Saudi Consensus Recommendations on the Management of Multiple Sclerosis: Symptom Management and Vaccination
Abstract
:1. Introduction
1.1. Symptoms of Multiple Sclerosis
1.2. Multiple Sclerosis and Vaccination
2. Management of Common and Troublesome Symptoms of Multiple Sclerosis
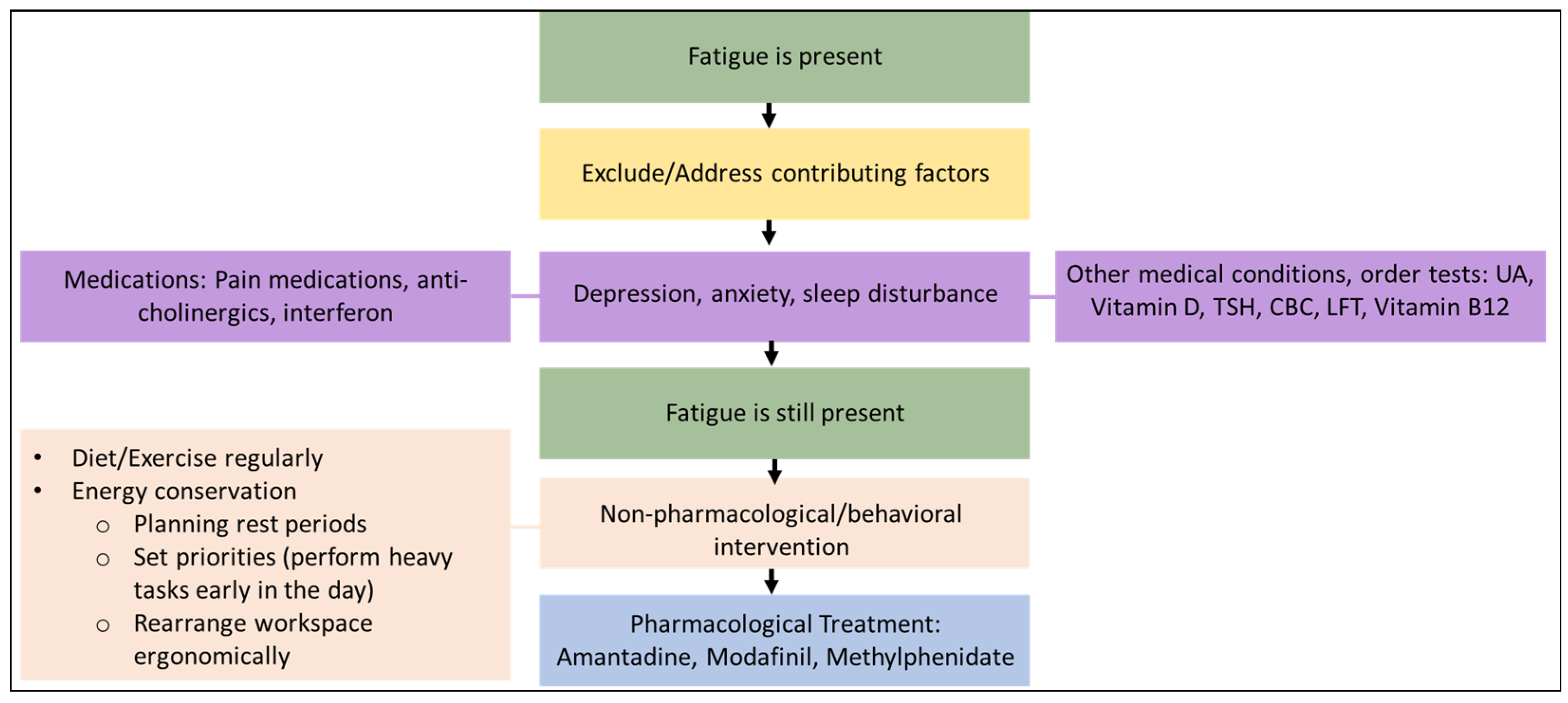
2.1. Fatigue
2.2. Depression
2.3. Cognitive Impairment
2.4. Lower Urinary Tract Symptoms/Bladder dysfunction
2.5. Bowel Dysfunction
2.6. Sexual Dysfunction
2.7. Paroxysmal Symptoms
2.8. Spasticity
2.9. Gait Impairment
2.10. Dysphagia
3. Vaccination in People with Multiple Sclerosis
3.1. General Considerations Regarding Vaccination
3.2. Potentially Immunosuppressive Therapies and Vaccination
3.3. Special Considerations with Respect to the Seasonal Influenza Vaccine in the MS Population
3.4. Special Considerations with Respect to the COVID-19 Vaccination in the MS Population [53,58,75]
3.5. Vaccination and MS Relapses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosure
References
- Al Malik, Y.M.; Al Thubaiti, I.A.; AlAmmari, M.A.; Al Fugham, N.; Ali, E.N.; Alissa, D.A.; Aljarallah, S.A.; Al-Jedai, A.H.; AlKathiri, M.A.; AlKhawajah, M.M.; et al. Saudi Consensus Recommendations on the Management of Multiple Sclerosis: Disease-Modifying Therapies and Management of Relapses. Clin. Transl. Neurosci. 2022, 6, 27. [Google Scholar] [CrossRef]
- Kister, I.; Bacon, T.E.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Kalina, J.T.; Herbert, J. Natural history of multiple sclerosis symptoms. Int. J. MS Care 2013, 15, 146–156. [Google Scholar] [CrossRef]
- Zwibel, H.L.; Smrtka, J. Improving quality of life in multiple sclerosis: An unmet need. Am. J. Manag. Care 2011, 17, S139. [Google Scholar]
- Zanghì, A.; Cimino, S.; Urzì, D.; Privitera, S.; Zagari, F.; Lanza, G.; Patti, F.; D’Amico, E. Pharmacotherapeutic management of lower urinary tract symptoms in Multiple Sclerosis patients. Expert Opin. Pharmacother. 2020, 21, 1449–1454. [Google Scholar] [CrossRef]
- Yong, K.P.; Kim, H. Disease modifying therapies and infection risks in multiple sclerosis-a decision-making conundrum. Ann. Transl. Med. 2020, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Farez, M.F.; Farez, M.F.; Correale, J.; Armstrong, M.J.; Rae-Grant, A.; Gloss, D.; Donley, D.; Holler-Managan, Y.; Kachuck, N.J.; Jeffery, D.; et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2019, 93, 584–594. [Google Scholar] [CrossRef]
- Reyes, S.A.-O.; Reyes, S.; Ramsay, M.; Ladhani, S.; Amirthalingam, G.; Singh, N.; Cores, C.; Lambourne, J.; Marta, M.; Turner, B.; et al. Protecting people with multiple sclerosis through vaccination. Pract. Neurol. 2020, 20, 435–445. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Neurology. AAN Practice Guideline Summary for Clinicians. Practice Guideline Update: Vaccine-Preventable Infections and Immunization in Multiple Sclerosis; AAN: Minneapolis, MN, USA, 2019. [Google Scholar]
- Ciottim, J.R.; Valtcheva, M.; Cross, A. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult. Scler. Relat. Disord. 2020, 45, 102439. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Young, C. A medical definition of fatigue in multiple sclerosis. QJM Int. J. Med. 2008, 101, 49–60. [Google Scholar] [CrossRef]
- Capone, F.A.-O.; Capone, F.; Collorone, S.; Cortese, R.; Di Lazzaro, V.; Moccia, M. Fatigue in multiple sclerosis: The role of thalamus. Mult. Scler. J. 2020, 26, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B.; Galea, M. Management of fatigue in persons with multiple sclerosis. Front. Neurol. 2014, 5, 177. [Google Scholar] [CrossRef] [Green Version]
- Tur, C. Fatigue Management in Multiple Sclerosis. Curr. Treat. Options Neurol. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Shangyan, H.; Shangyan, H.; Kuiqing, L.; Yumin, X.; Jie, C.; Weixiong, L. Meta-analysis of the efficacy of modafinil versus placebo in the treatment of multiple sclerosis fatigue. Mult. Scler. Relat. Disord. 2018, 19, 85–89. [Google Scholar] [CrossRef]
- Cohen, J.A.; Cohen, J.A.; Hunter, S.F.; Brown, T.R.; Gudesblatt, M.; Thrower, B.W.; Llorens, L.; Souza-Prien, C.J.; Ruby, A.E.; Chernoff, D.N.; et al. Safety and efficacy of ADS-5102 (amantadine) extended release capsules to improve walking in multiple sclerosis: A randomized, placebo-controlled, phase 2 trial. Mult. Scler. J. 2019, 25, 601–609. [Google Scholar] [CrossRef]
- Cameron, M.H.; McMillan, G. Methylphenidate is likely less effective than placebo for improving imbalance, walking, and fatigue in people with multiple sclerosis. Mult. Scler. J. 2017, 23, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Siegert, R.J.; Abernethy, D. Depression in multiple sclerosis: A review. J. Neurol. Neurosurg. Psychiatry 2005, 76, 469–475. [Google Scholar] [CrossRef] [PubMed]
- AlHadi, A.N.; AlHadi, A.N.; AlAteeq, D.A.; Al-Sharif, E.; Bawazeer, H.M.; Alanazi, H.; AlShomrani, A.T.; Shuqdar, R.M.; AlOwaybil, R. An arabic translation, reliability, and validation of Patient Health Questionnaire in a Saudi sample. Ann. Gen. Psychiatry 2017, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Koku, G. Beck Depression Inventory. Occup. Med. 2016, 66, 174–175. [Google Scholar] [CrossRef]
- Jongen, P.J.; Ter Horst, A.T.; Brands, A.M. Cognitive impairment in multiple sclerosis. Minerva Med. 2012, 103, 73–96. [Google Scholar]
- Oreja-Guevara, C.; Oreja-Guevara, C.; Ayuso Blanco, T.; Brieva Ruiz, L.; Hernández Pérez, M.Á.; Meca-Lallana, V.; Ramió-Torrentà, L. Cognitive Dysfunctions and Assessments in Multiple Sclerosis. Front. Neurol. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.; Kalb, R.; Beier, M.; Benedict, R.H.; Charvet, L.; Costello, K.; Feinstein, A.; Gingold, J.; Goverover, Y.; Halper, J.; et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult. Scler. J. 2018, 24, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Sandroff, B.M.; Benedict, R.H.B. Cognitive dysfunction and multiple sclerosis: Developing a rationale for considering the efficacy of exercise training. Mult. Scler. J. 2011, 17, 1034–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goverover, Y.; Goverover, Y.; Chiaravalloti, N.D.; O’Brien, A.R.; DeLuca, J. Evidenced-Based Cognitive Rehabilitation for Persons with Multiple Sclerosis: An Updated Review of the Literature From 2007 to 2016. Arch. Phys. Med. Rehabil. 2018, 99, 390–407. [Google Scholar] [CrossRef]
- Tornic, J.; Panicker, J.N. The Management of Lower Urinary Tract Dysfunction in Multiple Sclerosis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 371–381. [Google Scholar]
- Marrie, R.A.; Elliott, L.; Marriott, J.; Cossoy, M.; Blanchard, J.; Tennakoon, A.; Yu, N. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology 2014, 83, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Ghossein, N.; Kang, M.; Lakhkar, A.D. Anticholinergic Medications; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Moccia, M.; Affinito, G.; Ronga, B.; Giordana, R.; Fumo, M.G.; Lanzillo, R.; Petracca, M.; Carotenuto, A.; Triassi, M.; Brescia Morra, V.; et al. Emergency medical care for multiple sclerosis: A five-year population study in the Campania Region (South Italy). Mult. Scler. J. 2022, 28, 597–607. [Google Scholar] [CrossRef]
- Emmanuel, A. Neurogenic bowel dysfunction. F1000Res. 2019, 8, F1000. [Google Scholar] [CrossRef]
- Gulick, E.E. Neurogenic Bowel Dysfunction Over the Course of Multiple Sclerosis: A Review. Int. J. MS Care 2022, 24, 209–217. [Google Scholar] [CrossRef]
- Ashtari, F.; Rezvani, R.; Afshar, H. Sexual dysfunction in women with multiple sclerosis: Dimensions and contributory factors. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 228. [Google Scholar]
- Lew-Starowicz, M.; Gianotten, W.L. Sexual dysfunction in patients with multiple sclerosis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 357–370. [Google Scholar]
- Safarinejad, M.R. Evaluation of the safety and efficacy of sildenafil citrate for erectile dysfunction in men with multiple sclerosis: A double-blind, placebo controlled, randomized study. J. Urol. 2009, 181, 252–258. [Google Scholar] [CrossRef]
- Fowler, C.J.; Miller, J.R.; Sharief, M.K.; Hussain, I.F.; Stecher, V.J.; Sweeney, M. A double blind, randomised study of sildenafil citrate for erectile dysfunction in men with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 700–705. [Google Scholar] [CrossRef]
- D’Amico, E.; Zanghì, A.; Calogero, A.E.; Patti, F. Male fertility in relapsing-remitting multiple sclerosis patients treated with natalizumab and ocrelizumab: A prospective case-control study. Mult. Scler. J. 2021, 27, 2284–2287. [Google Scholar] [CrossRef] [PubMed]
- Lamaita, R.; Melo, C.; Laranjeira, C.; Barquero, P.; Gomes, J.; Silva-Filho, A. Multiple Sclerosis in Pregnancy and its Role in Female Fertility: A Systematic Review. JBRA Assist. Reprod. 2021, 25, 493. [Google Scholar] [CrossRef]
- Tüzün, E.; Akman-Demir, G.; Eraksoy, M. Paroxysmal attacks in multiple sclerosis. Mult. Scler. J. 2001, 7, 402–404. [Google Scholar] [CrossRef]
- Pöllmann, W.; Feneberg, W. Current management of pain associated with multiple sclerosis. CNS Drugs 2008, 22, 291–324. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Prieto González, J.M.; Vila Silván, C. Safety and tolerability of nabiximols oromucosal spray: A review of real-world experience in observational studies, registries, and case reports. Expert Rev. Neurother. 2021, 21, 547–558. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Goodman, A.D.; Brown, T.R.; Edwards, K.R.; Krupp, L.B.; Schapiro, R.T.; Cohen, R.; Marinucci, L.N.; Blight, A.R.; MSF204 Investigators. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann. Neurol. 2010, 68, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, P.; Ruoppolo, G.; Grasso, M.G.; De Vincentiis, M.; Paolucci, S. Dysphagia in multiple sclerosis—Prevalence and prognostic factors. Acta Neurol. Scand. 2002, 105, 40–43. [Google Scholar] [CrossRef]
- De Pauw, A.; Dejaeger, E.; D’hooghe, B.; Carton, H. Dysphagia in multiple sclerosis. Clin. Neurol. Neurosurg. 2002, 104, 345–351. [Google Scholar] [CrossRef]
- Lunde, H.M.B.; Assmus, J.; Myhr, K.M.; Bø, L.; Grytten, N. Survival and cause of death in multiple sclerosis: A 60-year longitudinal population study. J. Neurol. Neurosurg. Psychiatry 2017, 88, 621–625. [Google Scholar] [CrossRef]
- D’Amico, E.; Zanghi, A.; Serra, A.; Murabito, P.; Zappia, M.; Patti, F.; Cocuzza, S. Management of dysphagia in multiple sclerosis: Current best practice. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 47–54. [Google Scholar] [CrossRef]
- Hiss, S.G.; Postma, G.N. Fiberoptic endoscopic evaluation of swallowing. Laryngoscope 2003, 113, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Langer-Gould, A.; Qian, L.; Tartof, S.Y.; Brara, S.M.; Jacobsen, S.J.; Beaber, B.E.; Sy, L.S.; Chao, C.; Hechter, R.; Tseng, H.F. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014, 71, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M.; Chahin, S.; Berger, J.R. Vaccines in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Farez, M.F.; Correale, J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch. Neurol. 2011, 68, 1267–1271. [Google Scholar] [CrossRef]
- Lebrun, C.; Vukusic, S. Immunization and multiple sclerosis: Recommendations from the French multiple sclerosis society. Mult. Scler. Relat. Disord. 2019, 31, 173–188. [Google Scholar] [CrossRef]
- Abbasi, J. COVID-19 and mRNA Vaccines-First Large Test for a New Approach. JAMA 2020, 324, 1125–1127. [Google Scholar] [CrossRef]
- Al Jumah, M.; Abulaban, A.; Aggad, H.; Al Bunyan, R.; AlKhawajah, M.; Al Malik, Y.; Almejally, M.; Alnajashi, H.; Alshamrani, F.; Bohlega, S.; et al. Managing multiple sclerosis in the Covid19 era: A review of the literature and consensus report from a panel of experts in Saudi Arabia. Mult. Scler. Relat. Disord. 2021, 51, 102925. [Google Scholar] [CrossRef]
- von Hehn, C.; Howard, J.; Liu, S.; Meka, V.; Pultz, J.; Mehta, D.; Prada, C.; Ray, S.; Edwards, M.R.; Sheikh, S.I. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e409. [Google Scholar] [CrossRef] [PubMed]
- Aubagio (Teriflunomide) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202992s006lbl.pdf (accessed on 3 August 2020).
- European Medicines Agency. Tysabri. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tysabri (accessed on 29 July 2020).
- Society, U.N.M. Timing MS Medications with COVID-19 Vaccines. 2021. Available online: https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance/Timing-MS-Medications-with-COVID-19-Vaccines (accessed on 6 July 2021).
- Sharifian-Dorche, M.; Sahraian, M.A.; Fadda, G.; Osherov, M.; Sharifian-Dorche, A.; Karaminia, M.; Saveriano, A.W.; La Piana, R.; Antel, J.P.; Giacomini, P.S. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: A systematic review. Mult. Scler. Relat. Disord. 2021, 50, 102800. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.S.; Sellebjerg, F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419836913. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Ocrevus Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761053lbl.pdf (accessed on 3 August 2020).
- Li, Z.; Richards, S.; Surks, H.K.; Jacobs, A.; Panzara, M.A. Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis. Clin. Exp. Immunol. 2018, 194, 295–314. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Lemtrada. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lemtrada (accessed on 29 July 2020).
- European Medicines Agency. Mabthera. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/mabthera (accessed on 29 July 2020).
- Comi, G.; Cook, S.; Giovannoni, G.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Galazka, A.; Nolting, A.; Hicking, C.; Dangond, F. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 168–174. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Mavenclad. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/mavenclad (accessed on 29 July 2020).
- Food and Drug Administration. Mayzent Prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209884s000lbl.pdf (accessed on 3 August 2020).
- HSS.gov Immunization. Vaccine Types. Available online: https://www.vaccines.gov/basics/types (accessed on 25 November 2020).
- Bar-Or, A.; Freedman, M.S.; Kremenchutzky, M.; Menguy-Vacheron, F.; Bauer, D.; Jodl, S.; Truffinet, P.; Benamor, M.; Chambers, S.; O’Connor, P.W. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology 2013, 81, 552–558. [Google Scholar] [CrossRef]
- Kaufman, M.; Pardo, G.; Rossman, H.; Sweetser, M.T.; Forrestal, F.; Duda, P. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2014, 341, 22–27. [Google Scholar] [CrossRef]
- Kappos, L.; Mehling, M.; Arroyo, R.; Izquierdo, G.; Selmaj, K.; Curovic-Perisic, V.; Keil, A.; Bijarnia, M.; Singh, A.; von Rosenstiel, P. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology 2015, 84, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Calkwood, J.C.; Chognot, C.; Evershed, J.; Fox, E.J.; Herman, A.; Manfrini, M.; McNamara, J.; Robertson, D.S.; Stokmaier, D.; et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology 2020, 95, e1999–e2008. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Gilenya. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/gilenya (accessed on 29 July 2020).
- Riva, A.; Barcella, V.; Benatti, S.V.; Capobianco, M.; Capra, R.; Cinque, P.; Comi, G.; Fasolo, M.M.; Franzetti, F.; Galli, M.; et al. Vaccinations in patients with multiple sclerosis: A Delphi consensus statement. Mult. Scler. J. 2021, 27, 347–359. [Google Scholar] [CrossRef]
- Coyle, P.K.; Gocke, A.; Vignos, M.; Newsome, S.D. Vaccine Considerations for Multiple Sclerosis in the COVID-19 Era. Adv. Ther. 2021, 38, 3550–3588. [Google Scholar] [CrossRef]
- Beard, K.; Sriwastava, S. Insight in booster COVID-19 vaccine and disease modifying therapy in multiple sclerosis. J. Neurol. Sci. 2021, 430, 120034. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Immunization of Immunocompromised Persons: Canadian Immunization Guide. Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-8-immunization-immunocompromised-persons.html (accessed on 25 November 2020).
| Medication | Dose | Mechanism of Action | Side Effects |
|---|---|---|---|
| Oxybutynin | 5 mg twice to three times daily | Anti-cholinergic (anti-muscarinic M2-M3) causing smooth muscle relaxation | Dry mouth, constipation, difficulty in urination or retention, drowsiness, and rarely delirium |
| Tolterodine | 1–2 mg twice daily | New generation anti-cholinergic (anti-muscarinic) causing smooth muscle relaxation | Headache, dry mouth, constipation, difficulty in urination or retention and dizziness |
| Solifenacin | 5–10 mg daily | New generation anti-cholinergic (selective anti-muscarinic M3) causing smooth muscle relaxation | Dry mouth, less constipation, difficulty in urination or retention |
| Trospium | 20 mg twice daily | New generation anti-cholinergic (selective anti-muscarinic M3) causing smooth muscle relaxation | Dry mouth, less constipation, difficulty in urination or retention. |
| Tamsulosin | 0.4 mg daily | Blocks alpha 1 receptors on the urethral sphincter, decreasing the resistance on bladder smooth muscles. | Headache, orthostatic hypotension, and retrograde ejaculation in men. |
| Drug/Modality | Mechanism of Action | Side Effects |
|---|---|---|
| Psyllium (dietary fiber) [29] | Bulking agent | Abdominal bloating |
| Lactulose [29] | Osmotic agents | Abdominal bloating and cramps |
| Polyethylene glycol [29] | Osmotic agents | Nausea and abdominal bloating |
| Bisacodyl [29] | Stimulate enteric neurons | Nausea, diarrhoea, and cramps |
| Bisacodyl suppositories [29] | Rectal stimulants | |
| Prucalopride [29] | Selective 5-HT4 agonist; increases GI motility | Headache, nausea, abdominal pain, and diarrhoea. |
| Loperamide [29] | Anti-diarrheal; opioid-receptor agonist | Constipation, dizziness, nausea, cramps. |
| Transanal irrigation (retrograde irrigation) [29] | A device consists of a rubber catheter that is inserted in the rectum, with a balloon that is inflated to keep it in place and create a seal. Water is irrigated, and when the catheter is removed, a bowel action is obtained. Can be used for both constipation and fecal incontinence. | Requires a trained healthcare professional. Leaking of the remaining irrigated water. Rarely, perforation. Avoided in patients with previous pelvic surgeries. |
| Sacral neuromodulation [30] | Stimulation of the S2–S3 nerve roots or tibial nerve. Used for fecal incontinence | Limits MRI use. Efficacy on bowel dysfunction is not well established. |
| Therapy | Impact According to Available Evidence | Recommendation |
|---|---|---|
| Treatments that are unlikely to Impair the Effectiveness of Vaccinations | ||
| Interferon beta [6] | Unlikely to impair the efficacy of vaccinations (data available for vaccination against influenza, pneumococcus, meningococcus, diphtheria-tetanus) COVID-19 vaccination immune response is likely to be intact a | Apply Saudi Ministry of Health recommendations without modification For the COVID-19 vaccine, no washout is required; vaccinate immediately |
| Treatments with some concerns regarding their impact on vaccinations | ||
| Glatiramer acetate | Response to influenza vaccine may be reduced vs. healthy controls or untreated MS patients (no data on other vaccines) | Apply Saudi Ministry of Health recommendations without modification |
| Dimethyl fumarate [53,54] | Risk of lymphopenia with dimethyl fumarate An open-label, multicenter study, demonstrated lower response rates to some vaccines in patients receiving dimethyl fumarate vs. interferon
No data on other vaccines | Concomitant administration of non-live vaccines according to the MOH vaccination schedules may be considered during therapy. Do not administer live attenuated vaccines to patients undergoing therapy For the COVID-19 vaccine, no washout is required; vaccinate immediately |
| Teriflunomide [53,55] | Teriflunomide-treated patients mounted appropriate immune responses to seasonal influenza vaccination (TERIVA study; >90% achieved post-vaccination antibody titers consistent with seroprotection)EMA, Bar-Or No data on other vaccines COVID-19 vaccination immune response is probably intact a | Apply vaccine recommendations for immunocompromised individuals b Do not administer live attenuated vaccines during and for at least 6 months after treatment. For the COVID-19 Vaccine, no washout is required; vaccinate immediately |
| Natalizumab [53,56] | Vaccine response to influenza is reduced kaufman EMA Little effect on levels of anti-Tetanus toxoid IgG antibodies or antibodies to keyhole limpet hemocyanin No data on other vaccines COVID-19 vaccination immune response is probably intact a | Apply vaccine recommendations for immunocompromised individuals b Do not administer live attenuated vaccines during treatment For the COVID-19 vaccine, no washout is required; vaccinate immediately |
| Fingolimod [53,57,58] | Reduced response to vaccination vs. healthy controls, untreated patients, and patients on interferonβ: kappos ema Reduced responder rates for fingolimod vs placebo for influenza vaccine (54% vs. 85% at 3 weeks; 43% vs. 75% at 6 weeks post-vaccination) and for tetanus toxoid (40% vs. 61%; 38% vs. 49%, respectively) COVID-19 vaccination immune response is mostly diminisheda | Consider a complete vaccination schedule before starting the treatment Avoid live attenuated vaccines during and for at least 2 months after discontinuation due to risk of infection. Other vaccines may not work as well as usual if given during this period Assess patients for their immunity to varicella zoster virus (VZV)/(chickenpox) prior to treatment Vaccinate for varicella zoster in antibody-negative patients at least one month before treatment. For the COVID-19 vaccine, no washout is required to avoid MS rebound disease |
| Ocrelizumab [53,59,60] | Reduced vaccine response vs. healthy controls or patients on interferons for influenza, tetanus, pneumococcus However, patients on ocrelizumab mounted humoral responses to vaccination, although decreased vs. controls, for:
No available data on other vaccines | It is not recommended to receive live-attenuated or live vaccines during treatment and after discontinuation until B-cell repletion Immunization guidelines recommend a minimum of 6 weeks between immunization and treatment initiation, For COVID-19 vaccines, vaccinate 3–4 months after the last dose of the DMT, with the second shot delayed by 2–4 weeks. Vaccination should be done ≥3 months after the last infusion. |
| Mitoxantrone | Vaccine response to influenza is reduced compared to healthy controls No available data on other vaccines | Apply vaccine recommendations for immunocompromised individuals b |
| Treatments with insufficient or unavailable human data | ||
| Alemtuzumab [53,61,62] | Not enough data to evaluate the vaccine’s response COVID-19 vaccination response is possibly diminished a | Patients should complete any necessary immunizations at least 6 weeks prior to treatment. Do not administer live viral vaccines for at least 6 weeks before treatment, during treatment, or following a recent course of treatment Prior to treatment, assess patients for immunity to varicella zoster virus (VZV). Consider vaccination for varicella zoster in antibody-negative patients and postpone alemtuzumab until 6 weeks post-vaccination. For the COVID-19 vaccine, vaccination should be done only after the satisfactory recovery of lymphocyte counts Studies have shown that total lymphocytes remained below the lower limit of normal for around half a year after the first and second courses of treatment |
| Rituximab/other anti-CD20 [53,59,63] | No data on any vaccine for patients on rituximab or other anti-CD20 agents (other than ocrelizumab, see above) Response to inactivated vaccines may be reduced during and after treatment COVID-19 vaccination response is possibly diminished a | Vaccinations such as hepatitis vaccinations should be completed at least 4 weeks prior to the first administration of treatment. Live virus vaccines should not be administered prior to or during treatment For the COVID-19 vaccine, vaccinate 3-4 months after the last infusion. The second dose could be delayed by 2-4 weeks. Vaccination should occur ≥3 months after the last infusion. |
| Cladribine [53,64,65] | No available data for any vaccine COVID-19 vaccination response is possibly weakened a | To allow for the full effect of vaccination to occur, administer all immunizations following guidelines with a minimum of 4–6 weeks after starting treatment. It is recommended to vaccinate patients who are antibody-negative for the varicella zoster virus before treatment initiation. Live-attenuated or live vaccines should be administered a minimum of 4–6 weeks prior to starting due to the risk of active vaccine infection As long as the patient’s white blood cell counts are not within normal limits, vaccination with live or attenuated live vaccines should be avoided during and after cladribine treatment Timing of the COVID-19 vaccine in relation to cladribine treatment is not likely to significantly impact the vaccine response; therefore, the COVID-19 vaccine may be administered as soon as it is available to the patient any time after a course of cladribine (4 weeks gap is recommended). Resuming the next treatment course of cladribine should be 2–4 weeks after vaccine completion. |
| Siponimod [53,66] | No available data for any vaccine COVID-19 vaccination response is mostly weakened a | It is recommended to vaccinate patients who are antibody-negative for the varicella zoster virus before treatment initiation. Postponing treatment for at least 4 weeks to allow the full effect of vaccination to occur is recommended. Avoiding the use of live attenuated vaccines during treatment and for 4 weeks after discontinuing the treatment Other vaccines could be less effective if administered during treatment. It is recommended to discontinue treatment at least 1 week before and until 4 weeks after a scheduled vaccination. For the COVID-19 vaccine, vaccinate without washout, even if the response is possibly diminished, to avoid MS rebound disease |
| Methotrexate | No data for any vaccine | Apply vaccine recommendations for immunocompromised individuals b Avoid vaccination with live vaccines |
| Cyclophosphamide | No data for any vaccine | Apply vaccine recommendations for immunocompromised individuals b |
| Type | Examples |
|---|---|
| Live-attenuated vaccines | Measles, mumps, rubella (MMR combined vaccine) Rotavirus Smallpox Chickenpox Yellow fever |
| Inactivated vaccines | Hepatitis A Influenza (injection) Polio (injection) Rabies |
| Subunit, recombinant, polysaccharide, and conjugate vaccines | Hib (Haemophilus influenzae type b) disease Hepatitis B HPV (Human papillomavirus) Rubella (part of the DTaP combined vaccine) Pneumococcal disease Meningococcal disease Varicella zoster (shingles) |
| Toxoid vaccines | Diphtheria Tetanus |
| mRNA vaccines | SARS-CoV2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Thubaiti, I.A.; AlKhawajah, M.M.; Al Fugham, N.; Alissa, D.A.; Al-Jedai, A.H.; Al Malik, Y.M.; Almejally, M.A.; Al-Mudaiheem, H.Y.; Al-Omari, B.A.; AlOtaibi, H.S.; et al. Saudi Consensus Recommendations on the Management of Multiple Sclerosis: Symptom Management and Vaccination. Clin. Transl. Neurosci. 2023, 7, 6. https://doi.org/10.3390/ctn7010006
Al Thubaiti IA, AlKhawajah MM, Al Fugham N, Alissa DA, Al-Jedai AH, Al Malik YM, Almejally MA, Al-Mudaiheem HY, Al-Omari BA, AlOtaibi HS, et al. Saudi Consensus Recommendations on the Management of Multiple Sclerosis: Symptom Management and Vaccination. Clinical and Translational Neuroscience. 2023; 7(1):6. https://doi.org/10.3390/ctn7010006
Chicago/Turabian StyleAl Thubaiti, Ibtisam A., Mona M. AlKhawajah, Norah Al Fugham, Dema A. Alissa, Ahmed H. Al-Jedai, Yaser M. Al Malik, Mousa A. Almejally, Hajer Y. Al-Mudaiheem, Bedor A. Al-Omari, Hessa S. AlOtaibi, and et al. 2023. "Saudi Consensus Recommendations on the Management of Multiple Sclerosis: Symptom Management and Vaccination" Clinical and Translational Neuroscience 7, no. 1: 6. https://doi.org/10.3390/ctn7010006
APA StyleAl Thubaiti, I. A., AlKhawajah, M. M., Al Fugham, N., Alissa, D. A., Al-Jedai, A. H., Al Malik, Y. M., Almejally, M. A., Al-Mudaiheem, H. Y., Al-Omari, B. A., AlOtaibi, H. S., Al Yafeai, R. H., Babakkor, M. A., Bunyan, R. F., Cupler, E. J., Hakami, M., Kedah, H. M., Makkawi, S., Saeed, L. H., Saeedi, J. A., ... Al Jumah, M. A. (2023). Saudi Consensus Recommendations on the Management of Multiple Sclerosis: Symptom Management and Vaccination. Clinical and Translational Neuroscience, 7(1), 6. https://doi.org/10.3390/ctn7010006






