Could New Generations of Sensors Reshape the Management of Parkinson’s Disease?
Abstract
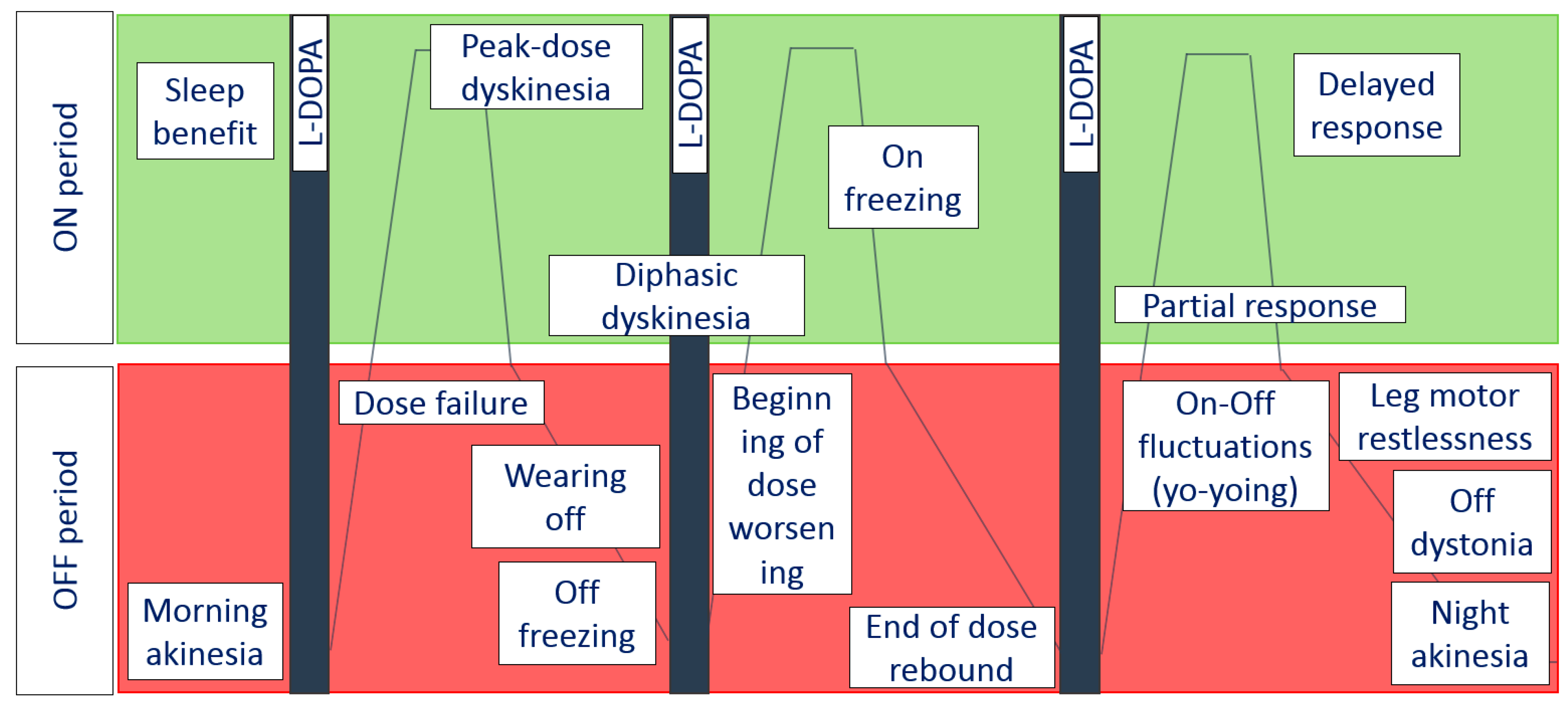
:1. A Long Day for a Patient with PD
2. Pathogenesis and Risk Factors of Motor Complications
- Short-duration response is characterized by motor improvement and coincides with the elevation of plasma L-dopa after drug consumption. It lasts from minutes to hours. Peak motor response also happens due to short-duration response.
- Long-duration response keeps the positive effect of L-dopa beyond the normal half-life of the individual dose: this kind of response usually dominates in early PD. During this period, patients are able to control motor symptoms using two or three daily doses of L-dopa [18].
3. Strategies of the Prevention and Management of Motor Complications
4. The World of Sensors
5. mHealth Applications for PD Monitoring
6. What Does an Ideal Sensor Look Like?
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Müller, T.; Russ, H. Levodopa, motor fluctuations and dyskinesia in Parkinson’s disease. Expert Opin. Pharmacother. 2006, 7, 1715–1730. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Violante, M.; Ospina-García, N.; Dávila-Avila, N.M.; Cruz-Fino, D.; Cruz-Landero, A.; Cervantes-Arriaga, A. Motor and non-motor wearing-off and its impact in the quality of life of patients with Parkinson’s disease. Arquivos Neuro-Psiquiatr. 2018, 76, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Bjornestad, A.; Forsaa, E.B.; Pedersen, K.F.; Tysnes, O.B.; Larsen, J.P.; Alves, G. Risk and course of motor complications in a population-based incident Parkinson’s disease cohort. Parkinsonism Relat. Disord. 2016, 22, 48–53. [Google Scholar] [CrossRef]
- Kim, H.J.; Mason, S.; Foltynie, T.; Winder-Rhodes, S.; Barker, R.A.; Williams-Gray, C.H. Motor complications in Parkinson’s disease: 13-year follow-up of the CamPaIGN cohort. Mov. Disord. 2020, 35, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Quinn, N.P. Classification of fluctuations in patients with Parkinson’s disease. Neurology 1998, 51 (Suppl. 2), S25–S29. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Sringean, J.; Trenkwalder, C. Mastering nocturnal jigsaws in Parkinson’s disease: A dusk-to-dawn review of night-time symptoms. J. Neural Transm. 2020, 127, 763–777. [Google Scholar] [CrossRef]
- Stocchi, F.; Coletti, C.; Bonassi, S.; Radicati, F.G.; Vacca, L. Early-morning OFF and levodopa dose failures in patients with Parkinson’s disease attending a routine clinical appointment using Time-to-ON Questionnaire. Eur. J. Neurol. 2019, 26, 821–826. [Google Scholar] [CrossRef]
- van Gilst, M.M.; Louter, M.; Baumann, C.R.; Bloem, B.R.; Overeem, S. Sleep benefit in Parkinson’s disease: Time to revive an enigma? J. Parkinsons Dis. 2012, 2, 167–170. [Google Scholar] [CrossRef]
- van Gilst, M.M.; Bloem, B.R.; Overeem, S. Sleep benefit” in Parkinson’s disease: A systematic review. Parkinsonism Relat. Disord. 2013, 19, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Högl, B.E.; Gómez-Arévalo, G.; García, S.; Scipioni, O.; Rubio, M.; Blanco, M.; Gershanik, O.S. A clinical, pharmacologic, and polysomnographic study of sleep benefit in Parkinson’s disease. Neurology 1998, 50, 1332–1339. [Google Scholar] [CrossRef]
- Zhong, R.; Chen, Q.; Zhang, X.; Zhang, X.; Lin, W. Correction: The related factors of sleep benefit in Parkinson’s disease: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0216524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, S.H.; Lang, A.E. Levodopa-related motor complications--phenomenology. Mov Disord. 2008, 23, S509–S514. [Google Scholar] [CrossRef] [PubMed]
- Kuoppamäki, M.; Al-Barghouthy, G.; Jackson, M.L.; Zeng, B.Y.; Quinn, N.; Jenner, P. Beginning-of-dose and rebound worsening in MPTP-treated common marmosets treated with levodopa. Mov. Disord. 2002, 17, 1312–1317. [Google Scholar] [CrossRef]
- Jankovic, J. Motor fluctuations and dyskinesias in Parkinson’s disease: Clinical manifestations. Mov. Disord. 2005, 20 (Suppl. 11), S11–S16. [Google Scholar] [CrossRef]
- Cucca, A.; Biagioni, M.C.; Fleisher, J.E.; Agarwal, S.; Son, A.; Kumar, P.; Brys, M.; Di Rocco, A. Freezing of gait in Parkinson’s disease: From pathophysiology to emerging therapies. Neurodegener. Dis. Manag. 2016, 6, 431–446. [Google Scholar] [CrossRef]
- Ginis, P.; Nackaerts, E.; Nieuwboer, A.; Heremans, E. Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann. Phys. Rehabil. Med. 2018, 61, 407–413. [Google Scholar] [CrossRef]
- Donaldson, I.; Marsden, C.D.; Schneider, S.A.; Bhatia, K. Parkinson’s disease. In Marsden’s Book of Movement Disorders; Oxford University Press: Oxford, UK, 2012; pp. 159–370. [Google Scholar]
- Burn, D. Parkinson’s Disease: Advanced Disease, Motor Complications, and Management. In Oxford Textbook of Movement Disorders; Oxford University Press: Oxford, UK, 2013; pp. 83–111. [Google Scholar]
- Nutt, J.G. Levodopa-induced dyskinesia: Review, observations, and speculations. Neurology 1990, 40, 340–345. [Google Scholar] [CrossRef]
- Luquin, M.R.; Scipioni, O.; Vaamonde, J.; Gershanik, O.; Obeso, J.A. Levodopa-induced dyskinesias in Parkinson’s disease: Clinical and pharmacological classification. Mov. Disord. 1992, 7, 117–124. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Mekawichai, P.; Jitkritsadakul, O.; Panyakaew, P.; Kaewwilai, L.; Boonrod, N.; Petchrutchatachart, S.; Jagota, P.; Boonpeng, K.; Singmaneesakulchai, S.; et al. Nocturnal journey of body and mind in Parkinson’s disease: The manifestations, risk factors and their relationship to daytime symptoms. Evidence from the NIGHT-PD study. J. Neural Transm. 2014, 121 (Suppl. 1), S59–S68. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Trenkwalder, C. Getting a good night sleep? The importance of recognizing and treating nocturnal hypokinesia in Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 50, 10–18. [Google Scholar] [CrossRef]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef]
- Moccia, M.; Erro, R.; Picillo, M.; Santangelo, G.; Spina, E.; Allocca, R.; Longo, K.; Amboni, M.; Palladino, R.; Assante, R.; et al. A Four-Year Longitudinal Study on Restless Legs Syndrome in Parkinson Disease. Sleep 2016, 39, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Gjerstad, M.D.; Tysnes, O.B.; Larsen, J.P. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology 2011, 77, 1941–1946. [Google Scholar] [CrossRef]
- Matsubara, T.; Suzuki, K.; Fujita, H.; Watanabe, Y.; Sakuramoto, H.; Matsubara, M.; Hirata, K. Restless legs syndrome, leg motor restlessness and their variants in patients with Parkinson’s disease and related disorders. J. Neurol. Sci. 2018, 393, 51–57. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, M.; Miyamoto, T.; Hirata, K. Restless Legs Syndrome and Leg Motor Restlessness in Parkinson’s Disease. Parkinsons Dis. 2015, 2015, 490938. [Google Scholar] [CrossRef]
- Brun, L.; Lefaucheur, R.; Fetter, D.; Derrey, S.; Borden, A.; Wallon, D.; Bourre, B.; Maltête, D. Non-motor fluctuations in Parkinson’s disease: Prevalence, characteristics and management in a large cohort of parkinsonian outpatients. Clin. Neurol. Neurosurg. 2014, 127, 93–96. [Google Scholar] [CrossRef]
- Seki, M.; Takahashi, K.; Uematsu, D.; Mihara, B.; Morita, Y.; Isozumi, K.; Ohta, K.; Muramatsu, K.; Shirai, T.; Nogawa, S.; et al. Clinical features and varieties of non-motor fluctuations in Parkinson’s disease: A Japanese multicenter study. Parkinsonism Relat. Disord. 2013, 19, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.E.; Lang, A.E. The spectrum of levodopa-related fluctuations in Parkinson’s disease. Neurology 1993, 43, 1459–1464. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Levin, O.S.; Ivanov, A.K.; Shindriaeva, N.N. Actual problems of inpatient psychiatric care in Russia. Zhurnal Nevrologii i Psikhiatrii imeni SS Korsakova 2011, 111, 38–42. [Google Scholar]
- Vázquez, A.; Jiménez-Jiménez, F.J.; García-Ruiz, P.; García-Urra, D. “Panic attacks” in Parkinson’s disease. A long-term complication of levodopa therapy. Acta Neurol. Scand. 1993, 87, 14–18. [Google Scholar] [CrossRef]
- Witjas, T.; Kaphan, E.; Azulay, J.P.; Blin, O.; Ceccaldi, M.; Pouget, J.; Poncet, M.; Chérif, A.A. Nonmotor fluctuations in Parkinson’s disease: Frequent and disabling. Neurology 2002, 59, 408–413. [Google Scholar] [CrossRef]
- Quinn, N.P.; Lang, A.E.; Koller, W.C.; Marsden, C.D. Painful Parkinson’s disease. Lancet 1986, 327, 1366–1369. [Google Scholar] [CrossRef]
- Factor, S.A.; Weiner, W.J. Parkinson’s Disease: Diagnosis & Clinical Management, 2nd ed.; Demos Medical Publishing: New York, NY, USA, 2008. [Google Scholar]
- Storch, A.; Schneider, C.B.; Wolz, M.; Stürwald, Y.; Nebe, A.; Odin, P.; Mahler, A.; Fuchs, G.; Jost, W.H.; Chaudhuri, K.R.; et al. Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology 2013, 80, 800–809. [Google Scholar] [CrossRef]
- Storch, A.; Schneider, C.B.; Klingelhöfer, L.; Odin, P.; Fuchs, G.; Jost, W.H.; Martinez-Martin, P.; Koch, R.; Reichmann, H.; Chaudhuri, K.R.; et al. Quantitative assessment of non-motor fluctuations in Parkinson’s disease using the Non-Motor Symptoms Scale (NMSS). J. Neural. Transm. 2015, 122, 1673–1684. [Google Scholar] [CrossRef]
- Funkiewiez, A.; Ardouin, C.; Caputo, E.; Krack, P.; Fraix, V.; Klinger, H.; Chabardes, S.; Foote, K.; Benabid, A.L.; Pollak, P. Long-term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 834–839. [Google Scholar] [CrossRef] [Green Version]
- Nissenbaum, H.; Quinn, N.P.; Brown, R.G.; Toone, B.; Gotham, A.M.; Marsden, C.D. Mood swings associated with the ‘on-off’ phenomenon in Parkinson’s disease. Psychol. Med. 1987, 17, 899–904. [Google Scholar] [CrossRef]
- Menza, M.A.; Sage, J.; Marshall, E.; Cody, R.; Duvoisin, R. Mood changes and “on-off” phenomena in Parkinson’s disease. Mov. Disord. 1990, 5, 148–151. [Google Scholar] [CrossRef]
- Stacy, M.A.; Murck, H.; Kroenke, K. Responsiveness of motor and nonmotor symptoms of Parkinson disease to dopaminergic therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 57–61. [Google Scholar] [CrossRef]
- Cools, R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev. 2006, 30, 1–23. [Google Scholar] [CrossRef]
- Kulisevsky, J.; Avila, A.; Barbanoj, M.; Antonijoan, R.; Berthier, M.L.; Gironell, A. Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain 1996, 119, 2121–2132. [Google Scholar] [CrossRef] [Green Version]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Raudino, F. Non motor off in Parkinson’s disease. Acta Neurol. Scand. 2001, 104, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Sage, J.I. Fluctuations of Nonmotor symptoms. In Parkinson’s Disease, Diagnosis and Clinical Management; Demos Medical Publishing: New York, NY, USA, 2002; Volume 41, pp. 455–463. [Google Scholar]
- Goetz, C.G.; Tanner, C.M.; Levy, M.; Wilson, R.S.; Garron, D.C. Pain in Parkinson’s disease. Mov. Disord. 1986, 1, 45–49. [Google Scholar] [CrossRef]
- Barbeau, A. The clinical physiology of side effects in long-term L-DOPA therapy. Adv. Neurol. 1974, 5, 347–365. [Google Scholar] [PubMed]
- Sage, J.I.; Mark, M.H. Drenching sweats as an off phenomenon in Parkinson’s disease: Treatment and relation to plasma levodopa profile. Ann. Neurol. 1995, 37, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Baratti, M.; Calzetti, S. Fluctuation of arterial blood pressure during end-of-dose akinesia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1241–1243. [Google Scholar] [CrossRef] [Green Version]
- Fahn, S.; Jankovic, J.; Hallett, M. Nonmotor problems in Parkinson disease. In Principles and Practice of Movement Disorders; Elsevier: Amsterdam, The Netherlands, 2011; pp. 183–196. [Google Scholar]
- Fox, S.H.; Lang, A.E. Motor and non-motor fluctuations. Handbook Clin. Neurol. 2007, 84, 157–184. [Google Scholar] [CrossRef]
- Milazzo, V.; Di Stefano, C.; Vallelonga, F.; Sobrero, G.; Zibetti, M.; Romagnolo, A.; Merola, A.; Milan, A.; Espay, A.J.; Lopiano, L.; et al. Reverse blood pressure dipping as marker of dysautonomia in Parkinson disease. Parkinsonism Relat. Disord. 2018, 56, 82–87. [Google Scholar] [CrossRef]
- Vallelonga, F.; Di Stefano, C.; Merola, A.; Milan, A.; Espay, A.J.; Lopiano, L.; Veglio, F.; Maule, S. Blood pressure circadian rhythm alterations in alpha-synucleinopathies. J. Neurol. 2019, 266, 1141–1152. [Google Scholar] [CrossRef]
- Hineno, T.; Mizobuchi, M.; Nishimatsu, O.; Horiguchi, J.; Kakimoto, Y. Day-night variation of urine volume in Parkinson’s disease. Jpn. J. Psychiatry Neurol. 1994, 48, 583–587. [Google Scholar] [CrossRef]
- Freitas, M.E.; Hess, C.W.; Fox, S.H. Motor Complications of Dopaminergic Medications in Parkinson’s Disease. Semin. Neurol. 2017, 37, 147–157. [Google Scholar] [CrossRef]
- Pahwa, R.; Lyons, K.E. Levodopa-related wearing-off in Parkinson’s disease: Identification and management. Curr. Med. Res. Opin. 2009, 25, 841–849. [Google Scholar] [CrossRef]
- Yoritaka, A.; Shimo, Y.; Takanashi, M.; Fukae, J.; Hatano, T.; Nakahara, T.; Miyamato, N.; Urabe, T.; Mori, H.; Hattori, N. Motor and non-motor symptoms of 1453 patients with Parkinson’s disease: Prevalence and risks. Parkinsonism Relat. Disord. 2013, 19, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenganatt, M.A.; Jankovic, J. Parkinson disease subtypes. JAMA Neurol. 2014, 71, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Virmani, T.; Tazan, S.; Mazzoni, P.; Ford, B.; Greene, P.E. Motor fluctuations due to interaction between dietary protein and levodopa in Parkinson’s disease. J. Clin. Mov. Disord. 2016, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Contin, M.; Martinelli, P. Pharmacokinetics of levodopa. J. Neurol. 2010, 257 (Suppl. 2), S253–S261. [Google Scholar] [CrossRef]
- Kostic, V.; Przedborski, S.; Flaster, E.; Sternic, N. Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson’s disease. Neurology 1991, 41, 202–205. [Google Scholar] [CrossRef]
- Warren Olanow, C.; Kieburtz, K.; Rascol, O.; Poewe, W.; Schapira, A.H.; Emre, M.; Nissinen, H.; Leinonen, M.; Stocchi, F.; Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease (STRIDE-PD) Investigators. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov. Disord. 2013, 28, 1064–1071. [Google Scholar] [CrossRef]
- Colombo, D.; Abbruzzese, G.; Antonini, A.; Barone, P.; Bellia, G.; Franconi, F.; Simoni, L.; Attar, M.; Zagni, E.; Haggiag, S.; et al. The “gender factor” in wearing-off among patients with Parkinson’s disease: A post hoc analysis of DEEP study. Sci. World J. 2015, 2015, 787451. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, T.; Nagayama, H.; Ota, T.; Nishiyama, Y.; Mishina, M.; Ueda, M. Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin. Neuropharmacol. 2014, 37, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Dekker, M.C.; Bonifati, V.; van Duijn, C.M. Parkinson’s disease: Piecing together a genetic jigsaw. Brain 2003, 126, 1722–1733. [Google Scholar] [CrossRef] [Green Version]
- Oliveri, R.L.; Annesi, G.; Zappia, M.; Civitelli, D.; Montesanti, R.; Branca, D.; Nicoletti, G.; Spadafora, P.; Pasqua, A.A.; Cittadella, R.; et al. Dopamine D2 receptor gene polymorphism and the risk of levodopa-induced dyskinesias in PD. Neurology 1999, 53, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, D.L.; Davies, A.D.; Playfer, J.R.; Turnbull, C.J. Circadian rest-activity rhythm is altered in Parkinson’s disease patients with hallucinations. Mov. Disord. 2008, 23, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, U.; Del Dotto, P.; Lucetti, C.; Petrozzi, L.; Bernardini, S.; Gambaccini, G.; Rossi, G.; Piccini, P. Diurnal motor variations to repeated doses of levodopa in Parkinson’s disease. Clin. Neuropharmacol. 2000, 23, 28–33. [Google Scholar] [CrossRef]
- Nutt, J.G. Motor fluctuations and dyskinesia in Parkinson’s disease. Parkinsonism Relat. Disord. 2001, 8, 101–108. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Barton, B.; de Bie, R.; Seppi, K.; Coelho, M.; Sampaio, C.; Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef]
- Erb, M.K.; Karlin, D.R.; Ho, B.K.; Thomas, K.C.; Parisi, F.; Vergara-Diaz, G.P.; Daneault, J.F.; Wacnik, P.W.; Zhang, H.; Kangarloo, T.; et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. NPJ Digit. Med. 2020, 3, 6. [Google Scholar] [CrossRef]
- Albani, G.; Ferraris, C.; Nerino, R.; Chimienti, A.; Pettiti, G.; Parisi, F.; Ferrari, G.; Cau, N.; Cimolin, V.; Azzaro, C.; et al. An Integrated Multi-Sensor Approach for the Remote Monitoring of Parkinson’s Disease. Sensors 2019, 19, 4764. [Google Scholar] [CrossRef] [Green Version]
- Rovini, E.; Maremmani, C.; Cavallo, F. Automated Systems Based on Wearable Sensors for the Management of Parkinson’s Disease at Home: A Systematic Review. Telemed. J. E Health 2019, 25, 167–183. [Google Scholar] [CrossRef]
- Santiago, A.; Langston, J.W.; Gandhy, R.; Dhall, R.; Brillman, S.; Rees, L.; Barlow, C. Qualitative Evaluation of the Personal KinetiGraphTM Movement Recording System in a Parkinson’s Clinic. J. Parkinsons Dis. 2019, 9, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ní Scanaill, C.; Carew, S.; Barralon, P.; Noury, N.; Lyons, D.; Lyons, G.M. A review of approaches to mobility telemonitoring of the elderly in their living environment. Ann. Biomed. Eng. 2006, 34, 547–563. [Google Scholar] [CrossRef]
- Thorp, J.E.; Adamczyk, P.G.; Ploeg, H.L.; Pickett, K.A. Monitoring Motor Symptoms during Activities of Daily Living in Individuals with Parkinson’s Disease. Front. Neurol. 2018, 9, 1036. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C.; Hsu, Y.L. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors. 2010, 10, 7772–7788. [Google Scholar] [CrossRef]
- Klapper, D.A.; Weaver, J.; Fernandez, H.; Ohno-Machado, L. Classification of movement states in Parkinson’s disease using a wearable ambulatory monitor. AMIA Annu. Symp. Proc. 2003, 2003, 896. [Google Scholar]
- Rodríguez-Molinero, A.; Samà, A.; Pérez-Martínez, D.A.; Pérez López, C.; Romagosa, J.; Bayés, À.; Sanz, P.; Calopa, M.; Gálvez-Barrón, C.; de Mingo, E.; et al. Validation of a portable device for mapping motor and gait disturbances in Parkinson’s disease. JMIR Mhealth Uhealth 2015, 3, e9. [Google Scholar] [CrossRef]
- Pfister, F.M.J.; Um, T.T.; Pichler, D.C.; Abedinpour, K.; Lang, M.; Endo, S.; Ceballos-Baumann, A.O.; Hirche, S.; Bischl, B.; Kulić, D.; et al. High-Resolution Motor State Detection in Parkinson’s Disease Using Convolutional Neural Networks. Sci. Rep. 2020, 10, 5860. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, R.I.; Kotschet, K.; Arfon, S.; Xu, Z.M.; Johnson, W.; Drago, J.; Evans, A.; Kempster, P.; Raghav, S.; Horne, M.K. Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J. Parkinsons Dis. 2012, 2, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Price, J.; Martin, H.; Ebenezer, L. A service evaluation by Parkinson’s disease nurse specialists, of Parkinson’s KinetiGraph (PKG) movement recording system use in routine clinical care of patients with Parkinson’s disease. In Proceedings of the 4th World Parkinson Congress, Portland, OR, USA, 20–23 September 2016; pp. 1–284. Available online: https://content.iospress.com/articles/journal-of-parkinsons-disease/jpd169900 (accessed on 3 July 2021).
- Spengler, D.; Velez-Aldahondo, V.A.; Singer, C.; Luca, C. Initial deep brain stimulation programming optimization using the Personal KinetiGraph (PKG) Movement Recording System. In Proceedings of the 68th AAN Annual Meeting Abstract, Vancouver, BC, Canada, 15–21 April 2016; Available online: http://www.abstractsonline.com/pp8/#!/4046/presentation/8131 (accessed on 3 July 2021).
- Salarian, A.; Russmann, H.; Vingerhoets, F.J.; Dehollain, C.; Blanc, Y.; Burkhard, P.R.; Aminian, K. Gait assessment in Parkinson’s disease: Toward an ambulatory system for long-term monitoring. IEEE Trans. Biomed. Eng. 2004, 51, 1434–1443. [Google Scholar] [CrossRef]
- Mera, T.O.; Burack, M.A.; Giuffrida, J.P. Objective motion sensor assessment highly correlated with scores of global levodopa-induced dyskinesia in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, D.; Pérez-López, C.; Samà, A.; Català, A.; Moreno Arostegui, J.M.; Cabestany, J.; Mestre, B.; Alcaine, S.; Prats, A.; Cruz Crespo, M.; et al. A Waist-Worn Inertial Measurement Unit for Long-Term Monitoring of Parkinson’s Disease Patients. Sensors 2017, 17, 827. [Google Scholar] [CrossRef] [Green Version]
- Tzallas, A.T.; Tsipouras, M.G.; Rigas, G.; Tsalikakis, D.G.; Karvounis, E.C.; Chondrogiorgi, M.; Psomadellis, F.; Cancela, J.; Pastorino, M.; Waldmeyer, M.T.; et al. PERFORM: A system for monitoring, assessment and management of patients with Parkinson’s disease. Sensors 2014, 14, 21329–21357. [Google Scholar] [CrossRef] [Green Version]
- Hoff, J.I.; van den Plas, A.A.; Wagemans, E.A.; van Hilten, J.J. Accelerometric assessment of levodopa-induced dyskinesias in Parkinson’s disease. Mov. Disord. 2001, 16, 58–61. [Google Scholar] [CrossRef]
- Keijsers, N.L.; Horstink, M.W.; van Hilten, J.J.; Hoff, J.I.; Gielen, C.C. Detection and assessment of the severity of levodopa-induced dyskinesia in patients with Parkinson’s disease by neural networks. Mov. Disord. 2000, 15, 1104–1111. [Google Scholar] [CrossRef]
- Keijsers, N.L.; Horstink, M.W.; Gielen, S.C. Automatic assessment of levodopa-induced dyskinesias in daily life by neural networks. Mov. Disord. 2003, 18, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, D.; Samà, A.; Pérez-López, C.; Català, A.; Moreno Arostegui, J.M.; Cabestany, J.; Bayés, À.; Alcaine, S.; Mestre, B.; Prats, A.; et al. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS ONE 2017, 12, e0171764. [Google Scholar] [CrossRef]
- Pérez-López, C.; Samà, A.; Rodríguez-Martín, D.; Moreno-Aróstegui, J.M.; Cabestany, J.; Bayes, A.; Mestre, B.; Alcaine, S.; Quispe, P.; Laighin, G.Ó.; et al. Dopaminergic-induced dyskinesia assessment based on a single belt-worn accelerometer. Artif. Intell. Med. 2016, 67, 47–56. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, A.; Pérez-López, C.; Samà, A.; Rodríguez-Martín, D.; Alcaine, S.; Mestre, B.; Quispe, P.; Giuliani, B.; Vainstein, G.; Browne, P.; et al. Estimating dyskinesia severity in Parkinson’s disease by using a waist-worn sensor: Concurrent validity study. Sci. Rep. 2019, 9, 13434. [Google Scholar] [CrossRef] [Green Version]
- Punin, C.; Barzallo, B.; Clotet, R.; Bermeo, A.; Bravo, M.; Bermeo, J.P.; Llumiguano, C. A Non-Invasive Medical Device for Parkinson’s Patients with Episodes of Freezing of Gait. Sensors 2019, 19, 737. [Google Scholar] [CrossRef] [Green Version]
- Zhan, A.; Mohan, S.; Tarolli, C.; Schneider, R.B.; Adams, J.L.; Sharma, S.; Elson, M.J.; Spear, K.L.; Glidden, A.M.; Little, M.A.; et al. Using Smartphones and Machine Learning to Quantify Parkinson Disease Severity: The Mobile Parkinson Disease Score. JAMA Neurol. 2018, 75, 876–880. [Google Scholar] [CrossRef]
- Fraiwan, L.; Khnouf, R.; Mashagbeh, A.R. Parkinson’s disease hand tremor detection system for mobile application. J. Med. Eng. Technol. 2016, 40, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Daneault, J.F.; Carignan, B.; Codère, C.É.; Sadikot, A.F.; Duval, C. Using a smart phone as a standalone platform for detection and monitoring of pathological tremors. Front. Hum. Neurosci. 2013, 6, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://apps.apple.com/us/app/cypd/id1472983543 (accessed on 3 July 2021).
- Available online: https://kinesiau.com/ (accessed on 3 July 2021).
- Silva de Lima, A.L.; Hahn, T.; Evers, L.J.W.; de Vries, N.M.; Cohen, E.; Afek, M.; Bataille, L.; Daeschler, M.; Claes, K.; Boroojerdi, B.; et al. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS ONE 2017, 12, e0189161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiouris, K.M.; Gatsios, D.; Rigas, G.; Miljkovic, D.; Koroušić Seljak, B.; Bohanec, M.; Arredondo, M.T.; Antonini, A.; Konitsiotis, S.; Koutsouris, D.D.; et al. PD_Manager: An mHealth platform for Parkinson’s disease patient management. Healthc. Technol. Lett. 2017, 4, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Hausdorff, J.M.; Sánchez-Ferro, Á.; Klucken, J.; Merola, A.; Bonato, P.; Paul, S.S.; Horak, F.B.; Vizcarra, J.A.; Mestre, T.A.; et al. Movement Disorder Society Task Force on Technology. A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov. Disord. 2019, 34, 657–663. [Google Scholar] [CrossRef]
- Shah, S.A.; Tinkhauser, G.; Chen, C.C.; Little, S.; Brown, P. Parkinsonian Tremor Detection from Subthalamic Nucleus Local Field Potentials for Closed-Loop Deep Brain Stimulation. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Honolulu, HI, USA, 17–21 July 2018; pp. 2320–2324. [Google Scholar] [CrossRef]
- O’Day, J.J.; Kehnemouyi, Y.M.; Petrucci, M.N.; Anderson, R.W.; Herron, J.A.; Bronte-Stewart, H.M. Demonstration of Kinematic-Based Closed-loop Deep Brain Stimulation for Mitigating Freezing of Gait in People with Parkinson’s Disease. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, online, 20–24 July 2020; pp. 3612–3616. [Google Scholar] [CrossRef]
- LeMoyne, R.; Mastroianni, T.; Whiting, D.; Tomycz, N. Parametric evaluation of deep brain stimulation parameter configurations for Parkinson’s disease using a conformal wearable and wireless inertial sensor system and machine learning. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, online, 20–24 July 2020; pp. 3606–3611. [Google Scholar] [CrossRef]
- Fisher, J.M.; Hammerla, N.Y.; Rochester, L.; Andras, P.; Walker, R.W. Body-Worn Sensors in Parkinson’s Disease: Evaluating Their Acceptability to Patients. Telemed. J. E Health 2016, 22, 63–69. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Variants | |
|---|---|---|
| Clinical symptom |
|
|
| Sensor type |
|
|
| Type of the way to use device |
| |
| Location of wearable sensor |
|
|
| Configuration of wearable sensor |
|
|
| Way of monitoring |
| |
| Operating system |
| |
| Data analysis |
|
|
| Functions |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levin, O.S.; Iakovleva, O.V.; Coloman, I.I.; Kuzmina, A.V. Could New Generations of Sensors Reshape the Management of Parkinson’s Disease? Clin. Transl. Neurosci. 2021, 5, 18. https://doi.org/10.3390/ctn5020018
Levin OS, Iakovleva OV, Coloman II, Kuzmina AV. Could New Generations of Sensors Reshape the Management of Parkinson’s Disease? Clinical and Translational Neuroscience. 2021; 5(2):18. https://doi.org/10.3390/ctn5020018
Chicago/Turabian StyleLevin, Oleg S., Olga V. Iakovleva, Irina I. Coloman, and Anastasia V. Kuzmina. 2021. "Could New Generations of Sensors Reshape the Management of Parkinson’s Disease?" Clinical and Translational Neuroscience 5, no. 2: 18. https://doi.org/10.3390/ctn5020018






