amine-Functionalized Silica Nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2021, 630, 127615. [Google Scholar] [CrossRef]
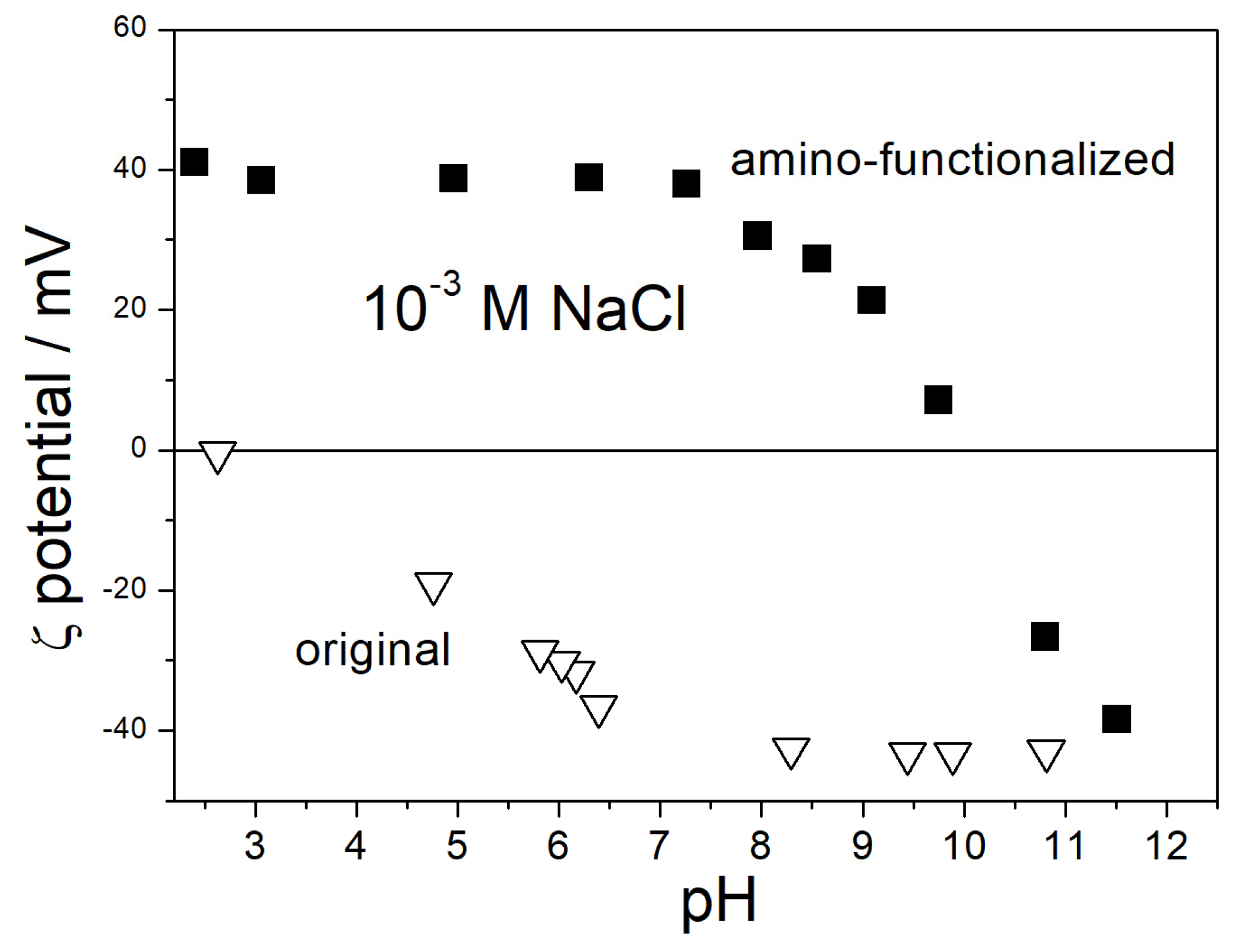
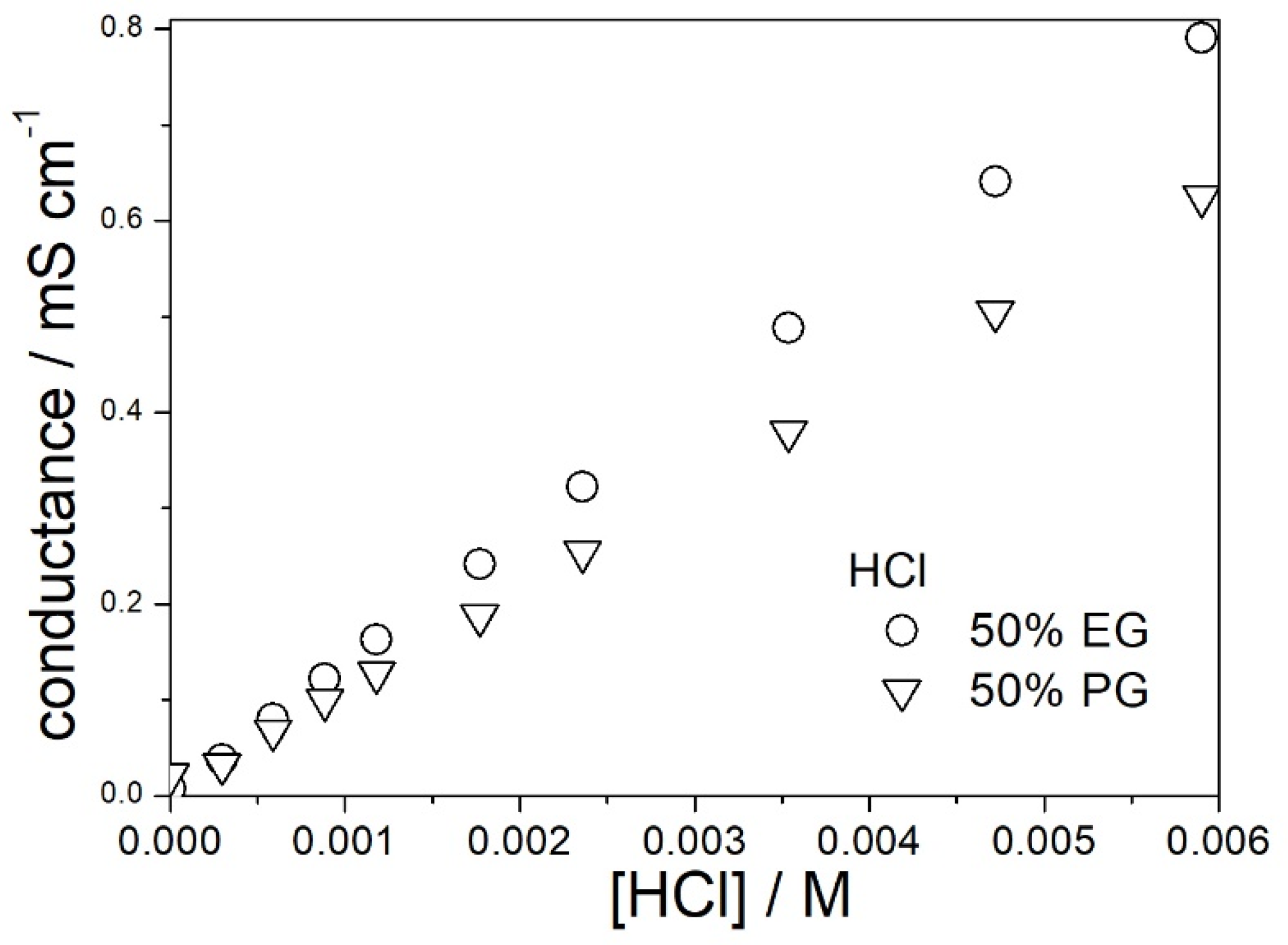



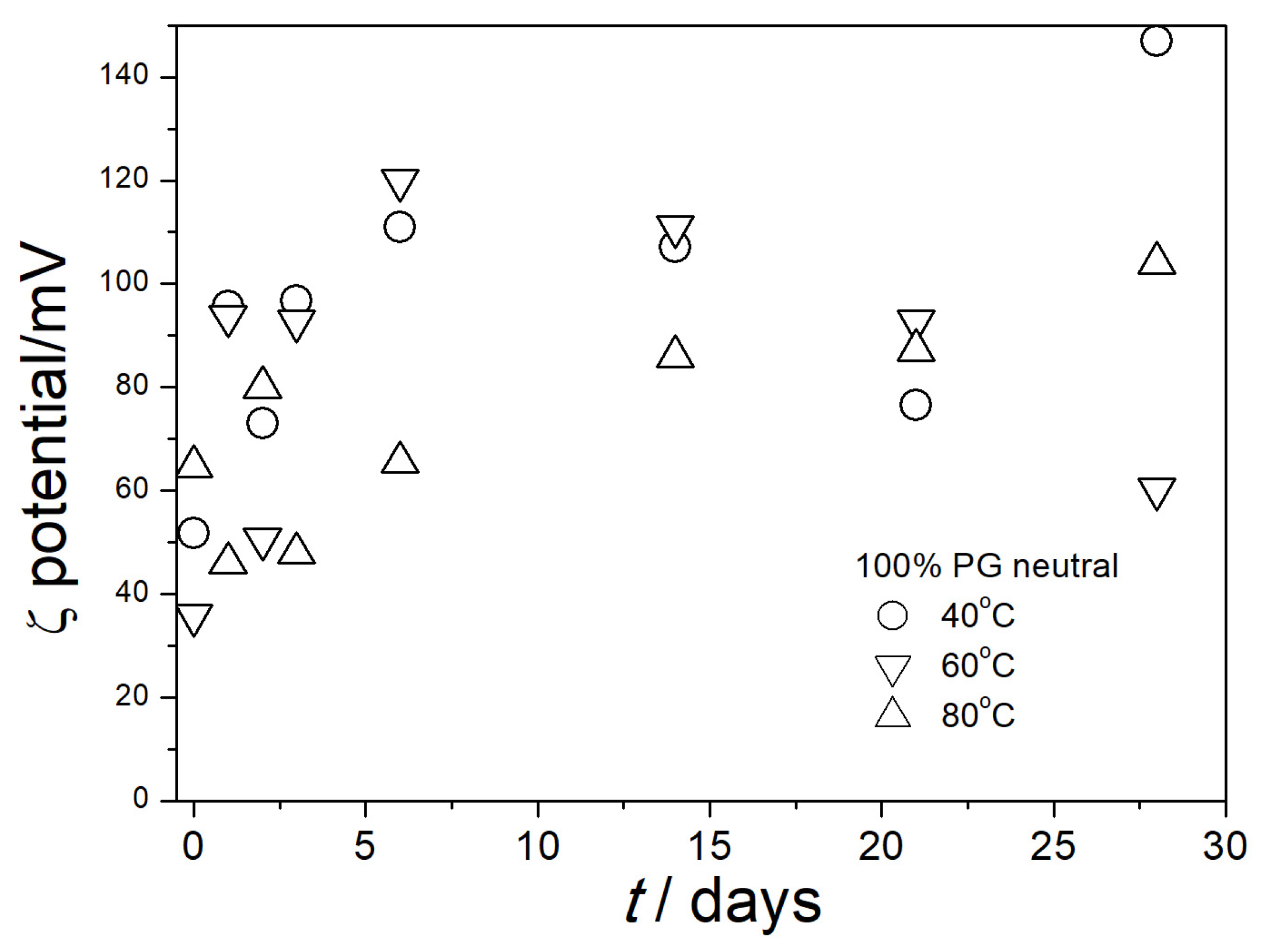
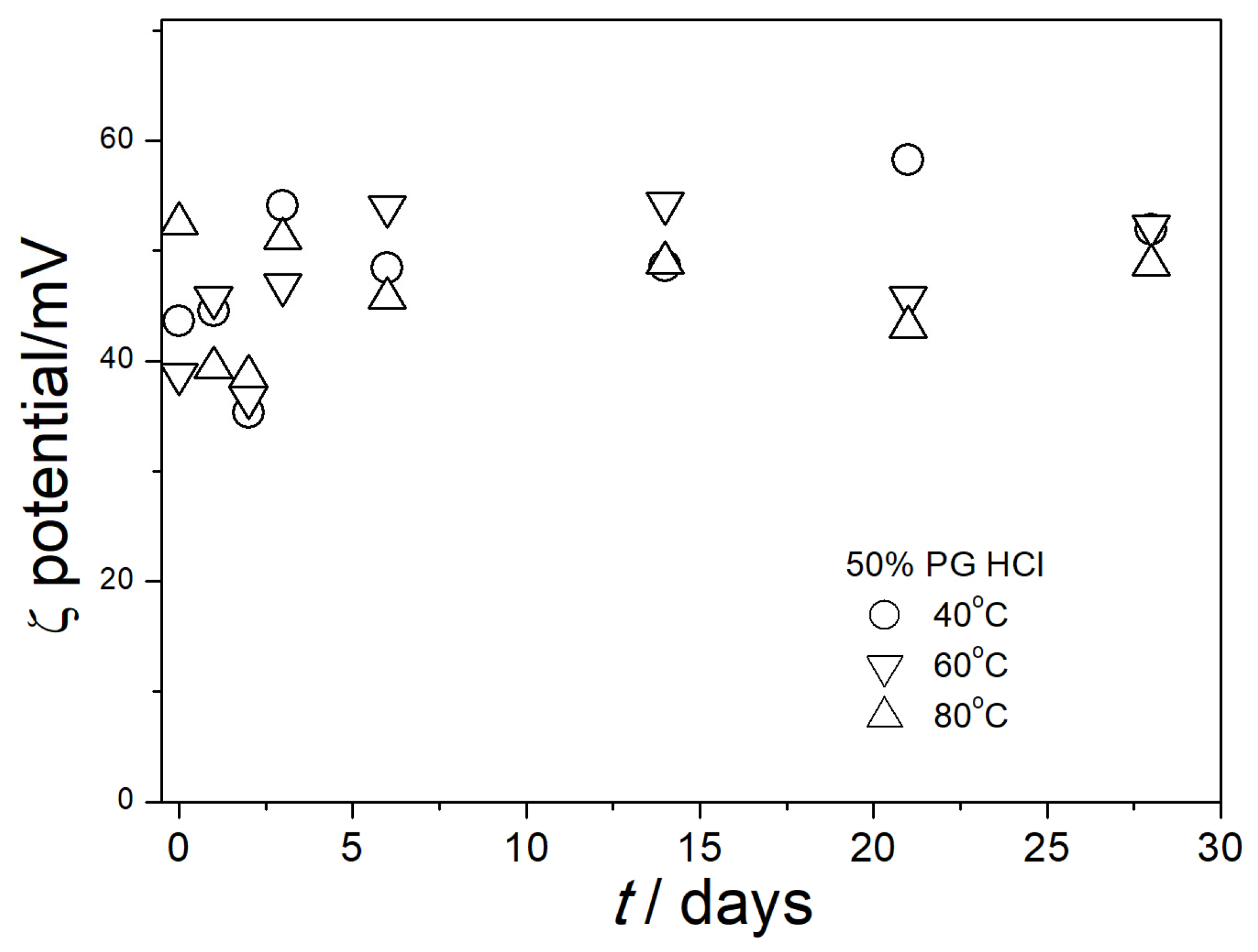



| % PG by Mass | ε0 [5] | η/mPa·s [6] | nD [7] |
|---|---|---|---|
| 0 | 78.8 | 0.891 | 1.3331 |
| 10 | 77.53 | 1.0531 | 1.3432 |
| 20 | 75.1 | 1.8011 | 1.3544 |
| 30 | 72.4 | 2.7481 | 1.3658 |
| 40 | 64.6 | 3.9785 | 1.377 |
| 50 | 58.8 | 5.6284 | 1.3878 |
| 60 | 55.5 | 7.9308 | 1.398 |
| 70 | 47.7 | 11.3144 | 1.4075 |
| 80 | 42.6 | 16.6447 | 1.4162 |
| 90 | 36.6 | 25.9109 | 1.4241 |
| 100 | 30.2 | 44.6335 | 1.4316 |
| % PG by Mass | Particles | Dispersant | Methods 1 | Ref. |
|---|---|---|---|---|
| 50 | SiO2 | various | Z | [10] |
| 100 | TiO2 | various | Z | [11] |
| 100 | Multiwall CNTs | various | V, T, H | [12] |
| 100 | SiO2 + TiO2 | none | V, T, Z | [13] |
| 100 | CeO2 | none | V, T | [14] |
| 30 | SiC | none | V, T, H | [15] |
| 50, 75, 100 | graphene | none | V, T, H | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalbarczyk, M.; Skupiński, S.; Kosmulski, M. Heat Transfer Fluids Based on Amino-Functionalized Silica Dispersed in 1,2-Propylene Glycol and in 50-50 Aqueous 1,2-Propylene Glycol. Colloids Interfaces 2024, 8, 43. https://doi.org/10.3390/colloids8040043
Kalbarczyk M, Skupiński S, Kosmulski M. Heat Transfer Fluids Based on Amino-Functionalized Silica Dispersed in 1,2-Propylene Glycol and in 50-50 Aqueous 1,2-Propylene Glycol. Colloids and Interfaces. 2024; 8(4):43. https://doi.org/10.3390/colloids8040043
Chicago/Turabian StyleKalbarczyk, Marta, Sebastian Skupiński, and Marek Kosmulski. 2024. "Heat Transfer Fluids Based on Amino-Functionalized Silica Dispersed in 1,2-Propylene Glycol and in 50-50 Aqueous 1,2-Propylene Glycol" Colloids and Interfaces 8, no. 4: 43. https://doi.org/10.3390/colloids8040043








