Unilateral Posterior Spinal Cord Ischemia Due to a Floating Aortic Thrombus: A Case Report
Abstract
1. Introduction
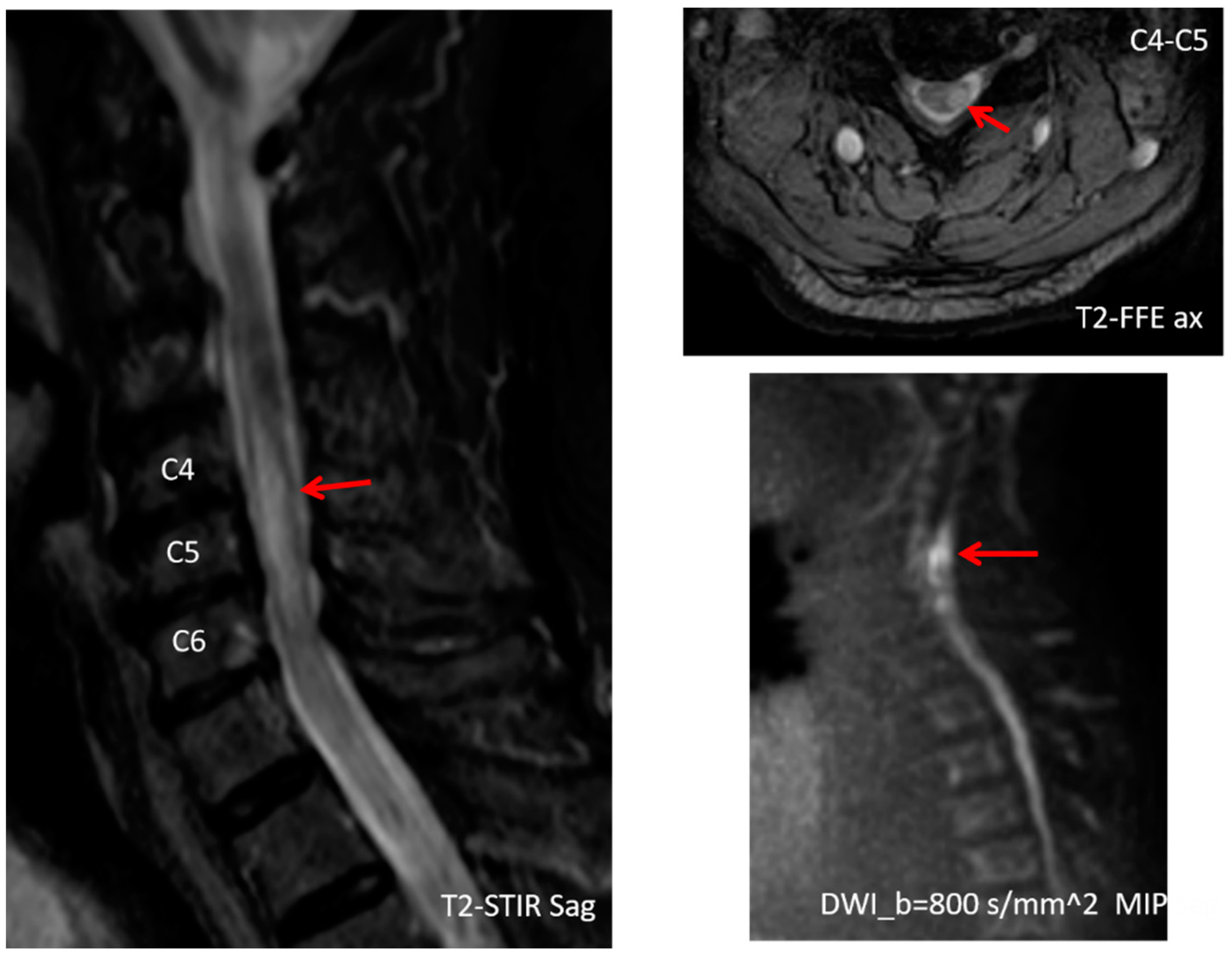
2. Case Presentation
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weidauer, S.; Nichtweiß, M.; Hattingen, E.; Berkefeld, J. Spinal cord ischemia: Aetiology, clinical syndromes and imaging features. Neuroradiology 2015, 57, 241–257. Available online: https://pubmed.ncbi.nlm.nih.gov/25398656/ (accessed on 27 September 2022). [CrossRef] [PubMed]
- Boddu, S.R.; Cianfoni, A.; Kim, K.W.; Banihashemi, M.A.; Pravatà, E.; Gobin, Y.P.; Patsalides, A. Spinal Cord Infarction and Differential Diagnosis. Neurovascular Imaging 2015, 2015, 1–64. [Google Scholar]
- Gülcü, A.; Gezer, N.S.; Men, S.; Öz, D.; Yaka, E.; Öztürk, V. Management of free-floating thrombus within the arcus aorta and supra-aortic arteries. Clin. Neurol. Neurosurg. 2014, 125, 198–206. Available online: https://pubmed.ncbi.nlm.nih.gov/25173962/ (accessed on 27 September 2022). [CrossRef] [PubMed]
- Yang, S.; Yu, J.; Zeng, W.; Yang, L.; Teng, L.; Cui, Y.; Shi, H. Aortic floating thrombus detected by computed tomography angiography incidentally: Five cases and a literature review. J. Thorac. Cardiovasc. Surg. 2017, 153, 791–803. Available online: https://pubmed.ncbi.nlm.nih.gov/28088428/ (accessed on 27 September 2022). [CrossRef] [PubMed]
- Weiss, S.; Bühlmann, R.; von Allmen, R.S.; Makaloski, V.; Carrel, T.P.; Schmidli, J.; Wyss, T.R. Management of floating thrombus in the aortic arch. J. Thorac. Cardiovasc. Surg. 2016, 152, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Noh, T.O.; Seo, P.W. Floating Thrombus in Aortic Arch. Korean J. Thorac. Cardiovasc. Surg. 2013, 46, 464. Available online: https://pmc/articles/PMC3868696/ (accessed on 14 August 2023). [CrossRef]
- Betz, R.; Biering-Sørensen, F.; Burns, S.P.; Donovan, W.; Graves, D.E.; Guest, J. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)—What’s new? Spinal Cord. 2019, 57, 815–817. Available online: https://pubmed.ncbi.nlm.nih.gov/31530900/ (accessed on 13 August 2023).
- Richard, S.; Abdallah, C.; Chanson, A.; Foscolo, S.; Baillot, P.A.; Ducrocq, X. Unilateral posterior cervical spinal cord infarction due to spontaneous vertebral artery dissection. J. Spinal Cord. Med. 2014, 37, 233–236. Available online: https://pubmed.ncbi.nlm.nih.gov/24090478/ (accessed on 27 September 2022). [CrossRef][Green Version]
- Nogueira, R.G.; Ferreira, R.; Grant, P.E.; Maier, S.E.; Koroshetz, W.J.; Gonzalez, R.G.; Sheth, K.N. Restricted Diffusion in Spinal Cord Infarction Demonstrated by Magnetic Resonance Line Scan Diffusion Imaging. Stroke 2012, 43, 532–535. [Google Scholar] [CrossRef]
- Alblas, C.L.; Bouvy, W.H.; Lycklama à Nijeholt, G.J.; Boitena, J. Acute Spinal-Cord Ischemia: Evolution of MRI Findings. J. Clin. Neurol. 2012, 8, 218. Available online: https://pmc/articles/PMC3469803/ (accessed on 28 September 2022). [CrossRef]
- Grassner, L.; Klausner, F.; Wagner, M.; McCoy, M.; Golaszewski, S.; Leis, S.; Aigner, L.; Couillard-Despres, S.; Trinka, E. Acute and chronic evolution of MRI findings in a case of posterior spinal cord ischemia. Spinal Cord. 2014, 52 (Suppl. S1), S23–S24. Available online: https://pubmed.ncbi.nlm.nih.gov/24418956/ (accessed on 28 September 2022). [CrossRef] [PubMed]
- Gondo, T.; Kurihara, M.; Sugiyama, Y.; Mano, T.; Mori, H.; Hayashi, T.; Tsuji, S. Longitudinally extensive vasogenic edema following spinal cord infarction. Neurol. Clin. Neurosci. 2018, 6, 143–145. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ncn3.12215 (accessed on 28 September 2022). [CrossRef]
- Jellema, K.; Tijssen, C.C.; Van Gijn, J. Spinal dural arteriovenous fistulas: A congestive myelopathy that initially mimics a peripheral nerve disorder. Brain 2006, 129 Pt 12, 3150–3164. Available online: https://pubmed.ncbi.nlm.nih.gov/16921175/ (accessed on 28 September 2022). [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. Available online: https://pubmed.ncbi.nlm.nih.gov/25173340/ (accessed on 27 September 2022). [PubMed]
- Gutowski, N.J.; Murphy, R.P.; Beale, D.J. Unilateral upper cervical posterior spinal artery syndrome following sneezing. J. Neurol. Neurosurg. Psychiatry 1992, 55, 841–843. [Google Scholar] [CrossRef][Green Version]
- Kaneki, M.; Inoue, K.; Shimizu, T.; Mannen, T. Infarction of the unilateral posterior horn and lateral column of the spinal cord with sparing of posterior columns: Demonstration by MRI. J. Neurol. Neurosurg. Psychiatry 1994, 57, 629–631. [Google Scholar] [CrossRef]
- Fukuda, H.; Kitani, M. [Unilateral posterior spinal artery syndrome of the upper cervical cord associated with vertebral artery occlusion]. Rinsho Shinkeigaku. 1994, 34, 1171–1174. (In Japanese) [Google Scholar]
- Izumi, Y.; Kurokawa, K.; Katayama, S.; Nakamura, S. [Monoparesis of the left leg with bilateral pyramidal signs due to posterior spinal artery syndrome]. Rinsho Shinkeigaku. 1998, 38, 686–688. (In Japanese) [Google Scholar]
- Manabe, Y.; Murase, T.; Iwatsuki, K.; Warita, H.; Hayashi, T.; Sakai, K.; Abe, K. Infarct presenting with a combination of Wallenberg and posterior spinal artery syndromes. J. Neurol. Sci. 2000, 176, 155–157. [Google Scholar] [CrossRef]
- Masson, C.; Colombani, J.M. Unilateral infarct in the posterior spinal artery territory presenting as a “clumsy hand”. J Neurol. 2002, 249, 1327–1328. [Google Scholar] [CrossRef]
- Kuga, A.; Mitani, M.; Funakawa, I.; Jinnai, K. [Posterior spinal cord infarction presenting Brown-Séquard syndrome]. Rinsho Shinkeigaku. 2005, 45, 730–734. (In Japanese) [Google Scholar] [PubMed]
- Iwanaka, Y.; Okada, K.; Tanaka, Y.; Takechi, U.; Tsuji, S. Unilateral upper cervical cord infarct restricted to the right funiculus cuneatus. Intern. Med. 2008, 47, 479. [Google Scholar] [CrossRef] [PubMed]
- Petruzzellis, M.; Fraddosio, A.; Giorelli, M.; Prontera, M.; Tinelli, A.; Lucivero, V.; Federico, F. Posterior spinal artery infarct due to patent foramen ovale: A case report. Spine (Phila. Pa. 1976) 2010, 35, E155–E158. [Google Scholar] [CrossRef]
- Struhal, W.; Seifert-Held, T.; Lahrmann, H.; Fazekas, F.; Grisold, W. Clinical core symptoms of posterior spinal artery ischemia. Eur. Neurol. 2011, 65, 183–186. [Google Scholar] [CrossRef]
- Wang, C.M.; Tsai, W.L.; Lo, Y.L.; Chen, J.Y.; Wong, A.M. Unilateral right occipital condyle to C2 level spinal cord infarction associated with ipsilateral vertebral artery stenosis and contralateral vertebral artery dissection: A case report. J. Spinal Cord. Med. 2011, 34, 118–121. [Google Scholar] [CrossRef]
- Vuillier, F.; Tatu, L.; Camara, A.; Muzard, E.; Moulin, T. Unusual sensory disturbances revealing posterior spinal artery infarct. Case Rep. Neurol. 2012, 4, 23–27. [Google Scholar] [CrossRef]
- Sakurai, T.; Wakida, K.; Nishida, H. Cervical Posterior Spinal Artery Syndrome: A Case Report and Literature Review. J. Stroke Cerebrovasc. Dis. 2016, 25, 1552–1556. [Google Scholar] [CrossRef]
- Elzamly, K.; Nobleza, C.; Parker, E.; Sugg, R. Unilateral Upper Cervical Posterior Spinal Cord Infarction after a Neuroendovascular Intervention: A Case Report. Case Rep. Neurol. Med. 2018, 2018, 5070712. [Google Scholar] [CrossRef]
- Caplan, L.R.; Chang, Y.M. Severe Unilateral Proprioceptive Loss in Medullary- Rostral Spinal Cord Infarction. A Posterior Spinal Artery Syndrome. J. Stroke Cerebrovasc. Dis. 2021, 30, 105882. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giammello, F.; Gardin, A.; Brizzi, T.; Casella, C.; Fazio, M.C.; Galletta, K.; Mormina, E.; Vinci, S.L.; Musolino, R.F.; La Spina, P.; et al. Unilateral Posterior Spinal Cord Ischemia Due to a Floating Aortic Thrombus: A Case Report. Clin. Transl. Neurosci. 2023, 7, 26. https://doi.org/10.3390/ctn7030026
Giammello F, Gardin A, Brizzi T, Casella C, Fazio MC, Galletta K, Mormina E, Vinci SL, Musolino RF, La Spina P, et al. Unilateral Posterior Spinal Cord Ischemia Due to a Floating Aortic Thrombus: A Case Report. Clinical and Translational Neuroscience. 2023; 7(3):26. https://doi.org/10.3390/ctn7030026
Chicago/Turabian StyleGiammello, Fabrizio, Anna Gardin, Teresa Brizzi, Carmela Casella, Maria Carolina Fazio, Karol Galletta, Enricomaria Mormina, Sergio Lucio Vinci, Rosa Fortunata Musolino, Paolino La Spina, and et al. 2023. "Unilateral Posterior Spinal Cord Ischemia Due to a Floating Aortic Thrombus: A Case Report" Clinical and Translational Neuroscience 7, no. 3: 26. https://doi.org/10.3390/ctn7030026
APA StyleGiammello, F., Gardin, A., Brizzi, T., Casella, C., Fazio, M. C., Galletta, K., Mormina, E., Vinci, S. L., Musolino, R. F., La Spina, P., & Toscano, A. (2023). Unilateral Posterior Spinal Cord Ischemia Due to a Floating Aortic Thrombus: A Case Report. Clinical and Translational Neuroscience, 7(3), 26. https://doi.org/10.3390/ctn7030026





