Virtual Reality in the Rehabilitation of Cognitive Impairment after Stroke
Abstract
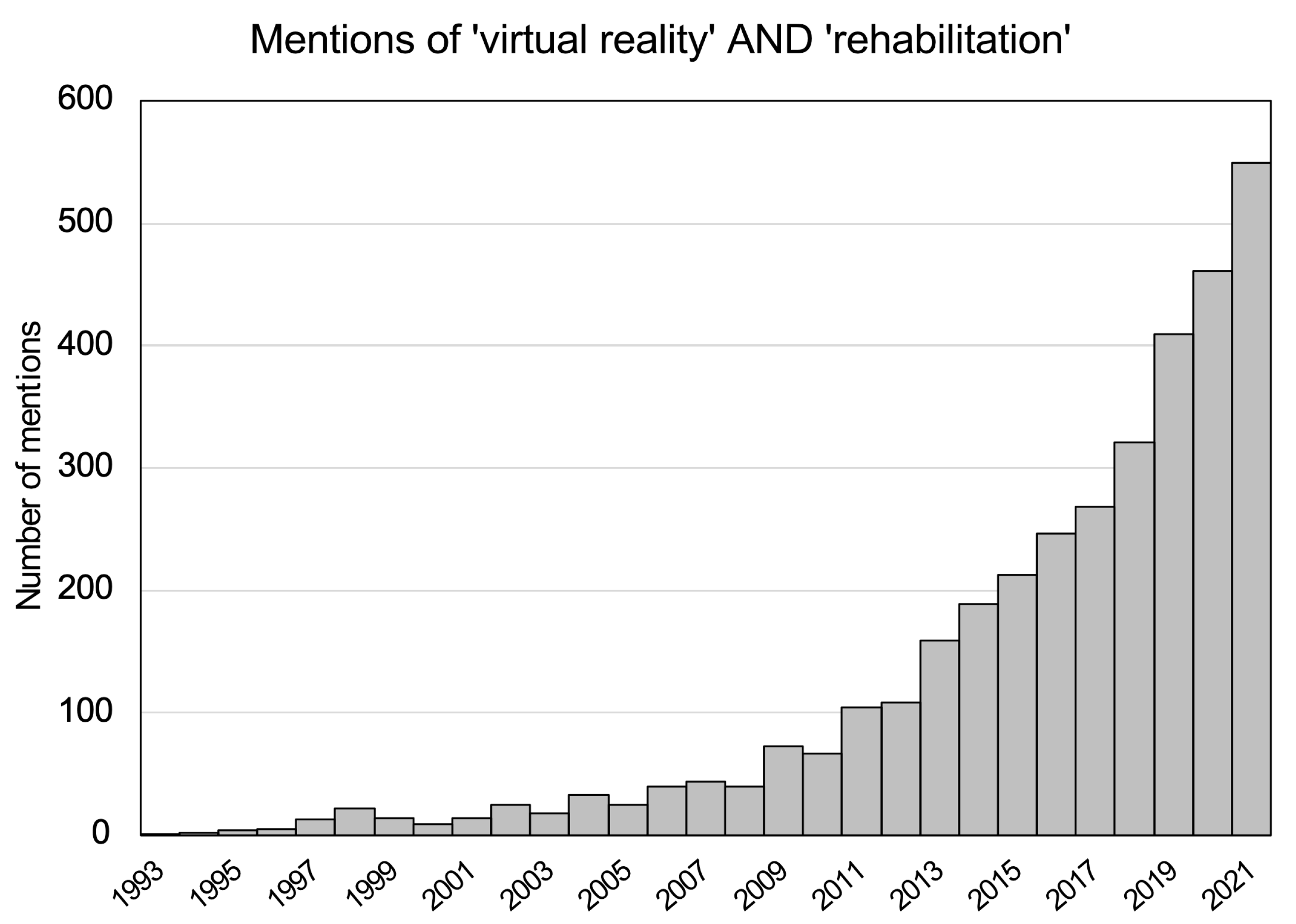
1. Introduction
2. Effects of VR on General Cognition
3. Effects of Training in Virtual Reality on Spatial Cognition
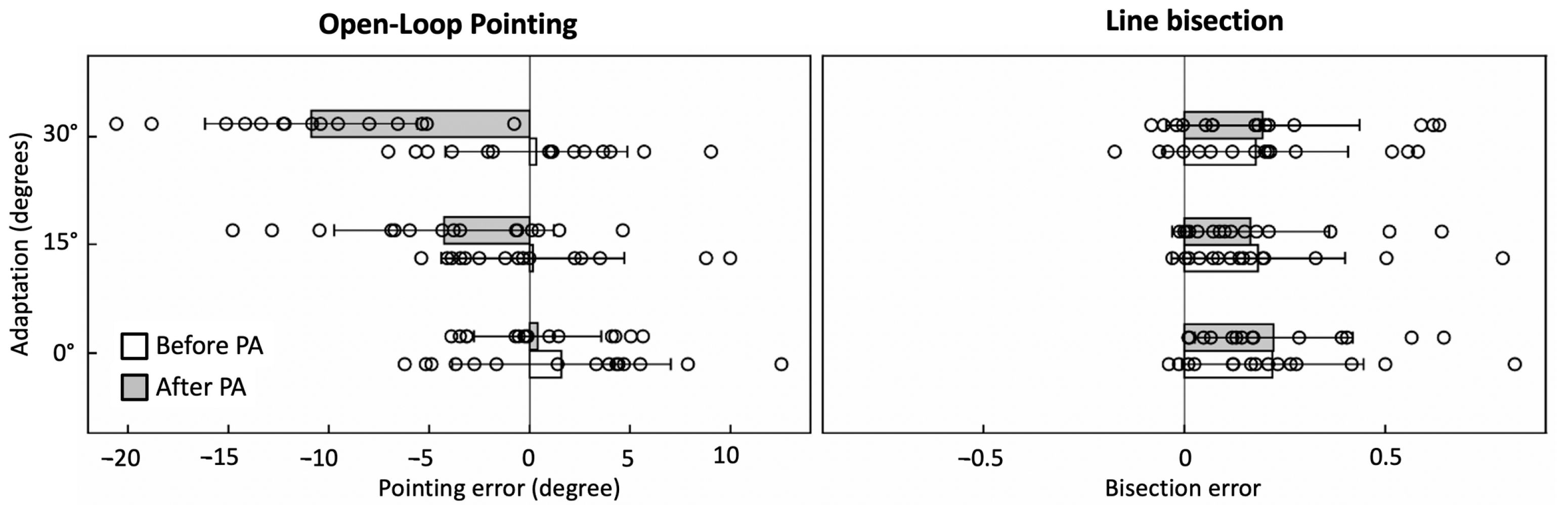
4. Effects of Prism Adaptation on Spatial Neglect
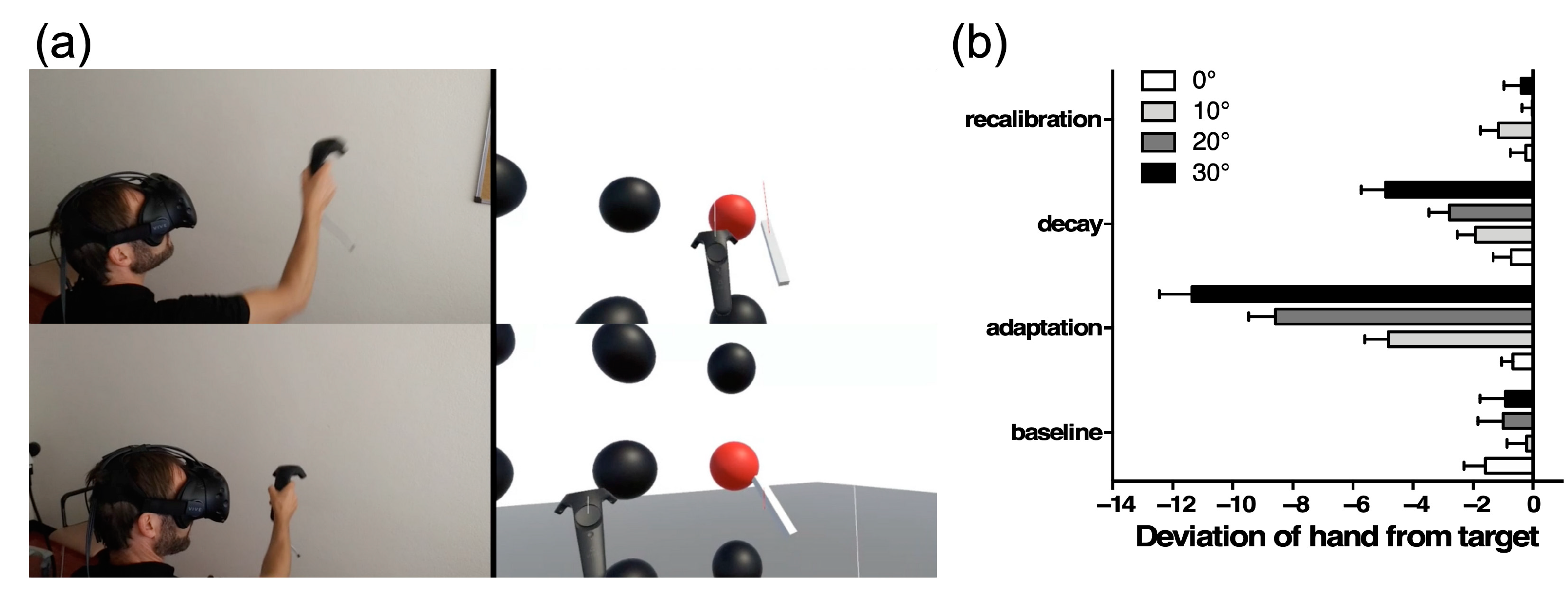
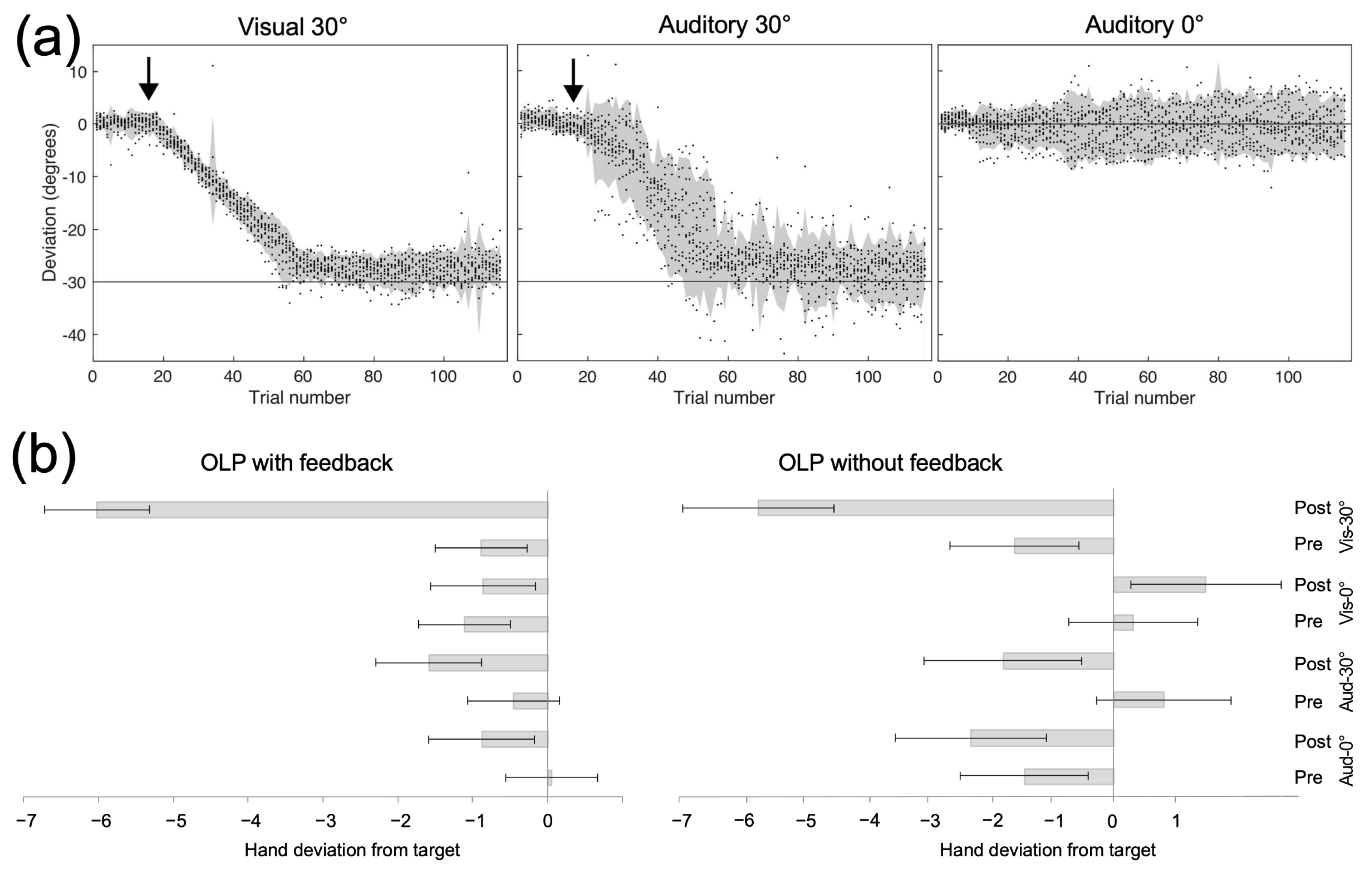
5. Mimicking Prism Adaptation with Virtual Reality
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef]
- Cano Porras, D.; Sharon, H.; Inzelberg, R.; Ziv-Ner, Y.; Zeilig, G.; Plotnik, M. Advanced virtual reality-based rehabilitation of balance and gait in clinical practice. Ther. Adv. Chronic. Dis. 2019, 10, 2040622319868379. [Google Scholar] [CrossRef]
- Dascal, J.; Reid, M.; Ishak, W.W.; Spiegel, B.; Recacho, J.; Rosen, B.; Danovitch, I. Virtual reality and medical inpatients: A systematic review of randomized, controlled trials. Innov. Clin. Neurosci. 2017, 14, 14–21. [Google Scholar]
- Patsaki, I.; Dimitriadi, N.; Despoti, A.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Nanas, S.; Karatzanos, E. The effectiveness of immersive virtual reality in physical recovery of stroke patients: A systematic review. Front. Syst. Neurosci. 2022, 16, 880447. [Google Scholar] [CrossRef]
- Rutkowski, S.; Kiper, P.; Cacciante, L.; Cieslik, B.; Mazurek, J.; Turolla, A.; Szczepanska-Gieracha, J. Use of virtual reality-based training in different fields of rehabilitation: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00121. [Google Scholar] [CrossRef]
- Keshner, E.A. Virtual reality and physical rehabilitation: A new toy or a new research and rehabilitation tool? J. Neuroeng. Rehabil. 2004, 1, 8. [Google Scholar] [CrossRef]
- Weiss, P.L.; Katz, N. The potential of virtual reality for rehabilitation. J. Rehabil. Res. Dev. 2004, 41, 7–10. [Google Scholar]
- Rose, F.D.; Brooks, B.M.; Rizzo, A.A. Virtual reality in brain damage rehabilitation: Review. Cyberpsychol. Behav. 2005, 8, 241–262, discussion 263–271. [Google Scholar] [CrossRef]
- Jonson, M.; Avramescu, S.; Chen, D.; Alam, F. The Role of Virtual Reality in Screening, Diagnosing, and Rehabilitating Spatial Memory Deficits. Front. Hum. Neurosci. 2021, 15, 628818. [Google Scholar] [CrossRef]
- Rizzo, A.A.; Schultheis, M.; Kerns, K.A.; Mateer, C. Analysis of assets for virtual reality applications in neuropsychology. Neuropsychol. Rehabil. 2004, 14, 207–239. [Google Scholar] [CrossRef]
- Huygelier, H.; Schraepen, B.; van Ee, R.; Vanden Abeele, V.; Gillebert, C.R. Acceptance of immersive head-mounted virtual reality in older adults. Sci. Rep. 2019, 9, 4519. [Google Scholar] [CrossRef] [PubMed]
- Borgnis, F.; Baglio, F.; Pedroli, E.; Rossetto, F.; Uccellatore, L.; Oliveira, J.A.G.; Riva, G.; Cipresso, P. Available Virtual Reality-Based Tools for Executive Functions: A Systematic Review. Front. Psychol. 2022, 13, 833136. [Google Scholar] [CrossRef]
- Garcia-Betances, R.I.; Jimenez-Mixco, V.; Arredondo, M.T.; Cabrera-Umpierrez, M.F. Using virtual reality for cognitive training of the elderly. Am. J. Alzheimer′s Dis. Other Dement. 2015, 30, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, W.; Chen, C.; Liu, C.; Yang, J.; Zhang, Y. A Review of the Application of Virtual Reality Technology in the Diagnosis and Treatment of Cognitive Impairment. Front. Aging Neurosci. 2019, 11, 280. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Chen, I.H.; Lin, Y.J.; Chen, Y.; Hsu, W.C. Effects of Virtual Reality-Based Physical and Cognitive Training on Executive Function and Dual-Task Gait Performance in Older Adults with Mild Cognitive Impairment: A Randomized Control Trial. Front. Aging Neurosci. 2019, 11, 162. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Y.; Ren, Z. Rehabilitative Effects of Virtual Reality Technology for Mild Cognitive Impairment: A Systematic Review with Meta-Analysis. Front. Psychol. 2020, 11, 1811. [Google Scholar] [CrossRef]
- Zhu, S.; Sui, Y.; Shen, Y.; Zhu, Y.; Ali, N.; Guo, C.; Wang, T. Effects of Virtual Reality Intervention on Cognition and Motor Function in Older Adults with Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 586999. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.L.; Wong, A.M.K.; Liang, K.C.; Tseng, K.C. Game-Based Virtual Reality System for Upper Limb Rehabilitation After Stroke in a Clinical Environment: Systematic Review and Meta-Analysis. Games Health J. 2022, 11, 277–297. [Google Scholar] [CrossRef]
- Shin, J.H.; Bog Park, S.; Ho Jang, S. Effects of game-based virtual reality on health-related quality of life in chronic stroke patients: A randomized, controlled study. Comput. Biol. Med. 2015, 63, 92–98. [Google Scholar] [CrossRef]
- Faria, A.L.; Andrade, A.; Soares, L.; Badia, S.B. Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: A randomized controlled trial with stroke patients. J. Neuroeng. Rehabil. 2016, 13, 96. [Google Scholar] [CrossRef]
- Faria, A.L.; Pinho, M.S.; Badia, S.B. A comparison of two personalization and adaptive cognitive rehabilitation approaches: A randomized controlled trial with chronic stroke patients. J. Neuroeng. Rehabil. 2020, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- De Luca, R.; Russo, M.; Naro, A.; Tomasello, P.; Leonardi, S.; Santamaria, F.; Desiree, L.; Bramanti, A.; Silvestri, G.; Bramanti, P.; et al. Effects of virtual reality-based training with BTs-Nirvana on functional recovery in stroke patients: Preliminary considerations. Int. J. Neurosci. 2018, 128, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Ballester, B.R.; Leiva Banuelos, N.; Oller, E.D.; Verschure, P. Adaptive conjunctive cognitive training (ACCT) in virtual reality for chronic stroke patients: A randomized controlled pilot trial. J. Neuroeng. Rehabil. 2020, 17, 42. [Google Scholar] [CrossRef]
- Manuli, A.; Maggio, M.G.; Latella, D.; Cannavo, A.; Balletta, T.; De Luca, R.; Naro, A.; Calabro, R.S. Can robotic gait rehabilitation plus Virtual Reality affect cognitive and behavioural outcomes in patients with chronic stroke? A randomized controlled trial involving three different protocols. J. Stroke Cerebrovasc. Dis. 2020, 29, 104994. [Google Scholar] [CrossRef]
- Maggio, M.G.; Latella, D.; Maresca, G.; Sciarrone, F.; Manuli, A.; Naro, A.; De Luca, R.; Calabro, R.S. Virtual Reality and Cognitive Rehabilitation in People with Stroke: An Overview. J. Neurosci. Nurs. 2019, 51, 101–105. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef]
- Howard, M.C. A meta-analysis and systematic literature review of virtual reality rehabilitation programs. Comput. Hum. Behav. 2017, 70, 317–327. [Google Scholar] [CrossRef]
- Mathews, M.; Mitrovic, A.; Ohlsson, S.; Holland, J.; McKinley, A. A Virtual Reality Environment for Rehabilitation of Prospective Memory in Stroke Patients. Procedia Comput. Sci. 2016, 96, 7–15. [Google Scholar] [CrossRef]
- Wiley, E.; Khattab, S.; Tang, A. Examining the effect of virtual reality therapy on cognition post-stroke: A systematic review and meta-analysis. Disabil. Rehabil. Assist. Technol. 2022, 17, 50–60. [Google Scholar] [CrossRef]
- Chen, X.; Liu, F.; Lin, S.; Yu, L.; Lin, R. Effects of Virtual Reality Rehabilitation Training on Cognitive Function and Activities of Daily Living of Patients with Poststroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2022, 103, 1422–1435. [Google Scholar] [CrossRef]
- Rose, T.; Nam, C.S.; Chen, K.B. Immersion of virtual reality for rehabilitation—Review. Appl. Ergon. 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef]
- van Kessel, M.E.; Geurts, A.C.; Brouwer, W.H.; Fasotti, L. Visual Scanning Training for Neglect after Stroke with and without a Computerized Lane Tracking Dual Task. Front. Hum. Neurosci. 2013, 7, 358. [Google Scholar] [CrossRef]
- Ogourtsova, T.; Souza Silva, W.; Archambault, P.S.; Lamontagne, A. Virtual reality treatment and assessments for post-stroke unilateral spatial neglect: A systematic literature review. Neuropsychol. Rehabil. 2017, 27, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Pedroli, E.; Serino, S.; Cipresso, P.; Pallavicini, F.; Riva, G. Assessment and rehabilitation of neglect using virtual reality: A systematic review. Front. Behav. Neurosci. 2015, 9, 226. [Google Scholar] [CrossRef]
- Kim, Y.M.; Chun, M.H.; Yun, G.J.; Song, Y.J.; Young, H.E. The effect of virtual reality training on unilateral spatial neglect in stroke patients. Ann. Rehabil. Med. 2011, 35, 309–315. [Google Scholar] [CrossRef]
- Fordell, H.; Bodin, K.; Eklund, A.; Malm, J. RehAtt—Scanning training for neglect enhanced by multi-sensory stimulation in Virtual Reality. Top Stroke Rehabil. 2016, 23, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Muroi, D.; Ohira, M.; Iwata, H. Validation of an immersive virtual reality system for training near and far space neglect in individuals with stroke: A pilot study. Top Stroke Rehabil. 2017, 24, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Redding, G.M.; Wallace, B. Prism adaptation and unilateral neglect: Review and analysis. Neuropsychologia 2006, 44, 1–20. [Google Scholar] [CrossRef]
- Parton, A.; Malhotra, P.; Husain, M. Hemispatial neglect. J. Neurol. Neurosurg. Psychiatry 2004, 75, 13–21. [Google Scholar] [PubMed]
- Halligan, P.W.; Fink, G.R.; Marshall, J.C.; Vallar, G. Spatial cognition: Evidence from visual neglect. Trends Cogn. Sci. 2003, 7, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ptak, R.; Fellrath, J. Spatial neglect and the neural coding of attentional priority. Neurosci. Biobehav. Rev. 2013, 37, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, L.J.; Ferraro, M.K.; Veramonti, T.; Farne, A.; Whyte, J.; Ladavas, E.; Frassinetti, F.; Coslett, H.B. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology 2004, 62, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Ptak, R.; Schnider, A. Neuropsychological Rehabilitation of Higher Cortical Functions after Brain Damage. In Oxford Textbook of Neurorehabilitation, 2nd ed.; Dietz, V., Ward, N., Eds.; Oxford University Press: Oxford, UK, 2020; pp. 262–271. [Google Scholar]
- Kerkhoff, G.; Schenk, T. Rehabilitation of neglect: An update. Neuropsychologia 2012, 50, 1072–1079. [Google Scholar] [CrossRef]
- Rossetti, Y.; Rode, G.; Pisella, L.; Farnè, A.; Li, L.; Boisson, D.; Perenin, M.T. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 1998, 395, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Vaes, N.; Nys, G.; Lafosse, C.; Dereymaeker, L.; Oostra, K.; Hemelsoet, D.; Vingerhoets, G. Rehabilitation of visuospatial neglect by prism adaptation: Effects of a mild treatment regime. A randomised controlled trial. Neuropsychol. Rehabil. 2018, 28, 899–918. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Barbiani, M.; Rinaldesi, M.L.; Ladavas, E. Effectiveness of prism adaptation in neglect rehabilitation: A controlled trial study. Stroke 2009, 40, 1392–1398. [Google Scholar] [CrossRef]
- Frassinetti, F.; Angeli, V.; Meneghello, F.; Avanzi, S.; Ladavas, E. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain J. Neurol. 2002, 125, 608–623. [Google Scholar] [CrossRef]
- Serino, A.; Bonifazi, S.; Pierfederici, L.; Ladavas, E. Neglect treatment by prism adaptation: What recovers and for how long. Neuropsychol. Rehabil. 2007, 17, 657–687. [Google Scholar] [CrossRef]
- Ten Brink, A.F.; Visser-Meily, J.M.A.; Schut, M.J.; Kouwenhoven, M.; Eijsackers, A.L.H.; Nijboer, T.C.W. Prism Adaptation in Rehabilitation? No Additional Effects of Prism Adaptation on Neglect Recovery in the Subacute Phase Poststroke: A Randomized Controlled Trial. Neurorehabil. Neural Repair 2017, 31, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Rode, G.; Lacour, S.; Jacquin-Courtois, S.; Pisella, L.; Michel, C.; Revol, P.; Alahyane, N.; Luaute, J.; Gallagher, S.; Halligan, P.; et al. Long-term sensorimotor and therapeutical effects of a mild regime of prism adaptation in spatial neglect. A double-blind RCT essay. Ann. Phys. Rehabil. Med. 2015, 58, 40–53. [Google Scholar] [CrossRef]
- Turton, A.J.; O’Leary, K.; Gabb, J.; Woodward, R.; Gilchrist, I.D. A single blinded randomised controlled pilot trial of prism adaptation for improving self-care in stroke patients with neglect. Neuropsychol. Rehabil. 2010, 20, 180–196. [Google Scholar] [CrossRef]
- Rousseaux, M.; Bernati, T.; Saj, A.; Kozlowski, O. Ineffectiveness of prism adaptation on spatial neglect signs. Stroke 2006, 37, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Ptak, R. What role for prism adaptation in the rehabilitation of pure neglect dyslexia? Neurocase 2017, 23, 193–200. [Google Scholar] [CrossRef]
- Mancuso, M.; Pacini, M.; Gemignani, P.; Bartalini, B.; Agostini, B.; Ferroni, L.; Caputo, M.; Capitani, D.; Mondin, E.; Cantagallo, A. Clinical application of prismatic lenses in the rehabilitation of neglect patients. A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2012, 48, 197–208. [Google Scholar]
- Mizuno, K.; Tsuji, T.; Takebayashi, T.; Fujiwara, T.; Hase, K.; Liu, M. Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: A randomized, controlled trial. Neurorehabil. Neural Repair 2011, 25, 711–720. [Google Scholar] [CrossRef]
- Nys, G.M.; de Haan, E.H.; Kunneman, A.; de Kort, P.L.; Dijkerman, H.C. Acute neglect rehabilitation using repetitive prism adaptation: A randomized placebo-controlled trial. Restor. Neurol. Neurosci. 2008, 26, 1–12. [Google Scholar] [PubMed]
- Li, J.; Li, L.; Yang, Y.; Chen, S. Effects of Prism Adaptation for Unilateral Spatial Neglect after Stroke: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2021, 100, 584–591. [Google Scholar] [CrossRef]
- Wilson, B.; Cockburn, J.; Halligan, P. Behavioural Inattention Test; Thames Valley Test Company: Suffolk, UK, 1987. [Google Scholar]
- Serino, A.; Angeli, V.; Frassinetti, F.; Ladavas, E. Mechanisms underlying neglect recovery after prism adaptation. Neuropsychologia 2006, 44, 1068–1078. [Google Scholar] [CrossRef]
- Azouvi, P.; Olivier, S.; de Montety, G.; Samuel, C.; Louis-Dreyfus, A.; Tesio, L. Behavioral assessment of unilateral neglect: Study of the psychometric properties of the Catherine Bergego Scale. Arch. Phys. Med. Rehabil. 2003, 84, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Tsujimoto, K.; Tsuji, T. Effect of Prism Adaptation Therapy on the Activities of Daily Living and Awareness for Spatial Neglect: A Secondary Analysis of the Randomized, Controlled Trial. Brain Sci. 2021, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Székely, O.; Ten Brink, A.F.; Mitchell, A.G.; Bultitude, J.H.; McIntosh, R.D. No immediate treatment effect of prism adaptation for spatial neglect: An inclusive meta-analysis. PsyArXiv 2022. [Google Scholar] [CrossRef]
- Facchin, A.; Daini, R.; Toraldo, A. Prismatic adaptation in the rehabilitation of neglect patients: Does the specific procedure matter? Front. Hum. Neurosci. 2013, 7, 137. [Google Scholar] [CrossRef]
- Ladavas, E.; Bonifazi, S.; Catena, L.; Serino, A. Neglect rehabilitation by prism adaptation: Different procedures have different impacts. Neuropsychologia 2011, 49, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.M.; Goedert, K.M.; Basso, J.C. Prism adaptation for spatial neglect after stroke: Translational practice gaps. Nat. Rev. Neurol. 2012, 8, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Lunven, M.; Rode, G.; Bourlon, C.; Duret, C.; Migliaccio, R.; Chevrillon, E.; de Schotten, M.T.; Bartolomeo, P. Anatomical predictors of successful prism adaptation in chronic visual neglect. Cortex 2019, 120, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzini, E.; Ptak, R. The neuroanatomy of spatial awareness: A large-scale region-of-interest and voxel-based anatomical study. Brain Imaging Behav. 2020, 14, 615–626. [Google Scholar] [CrossRef]
- Gammeri, R.; Turri, F.; Ricci, R.; Ptak, R. Adaptation to virtual prisms and its relevance for neglect rehabilitation: A single-blind dose-response study with healthy participants. Neuropsychol. Rehabil. 2020, 30, 753–766. [Google Scholar] [CrossRef]
- Carter, A.R.; Foreman, M.H.; Martin, C.; Fitterer, S.; Pioppo, A.; Connor, L.T.; Engsberg, J.R. Inducing Visuomotor Adaptation Using Virtual Reality Gaming with a Virtual Shift as a Treatment for Unilateral Spatial Neglect. J. Intellect. Disabil. Diagn. Treat. 2016, 4, 170–184. [Google Scholar]
- Cho, S.; Kim, W.S.; Park, S.H.; Park, J.; Paik, N.J. Virtual Prism Adaptation Therapy: Protocol for Validation in Healthy Adults. J. Vis. Exp. 2020, 156, e60639. [Google Scholar] [CrossRef] [PubMed]
- Wilf, M.; Cheraka, M.C.; Jeanneret, M.; Ott, R.; Perrin, H.; Crottaz-Herbette, S.; Serino, A. Combined virtual reality and haptic robotics induce space and movement invariant sensorimotor adaptation. Neuropsychologia 2021, 150, 107692. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Horning, E.C.; Wilms, I.L. Simulated prism exposure in immersed virtual reality produces larger prismatic after-effects than standard prism exposure in healthy subjects. PLoS ONE 2019, 14, e0217074. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Chang, W.K.; Park, J.; Lee, S.H.; Lee, J.; Han, C.E.; Paik, N.J.; Kim, W.S. Feasibility study of immersive virtual prism adaptation therapy with depth-sensing camera using functional near-infrared spectroscopy in healthy adults. Sci. Rep. 2022, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Golay, L.; Hauert, C.A.; Greber, C.; Schnider, A.; Ptak, R. Dynamic modulation of visual detection by auditory cues in spatial neglect. Neuropsychologia 2005, 43, 1258–1265. [Google Scholar] [CrossRef]
- Kerkhoff, G. Spatial hemineglect in humans. Prog. Neurobiol. 2001, 63, 1–27. [Google Scholar] [CrossRef]
- Jacquin-Courtois, S.; Rode, G.; Pavani, F.; O’Shea, J.; Giard, M.H.; Boisson, D.; Rossetti, Y. Effect of prism adaptation on left dichotic listening deficit in neglect patients: Glasses to hear better? Brain 2010, 133, 895–908. [Google Scholar] [CrossRef]
- Tissieres, I.; Elamly, M.; Clarke, S.; Crottaz-Herbette, S. For Better or Worse: The Effect of Prismatic Adaptation on Auditory Neglect. Neural Plast. 2017, 2017, 8721240. [Google Scholar] [CrossRef]
- Bourgeois, A.; Schmid, A.; Turri, F.; Schnider, A.; Ptak, R. Visual but Not Auditory-Verbal Feedback Induces Aftereffects Following Adaptation to Virtual Prisms. Front. Neurosci. 2021, 15, 658353. [Google Scholar] [CrossRef]
- Bourgeois, A.; Turri, F.; Schnider, A.; Ptak, R. Virtual prism adaptation for spatial neglect: A double-blind study. Neuropsychol Rehabil 2022, 32, 1033–1047. [Google Scholar] [CrossRef]
- Petitet, P.; O’Reilly, J.X.; O’Shea, J. Towards a neuro-computational account of prism adaptation. Neuropsychologia 2018, 115, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Shadmehr, R.; Krakauer, J.W. A computational neuroanatomy for motor control. Exp. Brain Res. 2008, 185, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Ptak, R.; Doganci, N.; Bourgeois, A. From Action to Cognition: Neural Reuse, Network Theory and the Emergence of Higher Cognitive Functions. Brain Sci. 2021, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgeois, A.; Schnider, A.; Turri, F.; Ptak, R. Virtual Reality in the Rehabilitation of Cognitive Impairment after Stroke. Clin. Transl. Neurosci. 2023, 7, 3. https://doi.org/10.3390/ctn7010003
Bourgeois A, Schnider A, Turri F, Ptak R. Virtual Reality in the Rehabilitation of Cognitive Impairment after Stroke. Clinical and Translational Neuroscience. 2023; 7(1):3. https://doi.org/10.3390/ctn7010003
Chicago/Turabian StyleBourgeois, Alexia, Armin Schnider, Francesco Turri, and Radek Ptak. 2023. "Virtual Reality in the Rehabilitation of Cognitive Impairment after Stroke" Clinical and Translational Neuroscience 7, no. 1: 3. https://doi.org/10.3390/ctn7010003
APA StyleBourgeois, A., Schnider, A., Turri, F., & Ptak, R. (2023). Virtual Reality in the Rehabilitation of Cognitive Impairment after Stroke. Clinical and Translational Neuroscience, 7(1), 3. https://doi.org/10.3390/ctn7010003





