Cognitive Neuroimaging Studies on Poverty and Socioeconomic Status Differences in Children and Families across the World: Translational Insights for Next Decade’s Policy, Health, and Education
Abstract
:1. Introduction
Deficit Thinking and the Turn to Implicit Deficit Attribution
- How strong are the reported effects of poverty and socioeconomic status on brain development outcomes, and are they the same in rich and less affluent countries?
- Are the effects of family poverty or low socioeconomic status on neurocognition, as reflected by neuroimaging results, consistently found to be detrimental (i.e., adverse outcome in the direction of low-SES/poor group)?
- Are the effects interpreted mainly in relation to an implicit-deficit attribution or in relation to a structural or environmental set of ecological factors beyond the individual children and families?
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
| Keywords | Search Interfaces/Platforms | ||
|---|---|---|---|
| PubMed | Web of Science | Scopus | |
| Socioeconomic status | 1 (socioeconomic status) 2 (ses) | 1 Socioeconomic status 2 SES | 1socioeconomic status 2 ses |
| Poverty | 3 (poverty) | 3 poverty | 3 poverty |
| Children | 4 (child *) | 4 (child *) | 4 (child *) |
| Neuroimaging | 5 (neuroimage *) 6 (eeg) 7 (erp) 8 (mri) 9 (fmri) | 5 neuroimag* 6 EEG 7 ERP 8 MRI 9 fMRI | 5 neuroimag * 6 eeg 7 erp 8 mri 9 fmri |
| Combination terms | (5 OR 6 OR 7 8 OR 9) AND (1 OR 2 OR 3) AND 4 | TS = 4 AND TS = (1 OR 2 OR 3) AND TS = (5 OR 6 OR 7 OR 8 OR 9) | TITLE-ABS-KEY (1 OR 2 OR 3) AND TITLE-ABS-KEY 4 AND TITLE-ABS-KEY (5 OR 6 OR 7 OR 8 OR 9) |
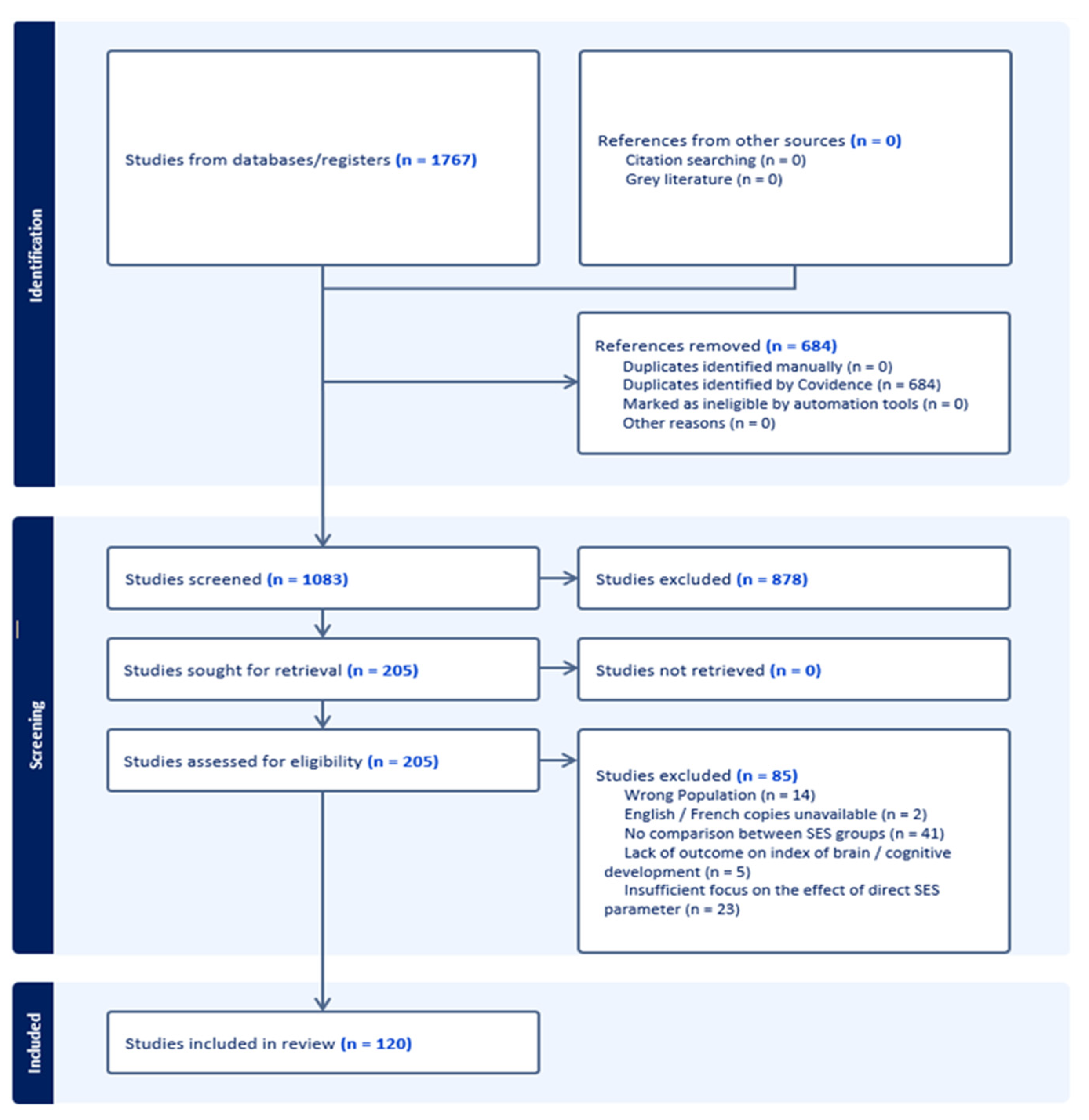
2.3. Selection Process
2.4. Data Collection Process
2.5. Synthesis Methods
3. Results
3.1. Descriptive Results
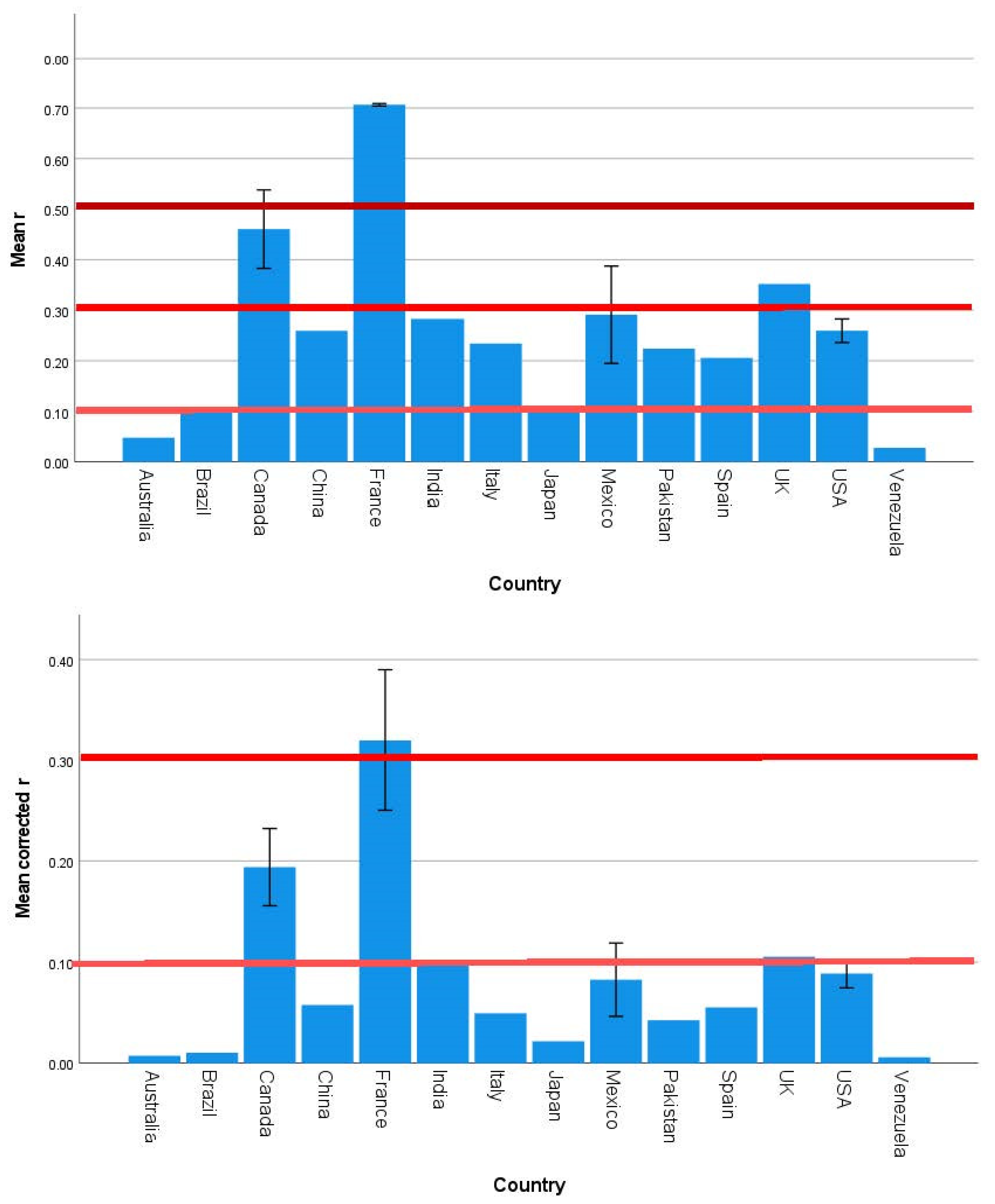
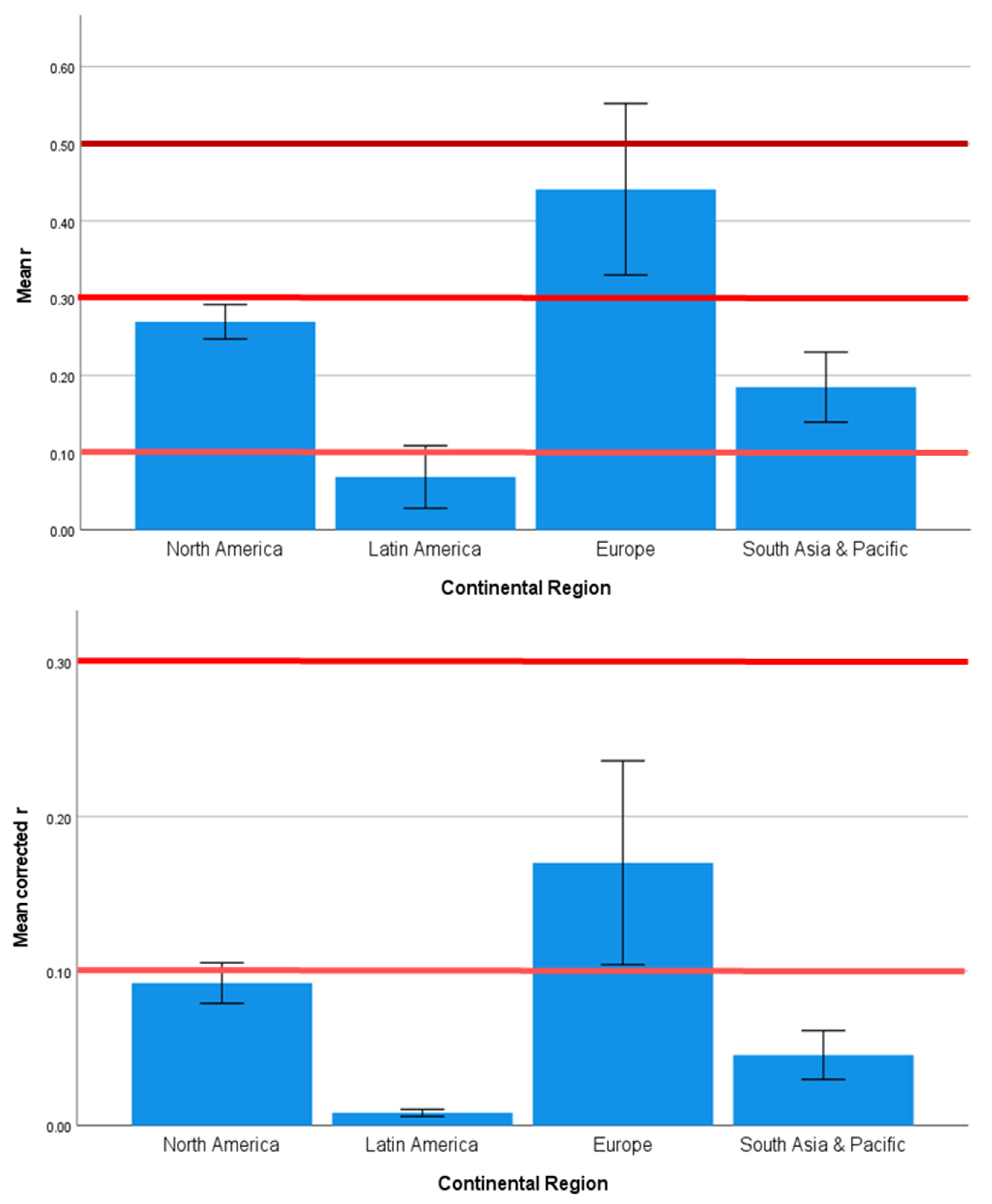
3.2. Strength and Significance of the Direct Effects of SES on Brain Development Outcomes Vary across Countries, Continents, and Country Income Level
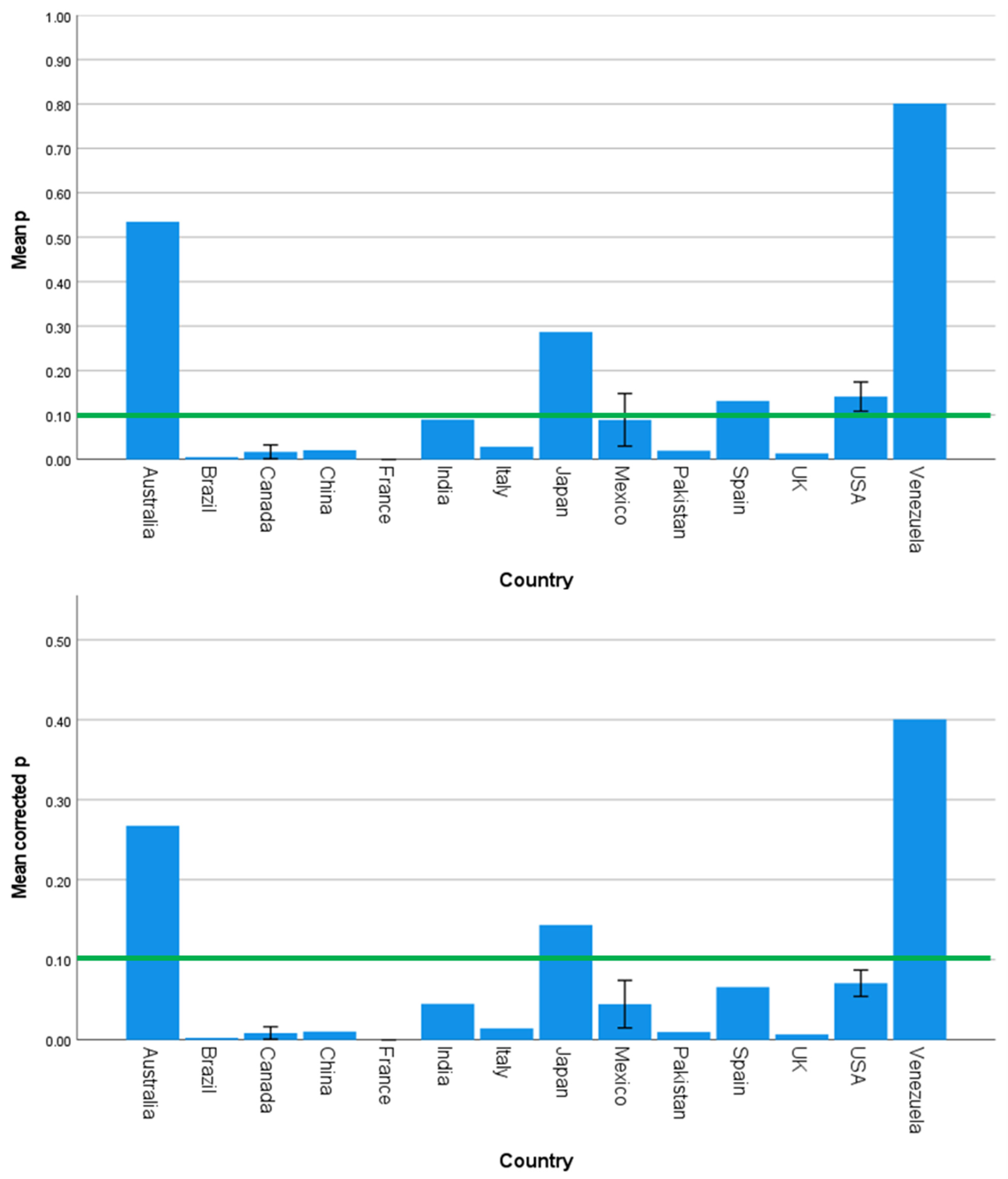
3.3. Are the Main Effects of SES Always Detrimental?
3.4. Effect Sizes and Significance Levels Differ by Neuroimaging Technique
3.5. Effect Sizes and Significance Levels Vary by Developmental Outcome
3.6. Evidence of Implicit Deficit Attribution within the Dataset
4. Discussion
4.1. General Summary of Results
4.2. Regional Geographic Differences in SES Effects on Health Outcomes
4.3. Specificity of SES Effects on Developmental Outcomes
4.4. SES-Related Differences in Brain Activation Do Not Necessarily Predict Behavioral Performance
4.5. Implicit Deficit Attribution and Neuroethical Considerations
5. Limitations and Considerations
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF. UNICEF’s Commitment to Ending Child Poverty and Achieving the SDGs: Measurement, Advocacy and Evidence Based Policies; UNICEF: New York, NY, USA, 2022; Available online: https://tinyurl.com/4n7n83y9 (accessed on 23 June 2023).
- The World Bank. Understanding Poverty; The World Bank Group: Washington, DC, USA, 2022; Available online: https://www.worldbank.org/en/topic/poverty/overview (accessed on 23 June 2023).
- Stringhini, S.; Carmeli, C.; Jokela, M.; Avendaño, M.; Muennig, P.; Guida, F.; Ricceri, F.; d’Errico, A.; Barros, H.; LIFEPATH Consortium; et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1·7 million men and women. Lancet 2017, 389, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Pollak, S.; Wolfe, B. How developmental neuroscience can help address the problem of child poverty. Dev. Psychopathol. 2020, 32, 1640–1656. [Google Scholar] [CrossRef] [PubMed]
- Raizada, R.D.; Richards, T.L.; Meltzoff, A.; Kuhl, P.K. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage 2008, 40, 1392–1401. [Google Scholar] [CrossRef]
- Wang, J.; Lytle, M.N.; Weiss, Y.; Yamasaki, B.L.; Booth, J.R. A longitudinal neuroimaging dataset on language processing in children ages 5, 7, and 9 years old. Sci. Data 2022, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- D’Angiulli, A.; Herdman, A.; Stapells, D.; Hertzman, C. Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology 2008, 22, 293. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.H.; Noble, K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, D.; Volkow, N.D. Associations of family income with cognition and brain structure in USA children: Prevention implications. Mol. Psychiatry 2021, 26, 6619–6629. [Google Scholar] [CrossRef]
- McDermott, C.L.; Seidlitz, J.; Nadig, A.; Liu, S.; Clasen, L.S.; Blumenthal, J.D.; Reardon, P.K.; Lalonde, F.; Greenstein, D.; Patel, R.; et al. Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. J. Neurosci. 2019, 39, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Magnuson, K. Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 2012, 3, 377–386. [Google Scholar] [CrossRef]
- Li, M.; Lindenmuth, M.; Tarnai, K.; Lee, J.; King-Casas, B.; Kim-Spoon, J.; Deater-Deckard, K. Development of cognitive control during adolescence: The integrative effects of family socioeconomic status and parenting behaviors. Dev. Cogn. Neurosci. 2022, 57, 101139. [Google Scholar] [CrossRef]
- Olson, L.; Chen, B.; Fishman, I. Neural correlates of socioeconomic status in early childhood: A systematic review of the literature. Child Neuropsychol. 2021, 27, 390–423. [Google Scholar] [CrossRef] [PubMed]
- Gianaros, P.J.; Kuan, D.C.; Marsland, A.L.; Sheu, L.K.; Hackman, D.A.; Miller, K.G.; Manuck, S.B. Community Socioeconomic Disadvantage in Midlife Relates to Cortical Morphology via Neuroendocrine and Cardiometabolic Pathways. Cereb. Cortex 2017, 27, 460–473. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Wei, D.; Liu, W.; Meng, J.; Wang, K.; Hao, L.; Qiu, J. Regional gray matter volume mediates the relationship between family socioeconomic status and depression-related trait in a young healthy sample. Cogn. Affect. Behav. Neurosci. 2016, 16, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Yaple, Z.A.; Yu, R. Functional and Structural Brain Correlates of Socioeconomic Status. Cereb. Cortex 2020, 30, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Tomalski, P.; Moore, D.G.; Ribeiro, H.; Axelsson, E.L.; Murphy, E.; Karmiloff-Smith, A.; Johnson, M.H.; Kushnerenko, E. Socioeconomic status and functional brain development—Associations in early infancy. Dev. Sci. 2013, 16, 676–687. [Google Scholar] [CrossRef]
- Ramphal, B.; Whalen, D.J.; Kenley, J.K.; Yu, Q.; Smyser, C.D.; Rogers, C.E.; Sylvester, C.M. Brain connectivity and socioeconomic status at birth and externalizing symptoms at age 2 years. Dev. Cogn. Neurosci. 2020, 45, 100811. [Google Scholar] [CrossRef] [PubMed]
- Tooley, U.A.; Mackey, A.P.; Ciric, R.; Ruparel, K.; Moore, T.M.; Gur, R.C.; Gur, R.E.; Satterthwaite, T.D.; Bassett, D.S. Associations between Neighborhood SES and Functional Brain Network Development. Cereb. Cortex 2020, 30, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Li, C.; Li, Y.; Wang, H.; Yu, D. The Effect of Socioeconomic Disparities on Prefrontal Activation in Initiating Joint Attention: A Functional Near-Infrared Spectroscopy Evidence from Two Socioeconomic Status Groups. Front. Hum. Neurosci. 2021, 15, 741872. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Shinohara, I. Socioeconomic disparity in prefrontal development during early childhood. Sci. Rep. 2019, 9, 2585. [Google Scholar] [CrossRef]
- Burgess, A.; Hernandez, S.M.; Li, X.; Bick, J.; Pollonini, L. Correlation between socioeconomic disadvantage in preschool children and brain organization: A functional NIRS connectivity study. In Optics and the Brain; Optica Publishing Group: Washington, DC, USA, 2021; p. BW4B-5. [Google Scholar]
- Louis, E.K.S.; Frey, L.C.; Britton, J.W.; Hopp, J.L.; Korb, P.; Koubeissi, M.Z.; Lievens, W.E.; Pestana-Knight, E.M. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; American Epilepsy Society: Chicago, IL, USA, 2016. [Google Scholar]
- Maguire, M.J.; Schneider, J.M. Socioeconomic status related differences in resting state EEG activity correspond to differences in vocabulary and working memory in grade school. Brain Cogn. 2019, 137, 103619. [Google Scholar] [CrossRef]
- Brito, N.H.; Fifer, W.P.; Myers, M.M.; Elliott, A.J.; Noble, K.G. Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Dev. Cogn. Neurosci. 2016, 19, 144–151. [Google Scholar] [CrossRef]
- Sur, S.; Sinha, V.K. Event-related potential: An overview. Ind. Psychiatry J. 2009, 18, 70–73. [Google Scholar] [CrossRef] [PubMed]
- D’Angiulli, A.; Van Roon, P.M.; Weinberg, J.; Oberlander, T.F.; Grunau, R.E.; Hertzman, C.; Maggi, S. Frontal EEG/ERP correlates of attentional processes, cortisol and motivational states in adolescents from lower and higher socioeconomic status. Front. Hum. Neurosci. 2012, 6, 306. [Google Scholar] [CrossRef]
- St. John, A.M.; Finch, K.; Tarullo, A.R. Socioeconomic status and neural processing of a go/no-go task in preschoolers: An assessment of the P3b. Dev. Cogn. Neurosci. 2019, 38, 100677. [Google Scholar] [CrossRef] [PubMed]
- Eilertsen, T.; Thorsen, A.L.; Holm, S.E.H.; Bøe, T.; Sørensen, L.; Lundervold, A.J. Parental socioeconomic status and child intellectual functioning in a Norwegian sample. Scand. J. Psychol. 2016, 57, 399–405. [Google Scholar] [CrossRef]
- Monzalvo, K.; Fluss, J.; Billard, C.; Dehaene, S.; Dehaene-Lambertz, G. Cortical networks for vision and language in dyslexic and normal children of variable socio-economic status. Neuroimage 2012, 61, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Tervo-Clemmens, B.; Simmonds, D.; Calabro, F.J.; Montez, D.F.; Lekht, J.A.; Day, N.L.; Richardson, G.A.; Luna, B. Early cannabis use and neurocognitive risk: A prospective functional neuroimaging study. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2018, 3, 713–725. [Google Scholar] [CrossRef]
- Noble, K.G.; McCandliss, B.D.; Farah, M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007, 10, 464–480. [Google Scholar] [CrossRef]
- Benischek, A.; Long, X.; Rohr, C.S.; Bray, S.; Dewey, D.; Lebel, C. Pre-reading language abilities and the brain’s functional reading network in young children. NeuroImage 2020, 217, 116903. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, S.A.; Espy, K.A.; Charak, D. Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Dev. Psychol. 2008, 44, 575–587. [Google Scholar] [CrossRef]
- Nentwich, M.; Ai, L.; Madsen, J.; Telesford, Q.K.; Haufe, S.; Milham, M.P.; Parra, L.C. Functional connectivity of EEG is subject-specific, associated with phenotype, and different from fMRI. NeuroImage 2020, 218, 117001. [Google Scholar] [CrossRef] [PubMed]
- Itahashi, T.; Okada, N.; Ando, S.; Yamasaki, S.; Koshiyama, D.; Morita, K.; Yahata, N.; Koike, S.; Nishida, A.; Kasai, K.; et al. Functional connectomes linking child-parent relationships with psychological problems in adolescence. Neuroimage 2020, 219, 117013. [Google Scholar] [CrossRef]
- Biazoli, C.E.; Salum, G.A.; Gadelha, A.; Rebello, K.; Moura, L.M.; Pan, P.M.; Brietzke, E.; Miguel, E.C.; Rohde, L.A.; Bressan, R.A.; et al. Socioeconomic status in children is associated with spontaneous activity in right superior temporal gyrus. Brain Imaging Behav. 2020, 14, 961–970. [Google Scholar] [CrossRef]
- Anum, A. Does socio-economic status have different impact on fluid and crystallized abilities? Comparing scores on Raven’s progressive matrices, Kaufman assessment battery for children II story completion and Kilifi naming test among children in Ghana. Front. Psychol. 2022, 13, 880005. [Google Scholar] [CrossRef]
- Julius, M.S.; Meir, R.; Shechter-Nissim, Z.; Adi-Japha, E. Children’s ability to learn a motor skill is related to handwriting and reading proficiency. Learn. Individ. Differ. 2016, 51, 265–272. [Google Scholar] [CrossRef]
- Bringas-Vega, M.L.; Michel, C.M.; Saxena, S.; White, T.; Valdes-Sosa, P.A. Neuroimaging and global health. NeuroImage 2022, 260, 119458. [Google Scholar] [CrossRef]
- Nisbett, R.E. Intelligence and How to Get It: Why Schools and Cultures Count; WW Norton and Company: New York, NY, USA, 2009. [Google Scholar]
- Valencia, R.R. Conceptualizing the notion of deficit thinking. In The Evolution of Deficit Thinking; Routledge: Abingdon-on-Thames, UK, 1997; pp. 1–12. [Google Scholar]
- Riojas-Cortéz, M. Mexican American Preschoolers Create Stories: Sociodramatic Play in a Dual Language Classroom. Biling. Res. J. 2000, 24, 295–307. [Google Scholar] [CrossRef]
- Menchaca, M. Early racist discourses: The roots of deficit thinking. In The Evolution of Deficit Thinking; Routledge: Abingdon-on-Thames, UK, 1997; pp. 13–40. [Google Scholar]
- Aikman, S.; Robinson-Pant, A.; McGrath, S.; Jere, C.M.; Cheffy, I.; Themelis, S.; Rogers, A. Challenging deficit discourses in international education and development. Compare 2016, 46, 314–334. [Google Scholar] [CrossRef]
- Mampaey, J.; Huisman, J. The reproduction of deficit thinking in times of contestation: The case of higher education. Br. J. Sociol. Educ. 2022, 43, 1233–1249. [Google Scholar] [CrossRef]
- Sleeter, C.E. Context-Conscious Portraits and Context-Blind Policy. Anthropol. Educ. Q. 2004, 35, 132–136. [Google Scholar] [CrossRef]
- James, C.E.; Turner, T. Towards Race Equity in Education: The Schooling of Black Students in the Greater Toronto Area; York University: York, UK, 2017; Available online: https://edu.yorku.ca/files/2017/04/Towards-Race-Equity-in-Education-April-2017.pdf (accessed on 15 May 2023).
- Perera, W.A.H.; Salehuddin, K.; Khairudin, R.; Schaefer, A. The Relationship Between Socioeconomic Status and Scalp Event-Related Potentials: A Systematic Review. Front. Hum. Neurosci. 2021, 15, 601489. [Google Scholar] [CrossRef] [PubMed]
- D’Angiulli, A.; Lipina, S.J.; Olesinska, A. Explicit and implicit issues in the developmental cognitive neuroscience of social inequality. Front. Hum. Neurosci. 2012, 6, 254. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Jodal, H.C.; Helsingen, L.M.; Anderson, J.C.; Lytvyn, L.; Vandvik, P.O.; Emilsson, L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: A systematic review and network meta-analysis. BMJ Open 2019, 9, e032773. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Hayes, A.F.; Krippendorff, K. Answering the Call for a Standard Reliability Measure for Coding Data. Commun. Methods Meas. 2007, 1, 77–89. [Google Scholar] [CrossRef]
- Krippendorff, K. Content analysis: An introduction to its methodology. In Content Analysis: An Introduction to Its Methodology, 2nd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2004; p. 23. [Google Scholar]
- Rosenthal, R. Meta-Analytic Procedures for Social Research, revised ed.; SAGE Publications: Thousand Oaks, CA, USA, 1991. [Google Scholar]
- Jeffreys, H. The Theory of Probability, 3rd ed.; Oxford University Press: Oxford, UK, 1961; p. 432. [Google Scholar]
- Murphy, K.R.; Myors, B.; Wolach, A. Statistical Power Analysis: A Simple and General Model for Traditional and Modern Hypothesis Tests, 4th ed.; Routledge/Taylor and Francis Group: Cambridge, UK, 2014. [Google Scholar]
- Van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring Scholarly Impact; Springer: Cham, Switzerland, 2010; pp. 285–320. [Google Scholar]
- The World Bank. World Bank Country and Lending Groups; The World Bank Group: Washington, DC, USA, 2022; Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 7 August 2023).
- Lenhard, W.; Lenhard, A. Computation of Effect Sizes. Psychometrica. 2016. Available online: https://www.psychometrica.de/effect_size.html (accessed on 26 May 2023).
- Eshetu, E.B.; Woldesenbet, S.A. Are there particular social determinants of health for the world’s poorest countries? Afr. Health Sci. 2011, 11, 108–115. [Google Scholar]
- Orach, C.G. Health equity: Challenges in low income countries. Afr. Health Sci. 2009, 9 (Suppl. S2), S49–S51. [Google Scholar] [PubMed]
- Lea, A.J.; Waigwa, C.; Muhoya, B.; Lotukoi, F.; Peng, J.; Henry, L.P.; Abhyankar, V.; Kamau, J.; Martins, D.; Gurven, M.; et al. Socioeconomic status effects on health vary between rural and urban Turkana. Evol. Med. Public Health 2021, 9, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, D.; Whittle, S. Socioeconomic status and the developing brain—A systematic review of neuroimaging findings in youth. Neurosci. Biobehav. Rev. 2021, 130, 379–407. [Google Scholar] [CrossRef]
- Dennis, E.; Manza, P.; Volkow, N.D. Socioeconomic status, BMI, and brain development in children. Transl. Psychiatry 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.A.; Farah, M.J. Socioeconomic status and the developing brain. Trends Cogn. Sci. 2009, 13, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.M.; Hook, C.J.; Farah, M.J. A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Dev. Sci. 2018, 21, 12529. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.J.; Shera, D.M.; Savage, J.H.; Betancourt, L.; Giannetta, J.M.; Brodsky, N.L.; Malmud, E.K.; Hurt, H. Childhood poverty: Specific associations with neurocognitive development. Brain Res. 2006, 1110, 166–174. [Google Scholar] [CrossRef]
- Noble, K.G.; Norman, M.F.; Farah, M.J. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 2005, 8, 74–87. [Google Scholar] [CrossRef]
- Engel, P.M.; Santos, F.H.; Gathercole, S.E. Are working memory measures free of socioeconomic influence? J. Speech Lang. Hear. Res. JSLHR 2008, 51, 1580–1587. [Google Scholar] [CrossRef]
- Lupien, S.J.; King, S.; Meaney, M.J.; McEwen, B.S. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 2001, 13, 653–676. [Google Scholar] [CrossRef]
- Ellwood-Lowe, M.E.; Whitfield-Gabrieli, S.; Bunge, S.A. What is an adaptive pattern of brain network coupling for a child? It depends on their environment. BioRxiv 2020, 12, 7183. [Google Scholar]
- Demir, Ö.E.; Prado, J.; Booth, J.R. Parental socioeconomic status and the neural basis of arithmetic: Differential relations to verbal and visuo-spatial representations. Dev. Sci. 2015, 18, 799–814. [Google Scholar] [CrossRef]
- Demir-Lira, Ö.E.; Prado, J.; Booth, J.R. Neural correlates of math gains vary depending on parental socioeconomic status (SES). Front. Psychol. 2016, 7, 892. [Google Scholar] [CrossRef]
- Frankenhuis, W.E.; de Weerth, C. Does early-life exposure to stress shape or impair cognition? Curr. Dir. Psychol. Sci. 2013, 22, 407–412. [Google Scholar] [CrossRef]
- Frankenhuis, W.E.; Nettle, D. The strengths of people in poverty. Curr. Dir. Psychol. Sci. 2020, 29, 16–21. [Google Scholar] [CrossRef]
- Mittal, C.; Griskevicius, V.; Simpson, J.A.; Sung, S.; Young, E.S. Cognitive adaptations to stressful environments: When childhood adversity enhances adult executive function. J. Personal. Soc. Psychol. 2015, 109, 604–621. [Google Scholar] [CrossRef]
- Ellis, B.J.; Bianchi, J.; Griskevicius, V.; Frankenhuis, W.E. Beyond Risk and Protective Factors: An Adaptation-Based Approach to Resilience. Perspect. Psychol. Sci. 2017, 12, 561–587. [Google Scholar] [CrossRef] [PubMed]
- Nweze, T.; Nwoke, M.B.; Nwufo, J.I.; Aniekwu, R.I.; Lange, F. Working for the future: Parentally deprived Nigerian Children have enhanced working memory ability. J. Child Psychol. Psychiatry Allied Discip. 2021, 62, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Young, E.S.; Griskevicius, V.; Simpson, J.A.; Waters, T.E.A.; Mittal, C. Can an unpredictable childhood environment enhance working memory? Testing the sensitized-specialization hypothesis. J. Personal. Soc. Psychol. 2018, 114, 891–908. [Google Scholar] [CrossRef]
- Schibli, K.; Wong, K.; Hedayati, N.; D’Angiulli, A. Attending, learning, and socioeconomic disadvantage: Developmental cognitive and social neuroscience of resilience and vulnerability. Ann. N. Y. Acad. Sci. 2017, 1396, 19–38. [Google Scholar] [CrossRef]
- Ginsburg, H.P. The myth of the deprived child: New thoughts on poor children. In The School Achievement of Minority Children; Routledge: Abingdon-on-Thames, UK, 1986; pp. 169–189. [Google Scholar]
- Cole, M. Differences and deficits in psychological research in historical perspective: A commentary on the special section. Dev. Psychol. 2013, 49, 84–91. [Google Scholar] [CrossRef]
- Wastell, D.; White, S. Blinded by Science: The Social Implications of Epigenetics and Neuroscience; Policy Press: Bristol, UK, 2017. [Google Scholar]
- Hoff, E. Interpreting the early language trajectories of children from low-SES and language minority homes: Implications for closing achievement gaps. Dev. Psychol. 2013, 49, 4–14. [Google Scholar] [CrossRef]
- Hall, W.S.; Tirre, W.C. The Communicative Environment of Young Children: Social Class, Ethnic, and Situational Differences; Technical Report No. 125; University of Illinois at Urbana: Champaign, IL, USA, 1979. [Google Scholar]
- Fagan, J.F.; Holland, C.R. Equal opportunity and racial differences in IQ. Intelligence 2002, 30, 361–387. [Google Scholar] [CrossRef]
- Fagan, J.F.; Holland, C.R. Racial equality in intelligence: Predictions from a theory of intelligence as processing. Intelligence 2007, 35, 319–334. [Google Scholar] [CrossRef]
- Gould, S.J. Mismeasure of Man; WW Norton and Company: New York, NY, USA, 1996. [Google Scholar]
- Blum, J.M. Pseudoscience and Mental Ability: The Origins and Fallacies of the IQ Controversy. Rev. Radic. Political Econ. 1978, 13, 72–73. [Google Scholar]
- Waffenschmidt, S.; Knelangen, M.; Sieben, W.; Bühn, S.; Pieper, D. Single screening versus conventional double screening for study selection in systematic reviews: A methodological systematic review. BMC Med. Res. Methodol. 2019, 19, 132. [Google Scholar] [CrossRef]
- Ford, C.B.; Kim, H.Y.; Brown, L.; Aber, J.L.; Sheridan, M.A. A cognitive assessment tool designed for data collection in the field in low-and middle-income countries. Res. Comp. Int. Educ. 2019, 14, 141–157. [Google Scholar] [CrossRef]
- Sternberg, R.J. Context-sensitive cognitive and educational testing. Educ. Psychol. Rev. 2018, 30, 857–884. [Google Scholar] [CrossRef]
- Van de Vijver, F.; Tanzer, N.K. Bias and equivalence in cross-cultural assessment: An overview. Eur. Rev. Appl. Psychol. 2004, 54, 119–135. [Google Scholar] [CrossRef]
- Ardila, A.; Keating, K. Cognitive abilities in different cultural contexts. In International Handbook of Cross-Cultural Neuropsychology; Routledge: Abingdon-on-Thames, UK, 2007; pp. 109–125. [Google Scholar]
- Fernández, A.L.; Abe, J. Bias in cross-cultural neuropsychological testing: Problems and possible solutions. Cult. Brain 2018, 6, 1–35. [Google Scholar] [CrossRef]
- Zuilkowski, S.S.; McCoy, D.C.; Serpell, R.; Matafwali, B.; Fink, G. Dimensionality and the development of cognitive assessments for children in Sub-Saharan Africa. J. Cross-Cult. Psychol. 2016, 47, 341–354. [Google Scholar] [CrossRef]
- Kranzler, J.H.; Flores, C.G.; Coady, M. Examination of the cross-battery approach for the cognitive assessment of children and youth from diverse linguistic and cultural backgrounds. Sch. Psychol. Rev. 2010, 39, 431–446. [Google Scholar] [CrossRef]
- Rosenthal, R. The file drawer problem and tolerance for null results. Psychol. Bull. 1979, 86, 638–641. [Google Scholar] [CrossRef]
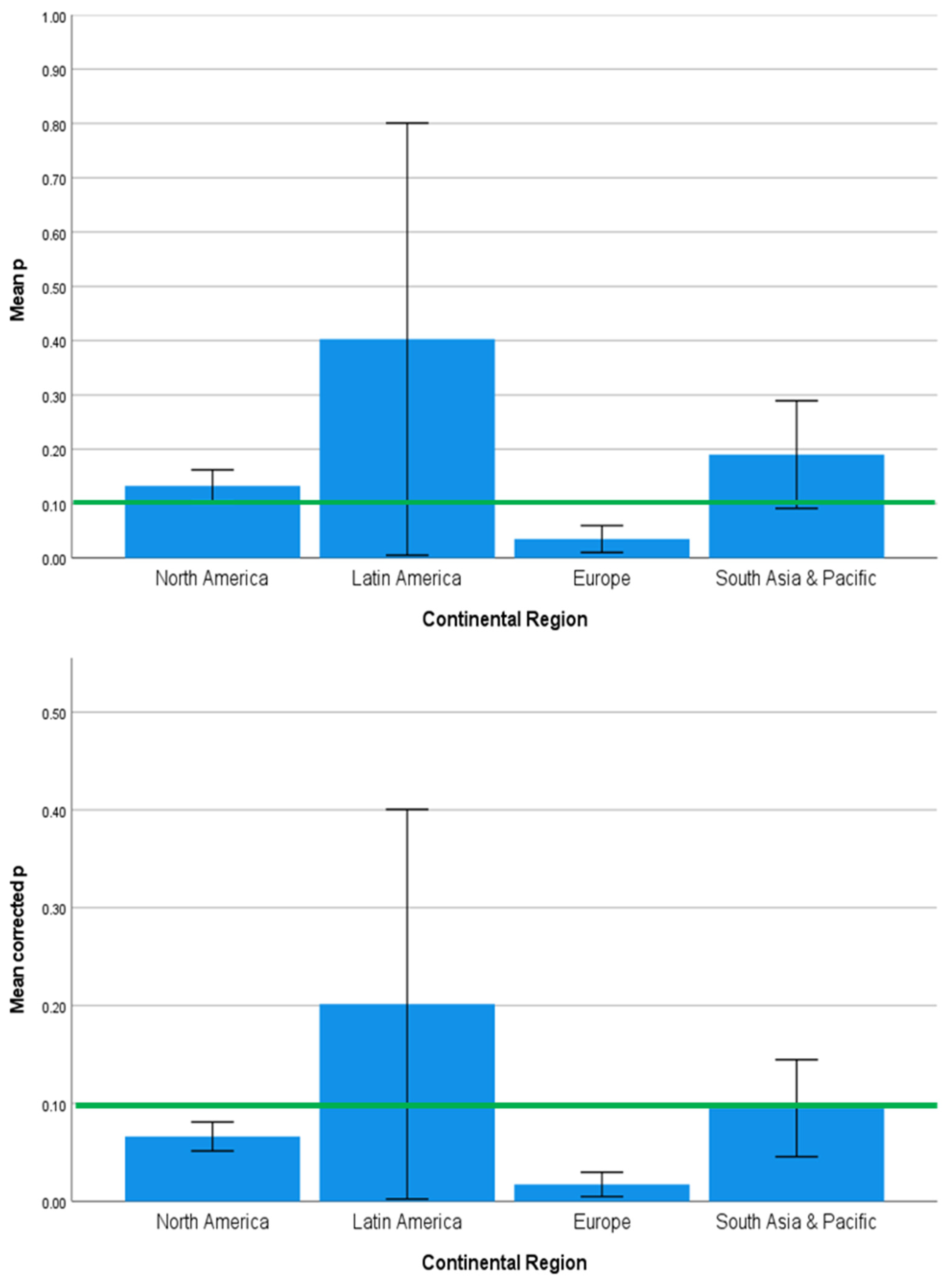



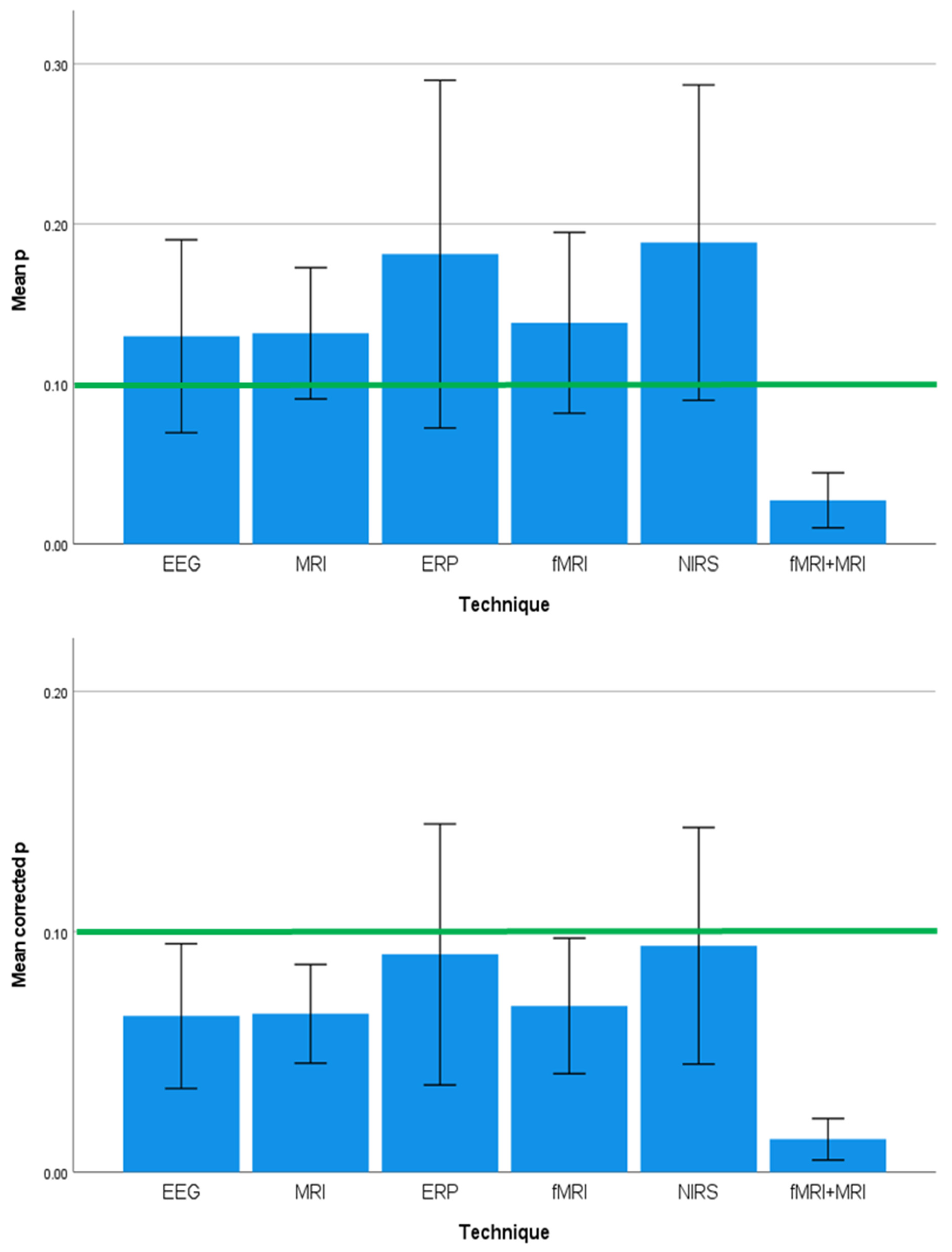
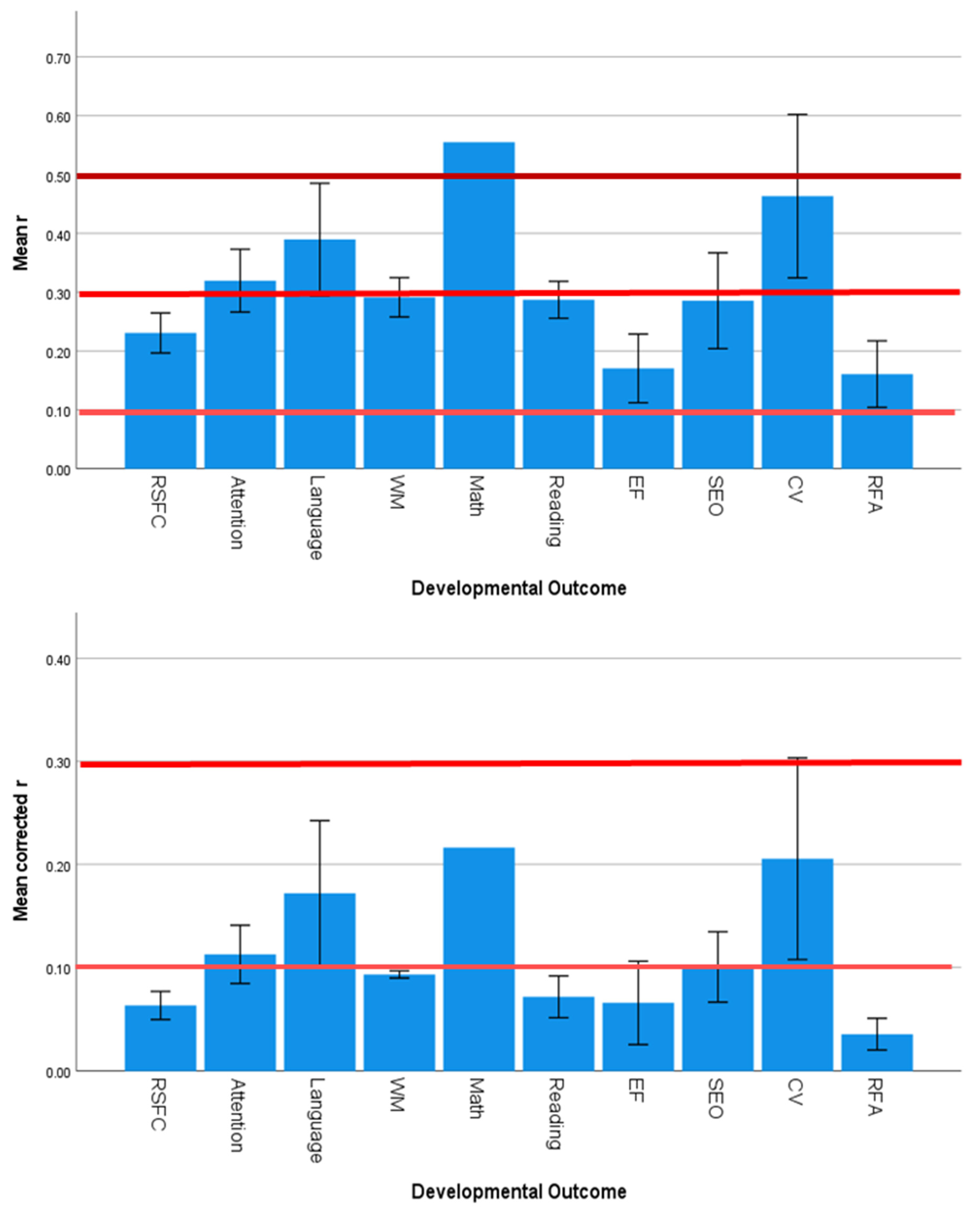
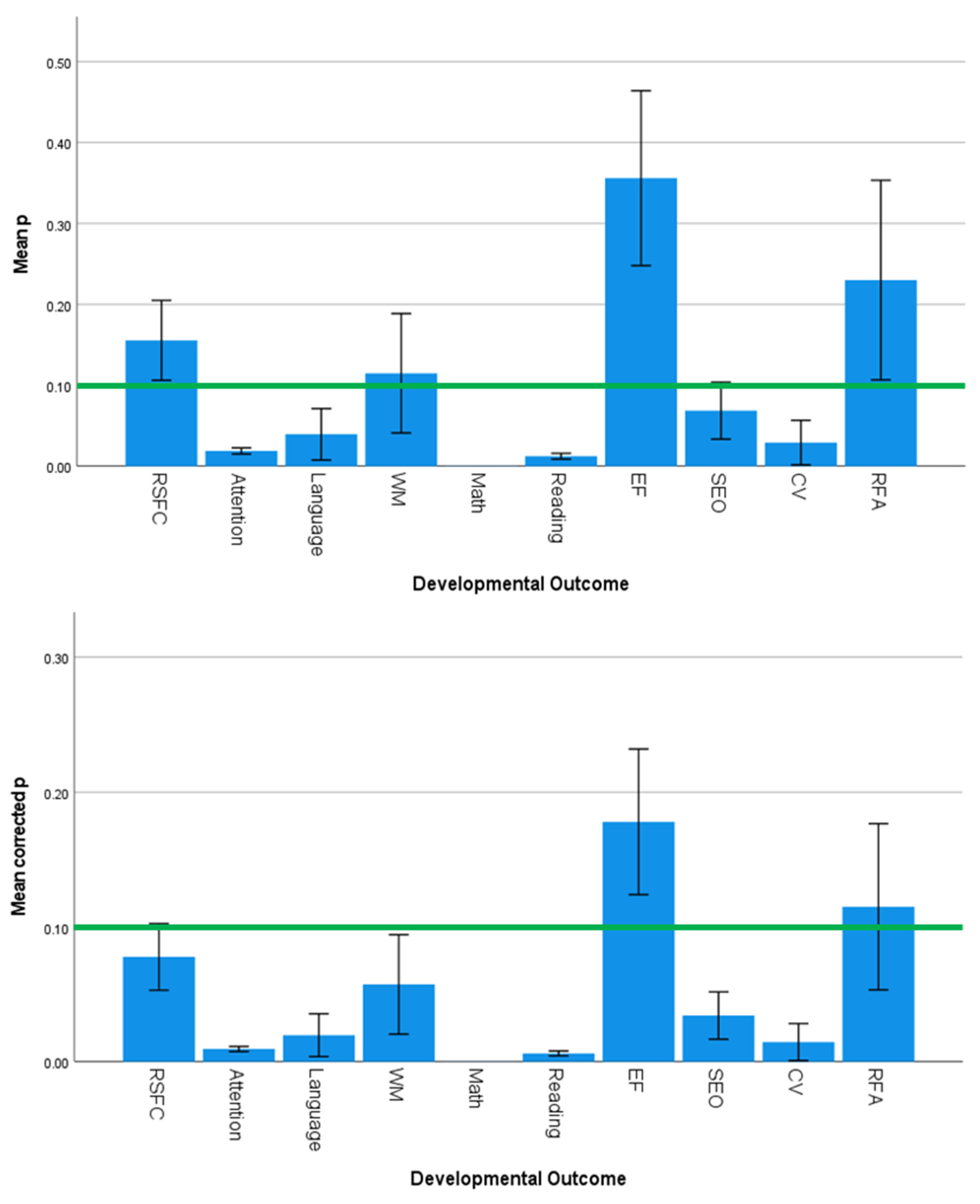
| Predictor Variables | Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean r (Unstandardized) | Mean p-Value (Two-Tailed) | Mean Zr | |||||||
| F | p | BF | F | p | BF | F | p | BF | |
| Country | 1.585 | 0.120 | 0.000 | 1.532 | 0.137 | 0.000 | 1.969 | 0.043 | 0.001 |
| Continent | 2.357 | 0.080 | 0.076 | 1.270 | 0.293 | 0.017 | 2.770 | 0.049 | 0.130 |
| Country Income Level | 0.272 | 0.763 | 0.019 | 1.385 | 0.258 | 0.055 | 0.225 | 0.799 | 0.018 |
| Developmental Outcome | 1.644 | 0.125 | 0.001 | 3.548 | 0.002 | 0.417 | 1.490 | 0.174 | 0.001 |
| Neuroimaging Technique | 3.161 | 0.013 | 0.161 | 0.335 | 0.890 | 0.000 | 2.958 | 0.019 | 0.108 |
| Predictor Variables | Outcome Variables | |||||
|---|---|---|---|---|---|---|
| Corrected Mean Zr | Corrected Mean p-Value (Two-Tailed) | |||||
| F | p | BF | F | p | BF | |
| Country | 1.571 | 0.124 | 0.000 | 1.538 | 0.135 | 0.000 |
| Continent | 2.342 | 0.082 | 0.097 | 1.316 | 0.277 | 0.008 |
| Country Income Level | 0.285 | 0.753 | 0.018 | 1.448 | 0.243 | 0.025 |
| Developmental Outcome | 1.306 | 0.255 | 0.000 | 3.080 | 0.005 | 0.107 |
| Neuroimaging Technique | 3.122 | 0.014 | 0.065 | 0.349 | 0.881 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamgang, S.; Lord, M.; Mishra, A.; D’Angiulli, A. Cognitive Neuroimaging Studies on Poverty and Socioeconomic Status Differences in Children and Families across the World: Translational Insights for Next Decade’s Policy, Health, and Education. Clin. Transl. Neurosci. 2023, 7, 24. https://doi.org/10.3390/ctn7030024
Kamgang S, Lord M, Mishra A, D’Angiulli A. Cognitive Neuroimaging Studies on Poverty and Socioeconomic Status Differences in Children and Families across the World: Translational Insights for Next Decade’s Policy, Health, and Education. Clinical and Translational Neuroscience. 2023; 7(3):24. https://doi.org/10.3390/ctn7030024
Chicago/Turabian StyleKamgang, Shanine, Meghan Lord, Aanchal Mishra, and Amedeo D’Angiulli. 2023. "Cognitive Neuroimaging Studies on Poverty and Socioeconomic Status Differences in Children and Families across the World: Translational Insights for Next Decade’s Policy, Health, and Education" Clinical and Translational Neuroscience 7, no. 3: 24. https://doi.org/10.3390/ctn7030024
APA StyleKamgang, S., Lord, M., Mishra, A., & D’Angiulli, A. (2023). Cognitive Neuroimaging Studies on Poverty and Socioeconomic Status Differences in Children and Families across the World: Translational Insights for Next Decade’s Policy, Health, and Education. Clinical and Translational Neuroscience, 7(3), 24. https://doi.org/10.3390/ctn7030024






