Is the Proof in the Pain? Association between Head and Neck Pain and Vessel Pathology at Follow-Up in Cervical Artery Dissection: A Retrospective Data Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| All n = 68 (100%) | Group A (+/−) n = 46 (68%) | Group B (−/−) n = 9 (13%) | Group C (+/+) n = 13 (19%) | p Value | |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age, median (IQR) | 48.6 (17) | 48.6 (18) | 55.4 (11) | 47.0 (13) | 0.104 |
| Female sex, n (%) | 23 (33.8%) | 17 (37%) | 1 (11.1%) | 5 (38.5%) | 0.367 |
| Location of dissection, n (%) | 0.131 | ||||
| Anterior circulation (carotid artery) | 39 (57.4%) | 24 (52.2%) | 8 (88.9%) | 7 (53.8%) | |
| Posterior circulation (vertebral or basilar artery) | 29 (42.6%) | 22 (48%) | 1 (11.1%) | 6 (46.2%) | |
| Initial vessel pathology, n (%) | 0.303 | ||||
| No vessel pathology | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (7.7%) | |
| Occlusion | 35 (51.5%) | 23 (50%) | 4 (44.4%) | 8 (61.5%) | |
| Stenosis | 32 (47.1%) | 23 (50%) | 5 (55.6%) | 4 (30.8%) | |
| More than one dissected vessel | 6 (8.8%) | 4 (8.7%) | 1 (11.1%) | 1 (7.7%) | 1.000 |
| Patient characteristic, median (IQR) | |||||
| First systolic blood pressure (mmHG) | 141 (25) | 141.0 (29) | 146.0 (33) | 134 (22) | 0.177 |
| BMI, median (IQR) | 23.9 (5) | 24.0 (6) | 23.1 (7) | 23.0 (5) | 0.736 |
| Clinical Scores, median (IQR) | |||||
| NIHSS on admission | 1 (5) | 2 (5) | 3 (8) | 0 (2) | 0.041 |
| mRS after 3 months (n = 67) | 0 (1) | 1 (1) | 0 (2) | 0 (1) | 0.137 |
| Treatment, n (%) | |||||
| Antiplatelet drugs | 39 (57.4%) | 26 (63%) | 4 (44.4%) | 9 (69.2%) | 0.528 |
| Anticoagulants | 11 (16.2%) | 8 (17.4%) | 0 (0%) | 3 (23.1%) | 0.410 |
| Intraarterial treatment | 11 (16.2%) | 8 (17.4%) | 3 (33.3%) | 0 (0%) | 0.090 |
| Intravenous rTPA | 17 (25%) | 12 (26.0%) | 4 (44.4%) | 1 (7.7%) | 0.154 |
| Outcome, n (%) | |||||
| Recurrent stroke | 1 (1.5%) | 0 (0%) | 1 (11.1%) | 0 (0%) | 0.132 |
| Persisting vessel pathology, n (%) | 0.272 | ||||
| No persisting vessel pathology | 36 (52.9%) | 26 (56.5%) | 3 (33.3%) | 7 (53.8%) | |
| Persisting stenosis | 17 (25.0%) | 10 (21.7%) | 2 (22.2%) | 5 (38.5%) | |
| Persisting occlusion | 15 (22.1%) | 10 (21.7%) | 4 (44.4%) | 1 (7.7%) | |
| Time between onset and pain follow-up (days), median (IQR) | 113 (54) | 116.0 (63) | 140.0 (76) | 94.0 (29) | 0.010 |
| Medical History, n (%) | |||||
| Migraine (n = 67) | 7 (10.4%) | 3 (6.7%) | 0 (0%) | 4 (30.8%) | 0.029 |
| Peripheral artery disease | 0 (0%) | ||||
| Low ejection fraction (n = 62) | 0 (0%) | ||||
| Prosthetic heart valves | 1 (1.5%) | 1 (2.2%) | 0 | 0 | 1.000 |
| Coronary heart disease | 2 (2.9%) | 2 (4.3%) | 0 | 0 | 1.000 |
| Atrial Fibrillation | 1 (1.5%) | 1 (2.2%) | 0 | 0 | 1.000 |
| Smoking | 14 (20.6%) | 9 (19.6%) | 2 (22.2%) | 3 (23.1%) | 1.000 |
| Hyperlipidemia | 34 (50%) | 25 (54.3%) | 3 (33.3%) | 6 (46.2%) | 0.506 |
| Diabetes | 3 (4.4%) | 1 (2.2%) | 1 (11.1%) | 1 (7.7%) | 0.243 |
| Hypertension | 19 (27.9%) | 15 (32.6%) | 2 (22.2%) | 2 (15.4%) | 0.438 |
| TIA | 0 (0%) | ||||
| Intracerebral hemorrhage | 0 (0%) | ||||
| Stroke | 2 (2.9%) | 1 (2.2%) | 1 (11.1%) | 0 (0%) | 0.283 |
| KERRYPNX | Regression Coefficient | Standard Error | Wald | df |
|---|---|---|---|---|
| Age | 0.049 | 0.025 | 3.760 | 1 |
| Groups (A = 1/0, B = 0/0, C = 1/1) | 0.276 | 0.335 | 0.679 | 1 |
| Sex | −0.216 | 0.576 | 0.141 | 1 |
| Initial Vessel Status | 0.708 | 0.525 | 1.820 | 1 |
| Medical History of Hypertension | 0.050 | 0.625 | 0.006 | 1 |
| Constant | −3.604 | 1.628 | 4.902 | 1 |
| Sig. | Exp(B) | 95% confidence interval for EXP(B) | ||
| Lower Bound | Upper Bound | |||
| Age (calc.) | 0.052 | 1.050 | 0.999 | 1.103 |
| Groups (A = 1/0, B = 0/0, C = 1/1) | 0.410 | 1.318 | 0.684 | 2.540 |
| Sex | 0.707 | 0.806 | 0.260 | 2.491 |
| Initial Vessel Status | 0.177 | 2.029 | 0.726 | 5.674 |
| Medical History of Hypertension | 0.936 | 1.051 | 0.309 | 3.581 |
| Constant | 0.027 | 0.027 | ||
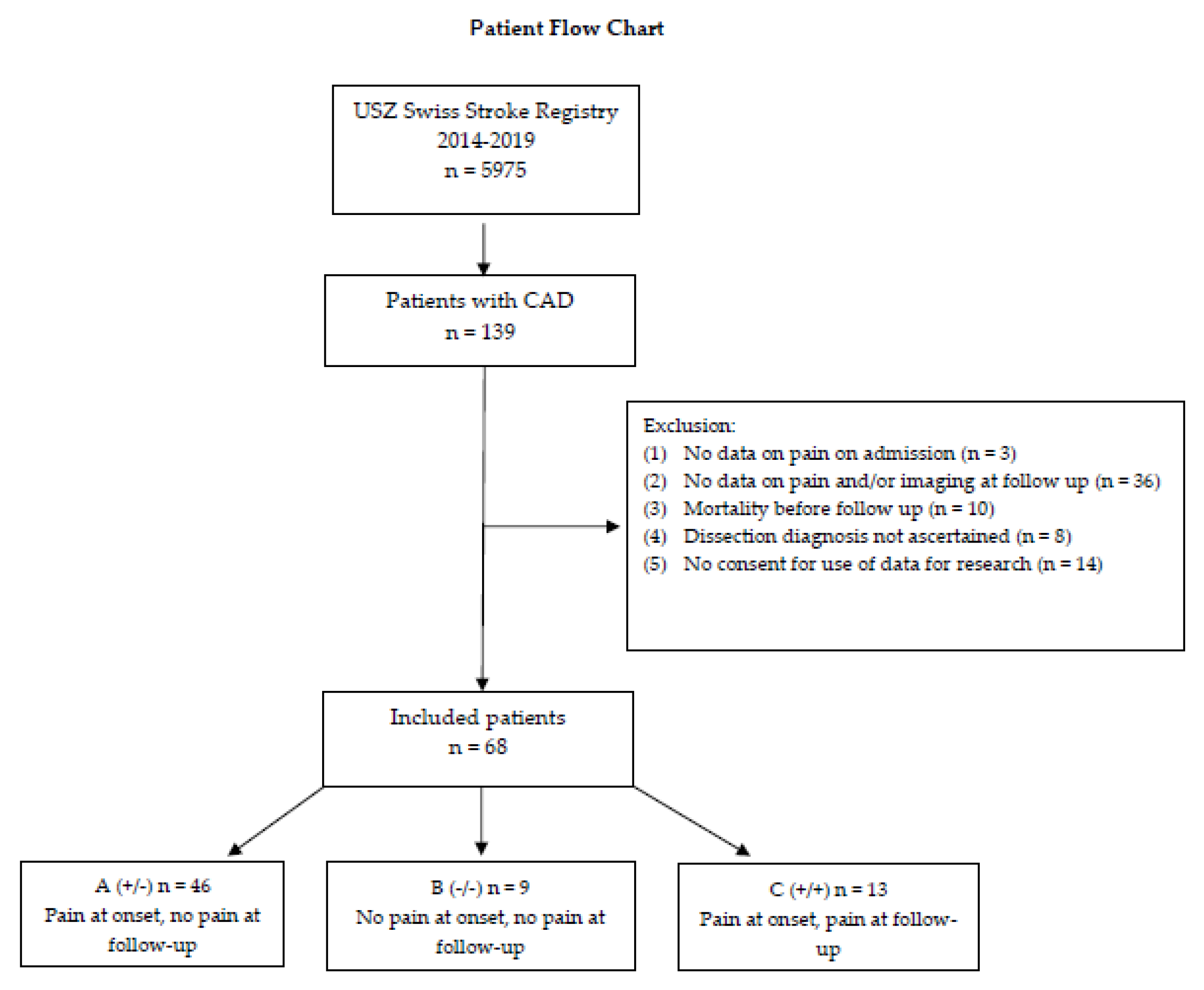
Patient Flow Chart
References
- Vidale, S. Headache in cervicocerebral artery dissection. Neurol. Sci. 2020, 41, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ahl, B.; Bokemeyer, M.; Ennen, J.C.; Kohlmetz, C.; Becker, H.; Weissenborn, K. Dissection of the brain supplying arteries over the life span. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1194. [Google Scholar] [CrossRef] [PubMed]
- Fusco, M.R.; Harrigan, M.R. Cerebrovascular Dissections—A Review Part I: Spontaneous Dissections. Neurosurgery 2011, 68, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Thanvi, B.; Munshi, S.K.; Dawson, S.L.; Robinson, T.G. Carotid and vertebral artery dissection syndromes. Postgrad. Med. J. 2005, 81, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haneline, M.T.; Rosner, A.L. The etiology of cervical artery dissection. J. Chiropr. Med. 2007, 6, 110–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, N.A.; Merkler, A.E.; Gialdini, G.; Kamel, H. Timing of Incident Stroke Risk After Cervical Artery Dissection Presenting Without Ischemia. Stroke 2018, 48, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debette, S.; Leys, D. Cervical-artery dissections: Predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009, 8, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Engelter, S.T.; Traenka, C.; Gensicke, H.; Schaedelin, S.A.; Luft, A.R.; Simonetti, B.G.; Fischer, U.; Michel, P.; Sirimarco, G.; Kägi, G.; et al. Aspirin versus anticoagulation in cervical artery dissection (TREAT-CAD): An open-label, randomised, non-inferiority trial. Lancet Neurol. 2021, 20, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Mazighi, M.; Bijlenga, P.; Pezzini, A.; Koga, M.; Bersano, A.; Kõrv, J.; Haemmerli, J.; Canavero, I.; Tekiela, P.; et al. ESO guideline for the management of extracranial and intracranial artery dissection. Eur. Stroke J. 2021, 6, XXXIX–LXXXVIII. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Cumurciuc, R.; Stapf, C.; Favrole, P.; Berthet, K.; Bousser, M.-G. Pain as the only symptom of cervical artery dissection. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer-Suess, L.; Frank, F.; Töll, T.; Boehme, C.; Gizewski, E.R.; Ratzinger, G.; Broessner, G.; Kiechl, S.; Knoflach, M. Head/neck pain characteristics after spontaneous cervical artery dissection in the acute phase and on a long-run. Cephalalgia 2022, 42, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Strunk, D.; Schwindt, W.; Wiendl, H.; Dittrich, R.; Minnerup, J. Long-Term Sonographical Follow-Up of Arterial Stenosis Due to Spontaneous Cervical Artery Dissection. Front. Neurol. 2022, 12, 792321. [Google Scholar] [CrossRef] [PubMed]
- Nedeltchev, K.; Bickel, S.; Arnold, M.; Sarikaya, H.; Georgiadis, D.; Sturzenegger, M.; Mattle, H.P.; Baumgartner, R.W. R2-recanalization of spontaneous carotid artery dissection. Stroke J. Cereb. Circ. 2008, 40, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biousse, V.; Mitsias, P. Carotid or Vertebral Artery Pain. In The Headaches; Olesen, J., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 911–917, Chapter 111. [Google Scholar]
- Maruyama, H.; Nagoya, H.; Kato, Y.; Deguchi, I.; Fukuoka, T.; Ohe, Y.; Horiuchi, Y.; Dembo, T.; Uchino, A.; Tanahashi, N. Spontaneous cervicocephalic arterial dissection with headache and neck pain as the only symptom. J. Headache Pain 2012, 13, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel Raj Ramabhai, M.D.; Adam Richard, M.D.; Maldjian Catherine, M.D.; Lincoln Christie, M.M.D.; Yuen Annie, M.D.; Arneja Amrita, B.A. Cervical Carotid Artery Dissection: Current Review of Diagnosis and Treatment. Cardiol. Rev. 2012, 20, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Abed, E.; Mohammed, N.H.; Elsheshiny, A.H.; Ahmed, S.; Rashad, M.H. Relation of post-stroke headache to cerebrovascular pathology and hemodynamics. Folia Neuropathol. 2022, 60, 221–227. [Google Scholar] [CrossRef] [PubMed]
- De Giuli, V.; Grassi, M.; Lodigiani, C.; Patella, R.; Zedde, M.; Gandolfo, C.; Zini, A.; DeLodovici, M.L.; Paciaroni, M.; Del Sette, M.; et al. Italian Project on Stroke in Young Adults Investigators. Association Between Migraine and Cervical Artery Dissection: The Italian Project on Stroke in Young Adults. JAMA Neurol. 2017, 74, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Mungoven, T.J.; Marciszewski, K.K.; Macefield, V.G.; Macey, P.M.; Henderson, L.A.; Meylakh, N. Alterations in pain processing circuitries in episodic migraine. J. Headache Pain 2022, 23, 9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumann, J.; Stattmann, M.; Wegener, S. Is the Proof in the Pain? Association between Head and Neck Pain and Vessel Pathology at Follow-Up in Cervical Artery Dissection: A Retrospective Data Analysis. Clin. Transl. Neurosci. 2023, 7, 15. https://doi.org/10.3390/ctn7020015
Baumann J, Stattmann M, Wegener S. Is the Proof in the Pain? Association between Head and Neck Pain and Vessel Pathology at Follow-Up in Cervical Artery Dissection: A Retrospective Data Analysis. Clinical and Translational Neuroscience. 2023; 7(2):15. https://doi.org/10.3390/ctn7020015
Chicago/Turabian StyleBaumann, Jil, Miranda Stattmann, and Susanne Wegener. 2023. "Is the Proof in the Pain? Association between Head and Neck Pain and Vessel Pathology at Follow-Up in Cervical Artery Dissection: A Retrospective Data Analysis" Clinical and Translational Neuroscience 7, no. 2: 15. https://doi.org/10.3390/ctn7020015
APA StyleBaumann, J., Stattmann, M., & Wegener, S. (2023). Is the Proof in the Pain? Association between Head and Neck Pain and Vessel Pathology at Follow-Up in Cervical Artery Dissection: A Retrospective Data Analysis. Clinical and Translational Neuroscience, 7(2), 15. https://doi.org/10.3390/ctn7020015






