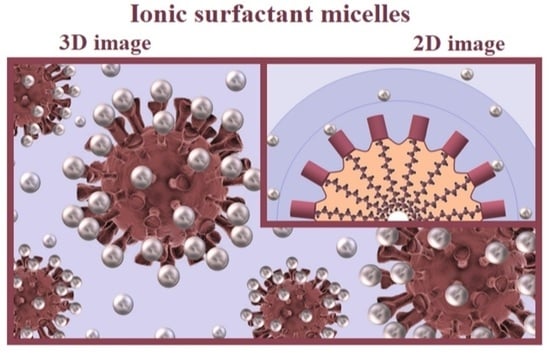
Nanosized Being of Ionic Surfactant Micelles: An Advanced View on Micellization Process
Abstract
1. Introduction
2. Conventional Model of Ionic Micelle Structure
3. Advanced Model of Ionic Micelle Structure
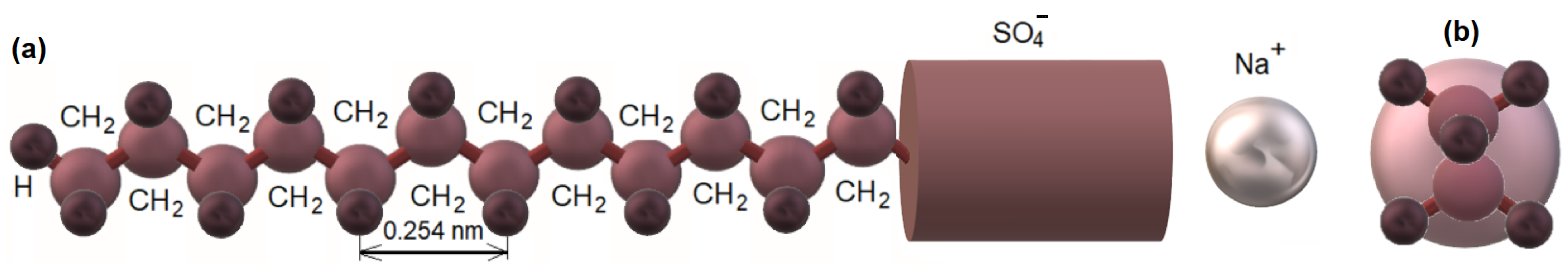
3.1. Hydrocarbon Core–Water Interface in Micelle
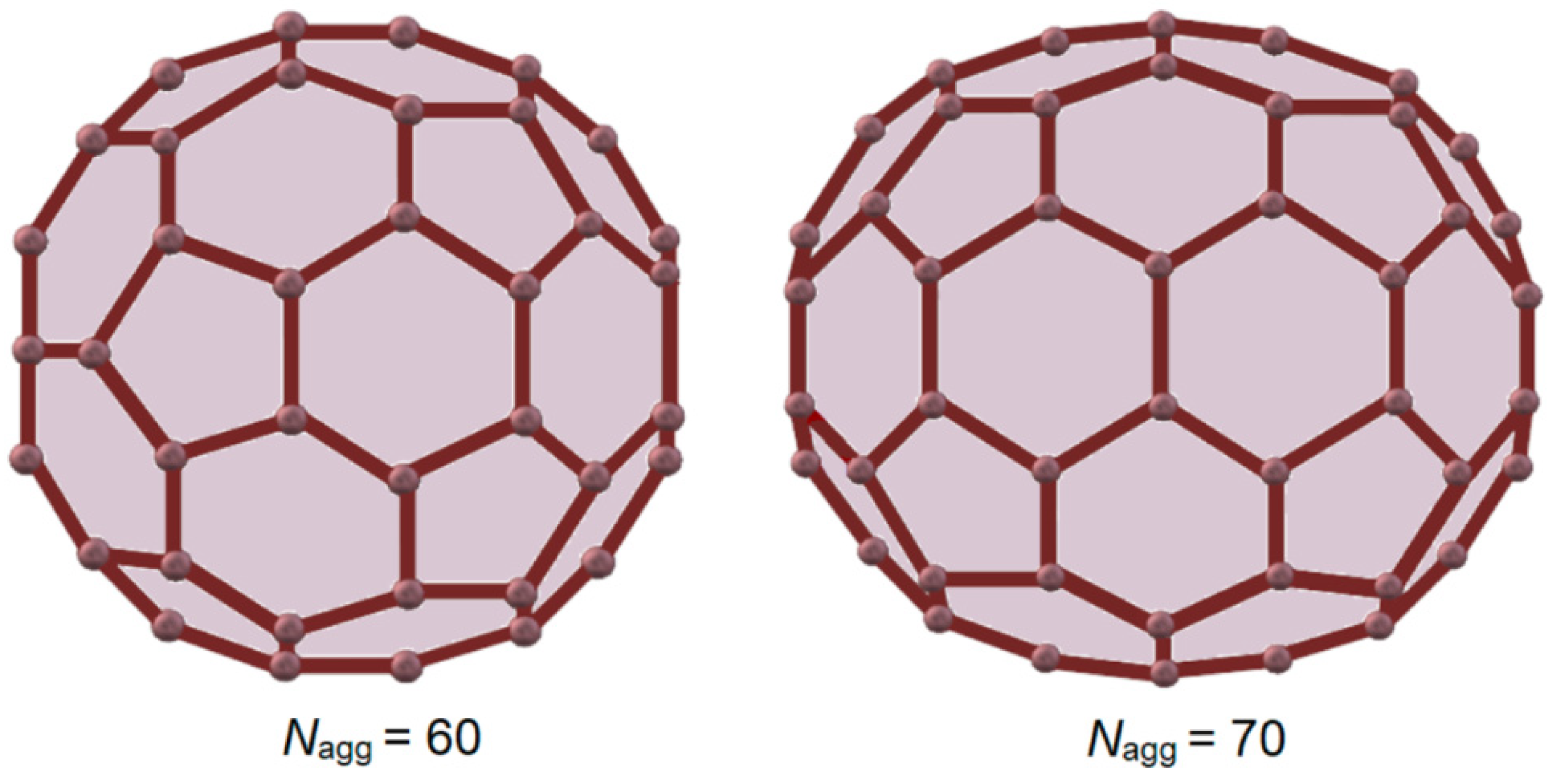
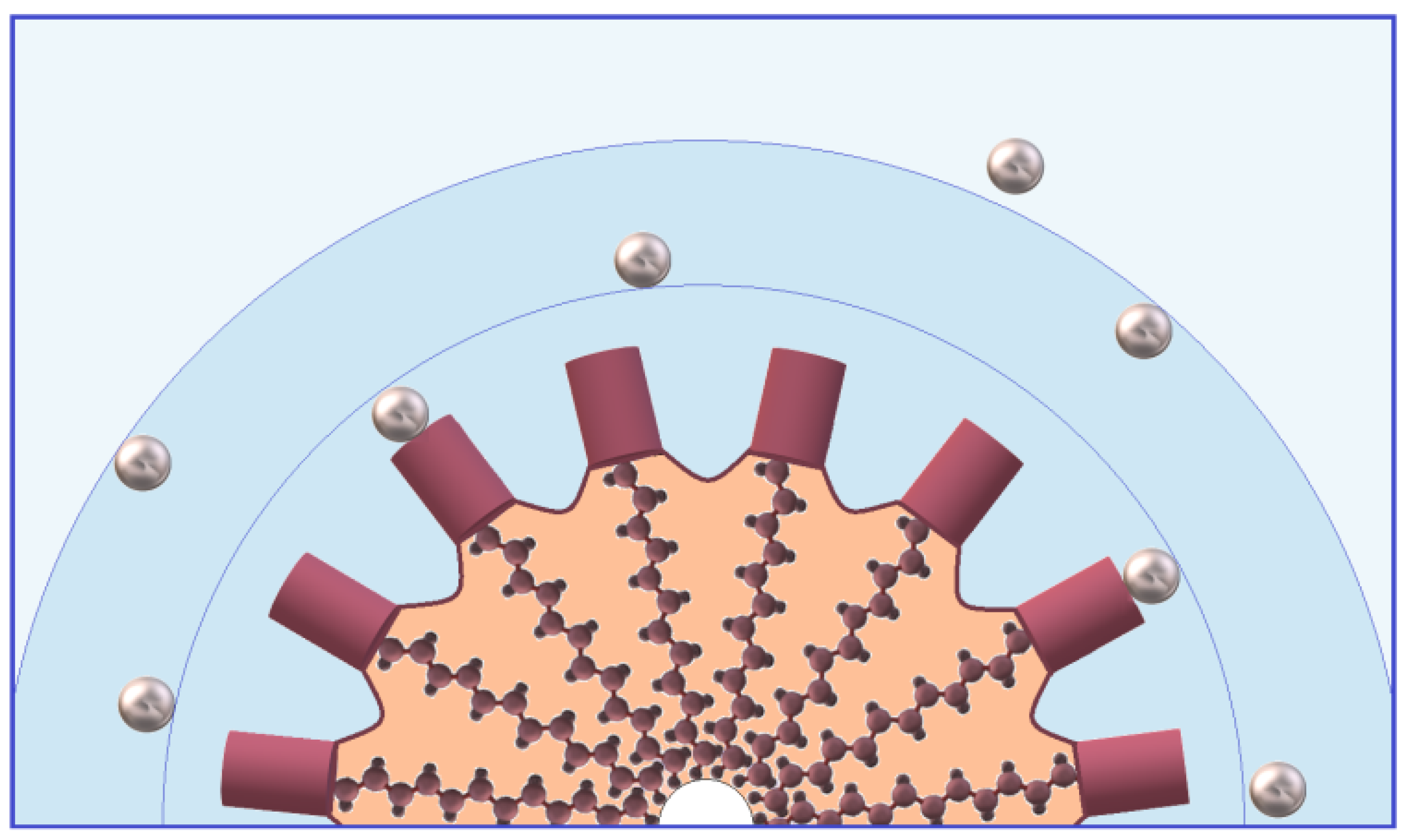
3.2. Fullerene-like Micelle
- (1)
- The traditional model is static in nature. The advanced model introduces the association–dissociation processes of head groups with times comparable to 10−10 s.
- (2)
- In the classical model, the adsorbed ions are allowed to move along the Helmholtz layer, participating in the processes of surface diffusion. In the advanced model, this process is also possible but most likely indirect. The above estimations of the distance between adjacent head groups indicate the existence of water-filled space between head groups. The discontinuity of contact ion pairs, even if located on the lateral side of the head group surface, will not lead to the creation of a similar pair on an adjacent head group due to the large distance and presence of water molecules. However, a similar process involving solvent-shared ion pairs may well take place.
- (3)
- The possible deflection of faces of the fullerene-like structure (Figure 3B) leads to the intricate shape of the hydrocarbon core surface and increased pseudophase volume and allows us to explain the penetration of water into the upper hydrocarbon layers.
3.3. Aggregation Number of Surfactant Ions in Micelle
3.4. Time Characteristics of Ionic Surfactant Solutions
3.5. Structure of the Micelle Hydrocarbon Core
3.6. Features of Structure and Properties of Surfactant Micelles
3.6.1. Penetration of Water Molecules up to Hydrocarbon Core
3.6.2. Gel-like Properties of Hydrocarbon Core
3.6.3. Surface Roughness of the Micelle Hydrocarbon Core
3.6.4. Increased Pseudophase Volume and Semi-Closed Hydrocarbon Surface Regions with Enhanced Hydrophobic Properties
3.6.5. Solubilization of Organic Compounds in Micellar Core
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romsted, L.S. (Ed.) Surfactants Science and Technology. Retrospects and Prospects; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxfordshire, UK, 2014. [Google Scholar]
- Bradburn, D.; Bittinger, J. (Eds.) Micelles: Structural Biochemistry, Formation and Functions & Usage; Nova Science Publishers: New York, NY, USA, 2013. [Google Scholar]
- Labuda, J.; Barek, J.; Gajdosechova, Z.; Goenaga-Infante, H.; Johnston, L.J.; Mester, Z.; Shtykov, S. Analytical chemistry of engineered nanomaterials: Part 1. Scope, regulation, legislation, and metrology (IUPAC Technical Report). Pure Appl. Chem. 2023, 95, 133–163. [Google Scholar] [CrossRef]
- Rusanov, A.I. The wonderful world of micelles. Colloid J. 2014, 76, 121–126. [Google Scholar] [CrossRef]
- Patist, A.; Jha, B.K.; Oh, S.G.; Shah, D.O. Importance of micellar relaxation time on detergent properties. J. Surfactants Deterg. 1999, 2, 317–324. [Google Scholar] [CrossRef]
- Oh, S.G.; Shah, D.O. Micellar lifetime: Its relevance to various technological processes. J. Dispers. Sci. Technol. 1994, 15, 297–316. [Google Scholar] [CrossRef]
- Ishchenko, A.A. (Ed.) Analytical Chemistry, Vol. 3: Instrumental Methods of Analysis, Part 2; FIZMATLIT: Moscow, Russia, 2020. (In Russian) [Google Scholar]
- Lloyd, S. Quantum coherence in biological systems. J. Phys. Conf. Ser. 2011, 302, 012037. [Google Scholar] [CrossRef]
- Engel, G.; Calhoun, T.; Read, E.; Ahn, T.-K.; Mančal, T.; Cheng, Y.-C.; Blankenship, R.E.; Fleming, G.R. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 2007, 446, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Collini, E.; Wong, C.Y.; Wilk, K.E.; Curmi, P.M.; Brumer, P.; Scholes, G.D. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 2010, 463, 644. [Google Scholar] [CrossRef]
- Ceriotti, M.; Fang, W.; Kusalik, P.G.; McKenzie, R.H.; Michaelides, A.; Morales, M.A.; Markland, T.E. Nuclear Quantum Effects in Water and Aqueous Systems: Experiment, Theory, and Current Challenges. Chem. Rev. 2016, 116, 7529–7550. [Google Scholar] [CrossRef] [PubMed]
- Mirgorod, Y.A.; Dolenko, T.A. Liquid polyamorphous transition and self-organization in aqueous solutions of ionic surfactants. Langmuir 2015, 31, 8535–8547. [Google Scholar] [CrossRef]
- Kundu, A.; Verma, P.K.; Cho, M. Water Structure and Dynamics in the Stern Layer of Micelles: Femtosecond Mid-Infrared Pump-Probe Spectroscopy Study. J. Phys. Chem. B 2019, 123, 5238–5245. [Google Scholar] [CrossRef] [PubMed]
- Buchachenko, A.L. Chemistry on the border of two centuries—Achievements and prospects. Russ. Chem. Rev. 1999, 68, 85–102. [Google Scholar] [CrossRef]
- Scholes, G.; Fleming, G.R.; Chen, L.X.; Aspuru-Guzik, A.; Buchleitner, A.; Coker, D.F.; Engel, G.; Van Grondelle, R.; Ishizaki, A.; Jonas, D.; et al. Using coherence to enhance function in chemical and biophysical systems. Nature 2017, 543, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G. Coherence from light harvesting to chemistry. J. Phys. Chem. Lett. 2018, 9, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G.D. Emergence of collective coherent states from strong-light coupling of disordered systems. J. Phys. Chem. A 2021, 125, 6739–6750. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Fein, Y.Y.; Geyer, P.; Zwick, P.; Kiałka, F.; Pedalino, S.; Mayor, M.; Gerlich, S.; Arndt, M. Quantum superposition of molecules beyond 25 kDa. Nat. Phys. 2019, 15, 1242–1245. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683–151705. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Zueva, O.S.; Rukhlov, V.S.; Zuev, Y.F. Morphology of ionic micelles as studied by numerical solution of Poisson equation. ACS Omega 2022, 7, 6174–6183. [Google Scholar] [CrossRef] [PubMed]
- Duplâtre, G.; Ferreira Marques, M.F.; da Gracüa Miguel, M. Size of Sodium Dodecyl Sulfate Micelles in Aqueous Solutions as Studied by Positron Annihilation Lifetime Spectroscopy. J. Phys. Chem. 1996, 100, 16608–16612. [Google Scholar] [CrossRef]
- Bockstahl, F.; Duplâtre, G. Quantitative determination of a sodium dodecyl sulfate micellar radius through positron annihilation lifetime spectroscopy. Phys. Chem. Chem. Phys. 1999, 1, 2767–2772. [Google Scholar] [CrossRef]
- Bockstahl, F.; Pachoud, E.; Duplâtre, G.; Billard, I. Size of sodium dodecyl sulphate micelles in aqueous NaCl solutions as studied by positron annihilation lifetime spectroscopy. Chem. Phys. 2000, 256, 307–313. [Google Scholar] [CrossRef]
- Hayter, J.B.; Penfold, J. Determination of micelle structure and charge by neutron small-angle scattering. Colloid Polym. Sci. 1983, 261, 1022–1030. [Google Scholar] [CrossRef]
- Hartland, G.V.; Grieser, F.; White, L.R. Surface potential measurements in pentanol–sodium dodecyl sulphate micelles. J. Chem. Soc. Faraday Trans. 1 1987, 83, 591–613. [Google Scholar] [CrossRef]
- Almgren, M.; Swarup, S. Size of sodium dodecyl sulfate micelles in the presence of additives i. alcohols and other polar compounds. J. Colloid Interface Sci. 1983, 91, 256–266. [Google Scholar] [CrossRef]
- Varela, A.S.; Sández, M.I.; Gil, A. The size of sodium dodecyl sulfate micelles in the presence of n-alcohols as determined by fluorescence quenching measurements. Colloid Polym. Sci. 1995, 273, 876–880. [Google Scholar] [CrossRef]
- Bastiat, G.; Grassl, B.; Khoukh, A.; Francois, J. Study of sodium dodecyl sulfate-poly(propylene oxide) methacrylate mixed micelles. Langmuir 2004, 20, 5759–5769. [Google Scholar] [CrossRef] [PubMed]
- Molero, M.; Andreu, R.; González, D.; Calvente, J.J.; López-Pérez, G. An isotropic model for micellar systems: Application to sodium dodecyl sulfate solutions. Langmuir 2001, 17, 314–322. [Google Scholar] [CrossRef]
- Ali, A.; Bhushan, V.; Malik, N.A.; Behera, K. Study of Mixed Micellar Aqueous Solutions of Sodium Dodecyl Sulfate and Amino Acids. Colloid J. 2013, 75, 357–365. [Google Scholar] [CrossRef]
- Mazer, N.A.; Benedek, G.B.; Carey, M.C. An investigation of the micellar phase of sodium dodecyl sulfate in aqueous sodium chloride solutions using quasielastic light scattering spectroscopy. J. Phys. Chem. 1976, 80, 1075–1085. [Google Scholar] [CrossRef]
- Corti, M.; Deglorgio, V. Quasielastic Light Scattering Study of Intermicellar Interactions in Aqueous Sodium Dodecyl Sulfate Solutions. J. Phys. Chem. 1981, 85, 711–717. [Google Scholar] [CrossRef]
- Colafemmina, G.; Fiorentino, D.; Ceglie, A.; Carretti, E.; Fratini, E.; Dei, L.; Baglioni, P.; Palazzo, G. Structure of SDS Micelles with Propylene Carbonate as Cosolvent: A PGSE-NMR and SAXS Study. J. Phys. Chem. B 2007, 111, 7184–7193. [Google Scholar] [CrossRef] [PubMed]
- Mirgorod, Y.; Chekadanov, A.; Dolenko, T. Structure of micelles of sodium dodecyl sulfate in water: An X-ray and dynamic light scattering study. Chem. J. Mold. 2019, 14, 107–119. [Google Scholar] [CrossRef]
- Dunstan, D.E.; White, L.R. An electrokinetic study of micellar solutions. J. Colloid Interface Sci. 1990, 134, 147–151. [Google Scholar] [CrossRef]
- Kumar, P.; Mittal, K.L. (Eds.) Handbook of Microemulsion Science and Technology; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2018. [Google Scholar]
- Gubaidullin, A.T.; Litvinov, I.A.; Samigullina, A.I.; Zueva, O.S.; Rukhlov, V.S.; Idiyatullin, B.Z.; Zuev, Y.F. Structure and dynamics of concentrated micellar solutions of sodium dodecyl sulfate. Russ. Chem. Bull. 2016, 65, 158–166. [Google Scholar] [CrossRef]
- Israelachvili, J. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Gruen, D.W.R. A model for the chains in amphiphilic aggregates. 1. Comparison with a molecular dynamics simulation of a bilayer. J. Phys. Chem. 1985, 89, 146–153. [Google Scholar] [CrossRef]
- Gruen, D.W.R. A model for the chains in amphiphilic aggregates. 2. Thermodynamic and experimental comparisons for aggregates of different shape and size. J. Phys. Chem. 1985, 89, 153–163. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Rajagopalan, R. (Eds.) Principles of Colloid and Surface Chemistry, 3rd ed.; Revised and Expanded; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Rusanov, A.I.; Shchekin, A.K. Micelle Formation in the Solutions of Surfactants. Lan: St. Peterburg, Russia, 2016. (In Russian) [Google Scholar]
- Stern, O. Zur Theorie der elektrolytischen Doppelschicht. Z. Electrochem. 1924, 30, 508–526. [Google Scholar] [CrossRef]
- Mittal, K.L.; Mukerjee, P. The Wide World of Micelles. In Micellization, Solubilization, and Microemulsions; Mittal, K.L., Ed.; Plenum Press: New York, NY, USA, 1977. [Google Scholar]
- Mukerjee, P. The nature of the association equilibria and hydrophobic bonding in aqueous solutions of association colloids. Adv. Colloid Interface Sci. 1967, 1, 242–275. [Google Scholar] [CrossRef]
- Mukerjee, P.; Cardinal, J.R.; Desai, N.R. The Nature of the Local Microenvironments in Aqueous Micellar Systems. In Micellization, Solubilization, and Microemulsions; Mittal, K.L., Ed.; Plenum Press: New York, NY, USA, 1977. [Google Scholar]
- Grahame, D.C. The Electrical Double Layer and the Theory of Electrocapillarity. Chem. Rev. 1947, 41, 441–501. [Google Scholar] [CrossRef] [PubMed]
- Bagotsky, V.S. Fundamentals of Electrochemistry; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Rusanov, A.I.; Shchekin, A.K.; Volkov, N.A. Diffusion in micellar systems: Theory and molecular modeling. Russ. Chem. Rev. 2017, 86, 567–588. [Google Scholar] [CrossRef]
- Barthel, J.; Hetzenauer, H.; Buchner, R. Dielectric relaxation of aqueous electrolyte solutions II. Ion-pair relaxation of 1:2, 2:1, and 2:2 electrolytes. Ber. Bunsenges. Phys. Chem. 1992, 96, 1424–1432. [Google Scholar] [CrossRef]
- Barthel, J.; Buchner, R.; Eberspächer, P.-N.; Münsterer, M.; Stauber, J.; Wurm, B. Dielectric relaxation spectroscopy of electrolyte solutions. Recent developments and prospects. J. Mol. Liq. 1998, 78, 83–109. [Google Scholar] [CrossRef]
- Akilan, C.; Hefter, G.; Rohman, N.; Buchner, R. Ion association and hydration in aqueous solutions of copper(II) sulfate from 5 to 65 C by dielectric spectroscopy. J. Phys. Chem. B 2006, 110, 14961–14970. [Google Scholar] [CrossRef]
- Marcus, Y.; Hefter, G. Ion-pairing. Chem. Rev. 2006, 106, 4585–4621. [Google Scholar] [CrossRef]
- Marcus, Y. Effect of ions on the structure of water: Structure making and breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef] [PubMed]
- Van der Vegt, F.A.; Haldrup, K.; Roke, S.; Zheng, J.; Lund, M.; Bakker, H.J. Water-mediated ion pairing: Occurrence and relevance. Chem. Rev. 2016, 116, 7626–7641. [Google Scholar] [CrossRef]
- Baar, C.; Buchner, R.; Kunz, W. Dielectric relaxation of cationic surfactants in aqueous solution. 2. Solute relaxation. J. Phys. Chem. B 2001, 105, 2914–2922. [Google Scholar] [CrossRef]
- Rohman, N.; Mohiuddin, T.; Khan, I. Dielectric Relaxation of Ion-pairs, Micelles and Hydration in Aqueous Hexyltrimethylammonium Bromide Solutions. Chem. Thermodyn. Therm. Anal. 2022, 8, 100098. [Google Scholar] [CrossRef]
- Van der Post, S.T.; Hunger, J.; Bonn, M.; Bakker, H.J. Observation of Water Separated Ion-Pairs between Cations and Phospholipid Headgroups. J. Phys. Chem. B 2014, 118, 4397–4403. [Google Scholar] [CrossRef] [PubMed]
- Iwahara, J.; Esadze, A.; Zandarashvili, L. Physicochemical Properties of Ion Pairs of Biological Macromolecules. Biomolecules 2015, 5, 2435–2463. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.E.; Gordon, L.G.M. Common problems with the double layer and ionic solutions. An introductory paper. J. Phys. Chem. 1969, 73, 3523–3527. [Google Scholar] [CrossRef]
- Friedrichsberg, D.A. Kurs Kolloidnoy Khimii [Course of Colloid Chemistry]; Lan: Saint-Petersburg, Russia, 2021. (In Russian) [Google Scholar]
- Moreira, L.; Firoozabadi, A. Molecular thermodynamic modeling of specific ion effects on micellization of ionic surfactants. Langmuir 2010, 26, 15177–15191. [Google Scholar] [CrossRef] [PubMed]
- Mchedlov-Petrosyan, N.O. Differentiation of Organic acid Strength in True and Organized Solutions; Khar’kovsk. Nats. Univ.: Kharkiv, Ukraine, 2004. [Google Scholar]
- Pimonov, V.V.; Konevtsova, O.V.; Rochal, S.B. Anomalous small viral shells and simplest polyhedral with icosahedral symmetry: The rhombic triacontahedron case. Acta Cryst. A 2019, 75, 135–141. [Google Scholar] [CrossRef]
- Volkov, N.A.; Tuzov, N.V.; Shchekin, A.K. Molecular dynamics study of salt influence on transport and structural properties of SDS micellar solutions. Fluid Phase Equilib. 2016, 424, 114–121. [Google Scholar] [CrossRef]
- Volkov, N.A.; Tuzov, N.V.; Shchekin, A.K.; Lebedeva, T.S.; Kazantseva, M.A. Molecular modeling of ionic aggregates at several concentrations of SDS in aqueous solution. J. Mol. Liq. 2017, 236, 414–421. [Google Scholar] [CrossRef]
- Farafonov, V.S.; Lebed, A.V.; Mchedlov-Petrossyan, N.O. Character of localization and microenvironment of solvatochromic reichardt’s betaine dye in sodium n-dodecyl sulfate and cetyltrimethylammonium bromide micelles: Molecular dynamics simulation study. Langmuir 2017, 33, 8342–8352. [Google Scholar] [CrossRef] [PubMed]
- Mchedlov-Petrossyan, N.O.; Vodolazkaya, N.A.; Kamneva, N.N. Acid-Base Equilibrium in Aqueous Micellar Solutions of Surfactants. In Micelles: Structural Biochemistry, Formation and Functions & Usage; Bradburn, D., Bittinger, T., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2013. [Google Scholar]
- Quina, F.H. Dynamics and prototropic reactivity of electronically excited states in simple surfactant aggregates. Curr. Opin. Colloid Interface Sci. 2013, 18, 35–39. [Google Scholar] [CrossRef]
- Zaev, E.E.; Mel’nikov, G.V.; Shtykov, S.N.; Shtykova, L.S. Adsorption of aliphatic alcohols on micelles of sodium dodecylsulfate as studied by fluorescence quenching. Russ. J. Phys. Chem. A 2002, 76, 809–811. [Google Scholar]
- Fowkes, F.M. Ideal two-dimensional solutions. II. A new isotherm for soluble and “gaseous” monolayers. J. Phys. Chem. 1962, 66, 385–389. [Google Scholar] [CrossRef]
- Svens, B.; Rosenholm, B. An investigation of the size and structure of the micelles in sodium octanoate solutions by small-angle X-ray scattering. J. Colloid Interface Sci. 1973, 44, 495–504. [Google Scholar] [CrossRef]
- Muller, N.; Birkhahn, R.H. Investigation of micelle structure by fluorine magnetic resonance. I. Sodium 10,10,10-trifluorocaprate and related compounds. J. Phys. Chem. 1967, 71, 957. [Google Scholar] [CrossRef]
- Glansdorff, P.; Prigogine, I. Thermodynamic Theory of Structure, Stability and Fluctuations; Wiley-Interscience: London, UK, 1971. [Google Scholar]
- Prigogine, I.; Nicolis, G. Self-Organization in Non-Equilibrium Systems; Wiley-Interscience: New York, NY, USA, 1977. [Google Scholar]
- Prigogine, I.; Stengers, I. Order Out of Chaos: Man’s New Dialogue with Nature; Bantam Books: New York, NY, USA, 1984. [Google Scholar]
- Toker, D.; Sommer, F.T.; D’Esposito, M. A simple method for detecting chaos in nature. Commun. Biol. 2020, 3, 11. [Google Scholar] [CrossRef]
- Glukhova, O.E. Printsipy Klassifikatsii Uglerodnykh Nanoklasterov s Pozitsii Primeneniya ikh v Elektronnykh Nanoustroystvakh [Principles of Classification of Carbon Nanoclusters from the Standpoint of Their Application in Electronic Nanodevices]; Saratov State University: Saratov, Russia, 2015. (In Russian) [Google Scholar]
- Missel, P.J.; Mazer, N.A.; Benedek, G.B.; Young, C.Y.; Carey, M.C. Thermodynamic analysis of the growth of sodium dodecyl sulfate micelles. J. Phys. Chem. 1980, 84, 1044–1057. [Google Scholar] [CrossRef]
- Aswal, V.K.; Goyal, P.S. Counterions in the growth of ionic micelles in aqueous electrolyte solutions: A small-angle neutron scattering study. Phys. Rev. E 2000, 61, 2947–2953. [Google Scholar] [CrossRef]
- Kwiatkowski, A.L.; Molchanov, V.S.; Philippova, O.E. Polymer-like Wormlike Micelles of Ionic Surfactants: Structure and Rheological Properties. Polym. Sci. Series A 2019, 61, 215–225. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevsky, P.A.; Stanimirova, R.D.; Stoyanov, S.D.; Cook, J.L.; Stott, I.P. Analytical modeling of micelle growth. 4. Molecular thermodynamics of wormlike micelles from ionic surfactants: Theory vs. experiment. J. Colloid Interface Sci. 2021, 584, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, T.; Fujii, S.; Miura, M. Acoustic study on the kinetics for the dissociation-recombination reaction between micelle and counterion in sodium dodecyl sulfate solution. J. Colloid Interface Sci. 1967, 23, 352–357. [Google Scholar] [CrossRef]
- Yasunaga, T.; Fujii, S.; Miura, M. Acoustic study of the kinetics of the dissociation-recombination reaction between micelle and counterion in solutions of sodium salts of fatty acids—Sodium caprylate and sodium caprate. J. Colloid Interface Sci. 1969, 30, 399–405. [Google Scholar] [CrossRef]
- Rassing, J.; Sams, P.J.; Wyn-Jones, E. Kinetics of micellization from ultrasonic relaxation studies. J. Chem. Soc. Faraday Trans. II 1974, 70, 1247–1258. [Google Scholar] [CrossRef]
- Aniansson, E.A.G.; Wall, S.N. Kinetics of step-wise micelle association. J. Phys. Chem. 1974, 78, 1024–1030. [Google Scholar] [CrossRef]
- Lang, J.; Tondre, C.; Zana, R.; Bauer, R.; Hoffmann, H.; Ulbricht, W. Chemical relaxation studies of micellar equilibriums. J. Phys. Chem. 1975, 79, 276–283. [Google Scholar] [CrossRef]
- Aniansson, E.A.G.; Wall, S.N.; Almgren, M.; Hoffmann, H.; Kielmann, I.; Ulbricht, W. Theory of the kinetics of micellar equilibria and quantitative interpretation of chemical relaxation studies of micellar solutions of ionic surfactants. J. Phys. Chem. 1976, 80, 905–922. [Google Scholar] [CrossRef]
- Kahlweit, M.; Teubner, M. On the kinetics of micellization in aqueous solutions. Adv. Colloid Interface Sci. 1980, 13, 1–64. [Google Scholar] [CrossRef]
- Mysona, J.A.; McCormic, A.V.; Morse, D.C. Nonlinear dynamics in micellar surfactant solutions. I. Kinetics. Phys. Rev. E 2022, 105, 034602. [Google Scholar] [CrossRef]
- Mysona, J.A.; McCormic, A.V.; Morse, D.C. Nonlinear dynamics in micellar surfactant solutions. II. Diffusion. Phys. Rev. E 2022, 105, 034603. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, A.I.; Shchekin, A.K.; Kuni, F.M. Thermodynamic and kinetic theory of ionic micellar systems: 1. Work of aggregation. Colloid J. 2009, 71, 816–825. [Google Scholar] [CrossRef]
- Rusanov, A.I.; Shchekin, A.K.; Kuni, F.M. Thermodynamic and kinetic theory of ionic micellar systems: 2. Statistical-thermodynamic relations. Colloid J. 2009, 71, 826–834. [Google Scholar] [CrossRef]
- Shchekin, A.K.; Kshevetskiy, M.S.; Pelevina, O.S. Micellization kinetics with allowance for fussion and fission of spherical and cylindrical micelles: 1. Set of nonlinear equations describing slow relaxation. Colloid J. 2011, 73, 406–417. [Google Scholar] [CrossRef]
- Zueva, O.S.; Kusova, A.M.; Makarova, A.O.; Iskhakova, A.; Salnikov, V.V.; Zuev, Y.F. Reciprocal effects of multi-walled carbon nanotubes and oppositely charged surfactants in bulk water and at interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125296. [Google Scholar] [CrossRef]
- Mortensen, K. Structural studies of aqueous solutions of PEO—PPO—PEO triblock copolymers, their micellar aggregates and mesophases; a small-angle neutron scattering study. J. Phys. Condens. Matter 1996, 8, 103–124. [Google Scholar] [CrossRef]
- Zueva, O.S.; Makarova, A.O.; Zueva, E.R.; Kurbanov, R.K.; Salnikov, V.V.; Turanov, A.N.; Zuev, Y.F. Industrial block copolymer surfactants: Diversity of associative forms and interaction with carbon nanomaterial. J. Mol. Liq. 2022, 359, 119267. [Google Scholar] [CrossRef]
- Zuev, Y.F.; Lunev, I.V.; Turanov, A.N.; Zueva, O.S. Micellization of sodium dodecyl sulfate in the vicinity of Krafft point: An NMR and dielectric spectroscopy study. Russ. Chem. Bull. 2024, 73, 529–535. [Google Scholar] [CrossRef]
- Gruen, D.W.R. The packing of amphiphile chain in a small spherical micelle. J. Colloid Interface Sci. 1981, 84, 281–283. [Google Scholar] [CrossRef]
- Almgren, M.; Swarup, S. Size of sodium dodecyl sulfate micelles in the presence of additives. 2. Aromatic and saturated hydrocarbons. J. Phys. Chem. 1982, 86, 4212–4216. [Google Scholar] [CrossRef]
- Gruen, D.W.R. The standard picture of ionic micelles. Prog. Colloid Polym. Sci. 1985, 70, 6–16. [Google Scholar] [CrossRef]
- Nagarajan, R. One Hundred Years of Micelles: Evolution of the Theory of Micellization. In Surfactant Science and Technology; Romsted, L.S., Ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Dill, K.A.; Flory, P.J. Molecular organization in micelles and vesicles. Proc. Natl. Acad. Sci. USA 1981, 78, 676–680. [Google Scholar] [CrossRef] [PubMed]
- WebElements: The Periodic Table of the Elements. Available online: https://www.webelements.com/ (accessed on 30 January 2025).
- Wikipedia: Kinetic Diameter. Available online: https://en.wikipedia.org/wiki/Kinetic_diameter (accessed on 30 January 2025).
- Morosin, B.; Assink, R.A.; Dunn, R.G.; Massis, T.M.; Schirber, J.E.; Kwei, G.H. Methane-intercalated C60: Preparation, orientational ordering, and structure. Phys. Rev. B 1997, 56, 13611. [Google Scholar] [CrossRef]
- Cholakova, D.; Denkov, N. Rotator phases in alkane systems: In bulk, surface layers and micro/nano-confinements. Adv. Colloid Interface Sci. 2019, 269, 7–42. [Google Scholar] [CrossRef] [PubMed]
- Cabane, B.; Duplessix, R.; Zemb, T. High resolution neutron scattering on ionic surfactant micelles: SDS in water. J. Phys. 1985, 46, 2161–2178. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Berr, S.S. Solvent isotope effects on alkyltrimethylammonium bromide micelles as a function of alkyl chain length. J. Phys. Chem. 1987, 91, 4760–4765. [Google Scholar] [CrossRef]
- Wikipedia: Truncated Icosahedron. Available online: https://en.wikipedia.org/wiki/Truncated_icosahedron (accessed on 30 January 2025).
- Menger, F.M. On the structure of micelles. Acc. Chem. Res. 1979, 12, 111–117. [Google Scholar] [CrossRef]
- Szleifer, I.; Ben-Shaul, A.; Gelbart, W.M. Chain statistics in micelles and bilayers: Effects of surface roughness and internal energy. J. Chem. Phys. 1986, 85, 5345–5358. [Google Scholar] [CrossRef]
- Dinh, T.D.; Phan, M.N.; Nguyen, D.T.; Le, T.M.D.; Nadda, A.K.; Srivastav, A.L.; Pham, T.N.M.; Pham, T.D. Removal of beta-lactam antibiotic in water environment by adsorption technique using cationic surfactant functionalized nanosilica rice husk. Environ. Res. 2022, 210, 112943. [Google Scholar] [CrossRef]
- Todorov, P.D.; Kralchevsky, P.A.; Denkov, N.D.; Broze, G.; Mehreteab, A. Kinetics of Solubilization of n-Decane and Benzene by Micellar Solutions of Sodium Dodecyl Sulfate. J. Colloid Interface Sci. 2002, 245, 371–382. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zueva, O.S.; Kazantseva, M.A.; Zuev, Y.F. Nanosized Being of Ionic Surfactant Micelles: An Advanced View on Micellization Process. Colloids Interfaces 2025, 9, 15. https://doi.org/10.3390/colloids9020015
Zueva OS, Kazantseva MA, Zuev YF. Nanosized Being of Ionic Surfactant Micelles: An Advanced View on Micellization Process. Colloids and Interfaces. 2025; 9(2):15. https://doi.org/10.3390/colloids9020015
Chicago/Turabian StyleZueva, Olga S., Mariia A. Kazantseva, and Yuriy F. Zuev. 2025. "Nanosized Being of Ionic Surfactant Micelles: An Advanced View on Micellization Process" Colloids and Interfaces 9, no. 2: 15. https://doi.org/10.3390/colloids9020015
APA StyleZueva, O. S., Kazantseva, M. A., & Zuev, Y. F. (2025). Nanosized Being of Ionic Surfactant Micelles: An Advanced View on Micellization Process. Colloids and Interfaces, 9(2), 15. https://doi.org/10.3390/colloids9020015