Abstract
The primary target was an investigation of the factors predicting a good clinical outcome of patients with acute ischemic stroke after treatment with mechanical thrombectomy. Additionally, we compared the treatment results in known and unknown symptom onset time groups. We retrospectively analyzed the data from 2012 to 2020 and divided 240 patients into the known and unknown symptom onset time groups. We looked for the variables predicting a good clinical outcome (NIHSS 0–4 at discharge) in both groups. In both groups, there was no statistically significant difference in good clinical outcomes (43% in the known symptom onset time group vs. 33.3% in the unknown symptom onset time group, p = 0.203). Factors predicting a good clinical outcome in both groups were lower NIHSS scores at admission, the presentation of pial arterial collaterals on admission CT angiography, and bridging intravenous thrombolysis. In the known symptom onset time group, lower age was also a factor predicting good outcome. Our clinical results of treatment by using mechanical thrombectomy were comparable in the known and unknown symptom onset time groups.
1. Introduction
The recanalization of affected vessel territory in acute ischemic stroke (AIS) patients treated with mechanical thrombectomy (MT) improves the outcome with no more complications in comparison to the intravenous administration of recombinant tissue plasminogen activator (rtPA) [1,2,3,4,5,6,7,8,9]. Lower symptom onset to vessel recanalization time can be an important factor supporting treatment success. Despite this fact, several studies have already highlighted the extension of the time window as an important indicator for treatment with MT [10,11]. However, in clinical practice, patient history, and especially symptom onset time, is often unknown. In these cases, the correct indication for MT can be complicated. Therefore, in our work, we aimed to find relevant good outcome predictors in a group of patients with known symptom onset time (KSOT) compared to those with unknown symptom onset time (USOT), particularly patients with wake-up stroke.
Patients with AIS treated with MT at the Neurosurgical and Neurointerventional Department of DONAUISAR Hospital in Deggendorf (Germany) from July 2012 to December 2020 were reviewed. We included 240 patients; 27 patients treated with the stent retriever technique using a SolitaireTM stent, 58 patients treated with the aspiration technique using a Penumbra ACE68TM aspiration catheter, and 155 patients treated with the aspiration technique using a Sophia 6FTM aspiration catheter.
MT was performed by hybrid neurosurgeons with good endovascular experience. Each of them performs approx. 30 cerebral endovascular interventions per year. The number of thrombectomies has increased every year. Since 2017, we have performed approximately 80 mechanical thrombectomies every year. Until 2015, we primarily used a stent retriever technique, particularly with SolitaireTM FR stents. In 2016, we moved stepwise to the ADAPT technique using aspiration catheters.
The baseline characteristics, good clinical outcome rates, TICI recanalization scores, and radiographic findings on Day 1 CT scans were statistically comparable between the KSOT and USOT groups of patients. In the KSOT cases, we identified lower age, good pial arterial collateral supply as evidenced by the CT scan at admission, and a lower NIHSS score at admission, but no lower symptom onset to vessel recanalization time, as predictors of a good clinical outcome in the group of treatment-independent variables. Except for the lower age, the predictors of a good clinical outcome in the USOT cases were the same. In the group of treatment-dependent variables, we identified the administration of intravenous bridging thrombolysis treatment as a factor with a positive predictive value.
2. Materials and Methods
AIS patients with identified thrombotic vessel occlusion without infarction demarcation confirmed on an admission head CT and CT angiography scan and initial NIHSS (National Institutes of Health Stroke Scale) [12] >4 were presented for treatment with MT and included in this study. AIS patients with infarct demarcation or intracerebral hemorrhage on admission CT scan, and those with an initial NIHSS score <4 were excluded. Intravenous bridging thrombolytic therapy with rtPA (0.9 mg/kg of body weight) was administered in case of no contraindications. The intervention was performed under general anesthesia. On Day 1, we performed a control head CT scan. An unbiased senior radiologist (P.K.) performed the radiographic analysis. CT angiography scans at admission were divided into groups with pial arterial collateralization (Figure 1) and without pial arterial collateralization (Figure 2), depending on the presentation of the peripheral arterial contrast filling of the affected vessel territory on the CT angiography scan upon admission. Using thrombolysis in the cerebral ischemia (TICI) [13] score, he assessed the recanalization rate. For the postinterventional CT scan analysis, we used the modified 1/3 MCA score [14]. The patients were divided into three groups: those with major infarction occupying more than 1/3 of the affected vessel territory, patients with minor infarction occupying less than 1/3 of the affected vessel territory or basal ganglia, and those without any infarction. Depending on the intracerebral hemorrhage (ICH) volume on the Day 1 head CT scan, we divided the patients into three groups: those with major ICH occupying more than 1/3 of the affected vessel territory, those with minor ICH occupying less than 1/3 of the affected vessel territory, and those without any ICH. Clinical outcome was evaluated based on the NIHSS score at discharge, with NIHSS 0–4 defined as a good outcome, as well as NIHSS improvement (NIHSS at admission minus NIHSS at discharge). In radiographic outcomes, we looked for major and minor ICH as well as major and minor infarction on Day 1 CT scan.
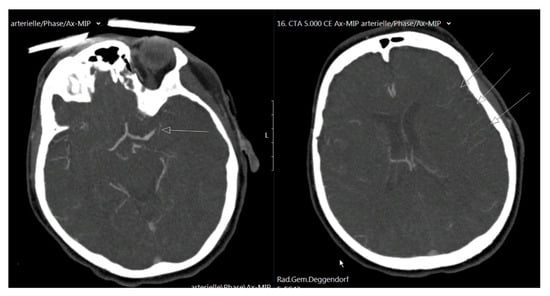
Figure 1.
On the left, a CT angiography scan is shown with left-sided proximal MCA occlusion (arrow), and on the right, peripheral vessel contrast filling is visible, with good pial arterial collaterals indicated as arrows.
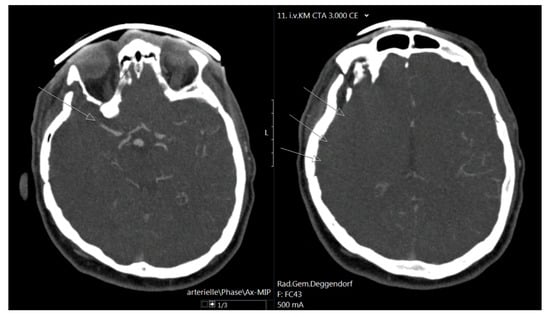
Figure 2.
On the right, a CT angiography scan is shown with right-sided MCA occlusion (arrow), and on the right, no peripheral vessel contrast filling is present, which is indicated with arrows.
SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. For differences in the means, an independent sample t-test was used for numeric variables. For nominal variables, Fisher’s exact test was used. The χ2-test was used for differences in the frequency distribution. The differences in the means and frequency distribution were statistically significant if the p value was <0.05. The results were obtained using mean values ± SD (standard deviation) and frequency tables. The variables predicting good clinical outcomes were determined using binary logistic regression and NIHSS at discharge as a dependent variable, with NIHSS 0–4 defined as a good outcome and NIHSS > 4 considered a bad outcome. The variables that were inputted in the logistic regression were divided into the treatment-dependent group and the treatment-independent group. The latter was calculated separately for the KSOT and USOT cases.
3. Results
3.1. Baseline Characteristics
In total, 240 patients with AIS who underwent MT were included. A SolitaireTM stent retriever (n = 27, 11.3%), Penumbra ACE68TM aspiration catheter (n = 58, 24.2%), and SophiaTM 6F aspiration catheter were used. The mean age was 72 ± 14 years, and the mean NIHSS score on admission was 19 ± 9. USOT was present in 54 (22.5%) cases. In the KSOT group, the mean symptom onset time to groin puncture time was 223 ± 116 min. Notably, 134 (55.8%) of the cases involved female patients, and bridging intravenous rtPA was administered in 150 (62.5%) cases. The thrombectomy system used, population characteristics, and the affected vessel territory are summarized in Table 1.

Table 1.
The thrombectomy system, population characteristics, and affected vessel territory.
3.2. KSOT and USOT Groups—Baseline Characteristics, Clinical Outcomes, and Radiographic Outcomes
Both groups were statistically comparable in terms of the mean age (72 ± 14 vs. 65 ± 13 years (p = 0.241)), sex (p = 0.231) and the mean NIHSS score on admission (19 ± 9 vs. 21 ± 10 (p = 0.307)). Bridging intravenous rtPA was administered in 66.7% of cases in the KSOT group and in 48.1% of cases in the USOT group (p = 0.013).
There was no difference in the mean NIHSS score at discharge between the groups; in the KSOT group, the mean NIHSS score was 14 ± 15, and in the USOT group, the mean NIHSS score was 17 ± 16 (p = 0.194). The mean NIHSS score improvement was 9 ± 9 in the KSOT group and 7 ± 8 in the USOT group (p = 0.082). Good clinical outcomes (NIHSS 0–4 at discharge) and the number needed to treat were 43% of cases and 2.3 in the KSOT group and 33.3% of cases and 3 in the USOT group (p = 0.203).
The frequency distribution of TICI recanalization scores was comparable in both groups (Fisher´s exact test, p = 0.516). We identified the TICI 3 recanalization in 166 cases (89.2%) in the KSOT group and in 46 cases (85.2%) in the USOT group. There was no significant difference in frequency distribution (χ2 = 3.077, p = 0.215) in the KSOT and USOT groups in terms of infarct demarcation on the Day 1 head CT scan. In the analysis of the ICH on the 24 h head CT scans, we did not find any difference in frequency distribution (χ2 = 2.652, p = 0.266) in the KSOT and USOT groups either. The results are presented in Table 2.

Table 2.
Known and unknown symptom onset time groups—baseline characteristics, clinical outcomes, and radiographic outcomes.
3.3. The Predicting Factors of the Clinical Outcome
The binary logistic regression model of treatment-independent variables was significant for KSOT cases (χ2 = 33.19, p = 0.001, n = 186). We identified age (OR 0.976, p = 0.043, all other things being equal), lower NIHSS score upon admission (OR 0.941, p = 0.004, all other things being equal), and the presentation of the pial arterial collateralization on CT angiography scan upon admission (OR 3.335, p = 0.002, all other things being equal) as the variables predicting a good clinical outcome (Table 3).

Table 3.
Factors predicting a good clinical outcome (NIHSS 0–4): binary logistic regression with treatment-independent variables, KSOT cases.
The model with treatment-independent variables using the USOT cases was significant as well (χ2 = 20.72, p = 0.001, n = 54). The presentation of the pial arterial collateralization on the CT angiography scan upon admission (OR 6.330, p = 0.041, all other things being equal) and lower NIHSS scores upon admission (OR 0.888, p = 0.027, all other things being equal) were the factors predicting a good clinical outcome (Table 4).

Table 4.
Factors predicting a good clinical outcome (NIHSS 0–4): binary logistic regression with treatment-independent variables, USOT cases.
The logistic regression model of treatment-dependent variables was significant (χ2 = 82.66, p < 0.001, n = 240). Intravenous rtPA administration (OR 2.077, p = 0.02, all other things being equal) was identified as a factor predicting a good clinical outcome. Cases with major infarction (>1/3 of vessel territory) on the Day 1 head CT scan had 25 times less chance for a good clinical outcome (OR 0.040, p < 0.001, all other things being equal). Major ICH was associated with six times less chance for a good clinical outcome (OR 0.167, p < 0.030, all other things being equal) (Table 5).

Table 5.
Factors predicting a good clinical outcome (NIHSS 0–4): binary logistic regression with treatment-dependent variables.
4. Discussion
According to our results, the presentation of the pial arterial collateralization on the CT angiography scan upon admission, as well as the lower admission NIHSS and bridging intravenous rtPA administration, were the factors predicting a good clinical outcome. Large ICH and infarction on the control CT scan had a negative predictive value. The clinical and radiographic outcomes of the treatment with MT in the KSOT and USOT groups were statistically comparable; however, overall, we observed slightly better results in the KSOT group of patients.
The frequency of the bridging intravenous rtPA administration was lower in the USOT group compared with the KSOT group (48.1% vs. 66.7%). This was to be expected and resulted from the limited off-label use of bridging intravenous rtPA in the USOT group. The remaining characteristics were comparable.
The clinical outcome measured according to the NIHSS at discharge, as well as improvement in the NIHSS score, showed no difference between both groups. Clinical outcomes of MT correspond with findings of other trials [7,9,15,16].
We observed no differences between the groups in the TICI 3 recanalization rate, which corresponds to published data [7,17] and the frequency of major infarct demarcation and major ICH on 24 h CT scans. The data in the literature differ in the incidence of infarction and bleeding complications [15,17,18,19,20].
The lower NIHSS at admission in both groups favors a good clinical outcome as well. The same finding is reported by Barral et al. [21], Gamba et al. [22], and Lu et al. [23].
In both KSOT and USOT groups, we identified the presentation of the pial arterial collateralization in the CT angiography scan at admission as the factor predicting a good clinical outcome. The presentation of the pial arterial collaterals on the CT angiography scan at admission in cases of successful vessel recanalization could be crucial for a good clinical outcome [24,25,26,27,28,29].
According to Rabinstein, perfusion brain imaging is necessary in patients with an extended time window (<6 h) prior to recanalization treatment [27]. In our opinion, the presentation of pial arterial collaterals on CT angiography at admission is sufficient for the estimation of collateral perfusion of the affected territory. That could help physicians make correct decisions for treatment with MT in patients with USOT, and it requires no additional time-consuming investigation like perfusion CT or MRI. At the same time, successful MT in patients with good pial arterial collaterals may result in a good functional outcome even with longer symptom onset to recanalization time and vice versa. In this case, the careful selection of patients for treatment with MT, especially those without infarction demarcation, is crucial. Infarct demarcation on CT scans at admission in cases with longer or unknown symptom onset to recanalization time corresponds with poor collateralization and automatically leads to disqualification for treatment with MT, or the MT has a poor clinical effect.
Could this mean that the time window in AIS patients is not a relevant prognostic criterion? This opinion could support the fact that, in this study, the time from symptom onset to groin puncture in the KSOT group had no prediction value regarding the good functional outcome. An analogous finding is presented in a paper published by Krajickova et al. In their study, neither the recanalization success nor the clinical outcome was significantly different between patients treated at different time windows [30]. At the same time, 2017 published trials successfully extended time windows up to 16 [10] and 24 h [11]. The restriction of cerebral perfusion on 18 mL/100 g of brain tissue per minute leads to the failure of neuronal excitability, but not neuronal death, which does not occur until perfusion restriction under 8 mL/100 g of brain tissue [31]. This then leads to the loss of 1.9 million neurons per minute in AIS patients [32]. In our opinion, the time window is an important prognostic factor, but even more relevant for a good clinical outcome is a sufficient collateralization of the affected vessel territory.
Expectedly, in the KSOT group, higher age reduced the odds of a good clinical outcome as well, whereas, in the USOT group, age had no predictive value.
In the treatment-dependent variable, the intravenous thrombolysis prior to MT using rtPA was the factor predicting a good clinical outcome. This finding correlates with data from two meta-analyses from 2017 and 2020 [33,34]. Both concluded that the additional intravenous thrombolysis treatment improves functional outcomes and reduces mortality compared with MT alone. As expected, major infarction and large ICH on the control CT scan reduced the chance of a good clinical outcome.
This study aimed to evaluate the predictive factors for a good functional outcome, considering both KSOT and USOT patients. This could be helpful in certain clinical situations, especially in cases of insufficient patient history. We acknowledge the limitations of this clinical study. These limitations are due to the retrospective nature of this research with the corresponding biases that can be associated with the study design and its monocentric nature. Our results should therefore be reviewed in the context of further studies.
5. Conclusions
The presentation of pial arterial collaterals on CT angiography scan at admission and lower NIHSS score at admission could be the treatment-independent factors predicting a good functional outcome in the case of successful treatment using MC in patients with AIS, even in cases of unknown symptom onset time. Additional bridging intravenous thrombolysis can also increase the odds of good clinical outcome. The clinical results of MT in the KSOT and USOT groups of patients were found to be comparable. Symptom onset to vessel recanalization time does not seem to be a main factor influencing a good functional outcome. This does not mean that the phrase “time is brain” is no longer valid; rather, it is more the case that we should say “time and collateralization is brain”. Time alone is not enough.
Author Contributions
Conceptualization, D.S. and M.M.; methodology, D.S., M.V. and M.M.; software, M.V. and D.S.; formal analysis, M.M. and P.K.; writing—original draft preparation, D.S.; writing—review and editing, F.C. and S.R.; supervision, S.R. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study due to the retrospective nature of this study. Ethical board approval was not mandatory according to local legislation (Ethics Committee of the Bavarian Medical Association).
Informed Consent Statement
Patient consent was waived due to the retrospective type of study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albers, G.W.; Goyal, M.; Jahan, R.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Cognard, C.; Yavagal, D.R.; Saver, J.L. Relationships Between Imaging Assessments and Outcomes in Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke. Stroke 2015, 46, 2786–2794. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Roman, L.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Lapergue, B.; Blanc, R.; Gory, B.; Labreuche, J.; Duhamel, A.; Marnat, G.; Saleme, S.; Costalat, V.; Bracard, S.; Desal, H.; et al. Effect of Endovascular Contact Aspiration vs Stent Retriever on Revascularization in Patients With Acute Ischemic Stroke and Large Vessel Occlusion: The ASTER Randomized Clinical Trial. JAMA 2017, 318, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef]
- Turk, A.S., 3rd; Siddiqui, A.; Fifi, J.T.; De Leacy, R.A.; Fiorella, D.J.; Gu, E.; Levy, E.I.; Snyder, K.V.; Hanel, R.A.; Aghaebrahim, A.; et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): A multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet 2019, 393, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Lazzaro, M.A.; Liebeskind, D.S.; Janjua, N.; Wechsler, L.; Nogueira, R.G.; Edgell, R.C.; Kalia, J.S.; Badruddin, A.; English, J.; et al. Revascularization grading in endovascular acute ischemic stroke therapy. Neurology 2012, 79, S110–S116. [Google Scholar] [CrossRef]
- Mak, H.K.; Yau, K.K.; Khong, P.L.; Ching, A.S.; Cheng, P.W.; Au-Yeung, P.K.; Pang, P.K.; Wong, K.C.; Chan, B.P.; Alberta Stroke Programme Early, C.T.S. Hypodensity of >1/3 middle cerebral artery territory versus Alberta Stroke Programme Early CT Score (ASPECTS): Comparison of two methods of quantitative evaluation of early CT changes in hyperacute ischemic stroke in the community setting. Stroke 2003, 34, 1194–1196. [Google Scholar] [CrossRef]
- Primiani, C.T.; Vicente, A.C.; Brannick, M.T.; Turk, A.S.; Mocco, J.; Levy, E.I.; Siddiqui, A.H.; Mokin, M. Direct Aspiration versus Stent Retriever Thrombectomy for Acute Stroke: A Systematic Review and Meta-Analysis in 9127 Patients. J. Stroke Cerebrovasc. Dis. 2019, 28, 1329–1337. [Google Scholar] [CrossRef]
- Stapleton, C.J.; Leslie-Mazwi, T.M.; Torok, C.M.; Hakimelahi, R.; Hirsch, J.A.; Yoo, A.J.; Rabinov, J.D.; Patel, A.B. A direct aspiration first-pass technique vs stentriever thrombectomy in emergent large vessel intracranial occlusions. J. Neurosurg. 2018, 128, 567–574. [Google Scholar] [CrossRef]
- Turk, A.S.; Frei, D.; Fiorella, D.; Mocco, J.; Baxter, B.; Siddiqui, A.; Spiotta, A.; Mokin, M.; Dewan, M.; Quarfordt, S.; et al. ADAPT FAST study: A direct aspiration first pass technique for acute stroke thrombectomy. J. Neurointerv. Surg. 2018, 10, i4–i7. [Google Scholar] [CrossRef] [PubMed]
- Harsany, J.; Haring, J.; Hoferica, M.; Mako, M.; Janega, P.; Krastev, G.; Klepanec, A. Aspiration thrombectomy as the first-line treatment of M2 occlusions. Interv. Neuroradiol. 2020, 26, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wang, C.; Buell, T.J.; Ding, D.; Raper, D.M.; Ironside, N.; Paisan, G.M.; Starke, R.M.; Southerland, A.M.; Liu, K.; et al. Endovascular Mechanical Thrombectomy for Acute Middle Cerebral Artery M2 Segment Occlusion: A Systematic Review. World Neurosurg. 2017, 107, 684–691. [Google Scholar] [CrossRef]
- Saber, H.; Narayanan, S.; Palla, M.; Saver, J.L.; Nogueira, R.G.; Yoo, A.J.; Sheth, S.A. Mechanical thrombectomy for acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery: A meta-analysis. J. Neurointerv. Surg. 2018, 10, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.; Lassalle, L.; Dargazanli, C.; Mazighi, M.; Redjem, H.; Blanc, R.; Rodesch, G.; Lapergue, B.; Piotin, M. Predictors of favorable outcome after mechanical thrombectomy for anterior circulation acute ischemic stroke in octogenarians. J. Neuroradiol. 2018, 45, 211–216. [Google Scholar] [CrossRef]
- Gamba, M.; Gilberti, N.; Premi, E.; Costa, A.; Frigerio, M.; Mardighian, D.; Vergani, V.; Spezi, R.; Delrio, I.; Morotti, A.; et al. Intravenous fibrinolysis plus endovascular thrombectomy versus direct endovascular thrombectomy for anterior circulation acute ischemic stroke: Clinical and infarct volume results. BMC Neurol. 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Young, C.C.; Chen, S.H.; O’Connor, K.P.; Silva, M.A.; Starke, R.M. Presenting NIHSS predicts 90-day functional outcome after mechanical thrombectomy for basilar artery occlusion: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2020, 197, 106199. [Google Scholar] [CrossRef]
- Christoforidis, G.A.; Mohammad, Y.; Kehagias, D.; Avutu, B.; Slivka, A.P. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am. J. Neuroradiol. 2005, 26, 1789–1797. [Google Scholar] [PubMed]
- Liebeskind, D.S.; Jahan, R.; Nogueira, R.G.; Zaidat, O.O.; Saver, J.L.; Investigators, S. Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke 2014, 45, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A. Treatment of Acute Ischemic Stroke. Contin. Lifelong Learn. Neurol. 2017, 23, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A. Update on Treatment of Acute Ischemic Stroke. Contin. Lifelong Learn. Neurol. 2020, 26, 268–286. [Google Scholar] [CrossRef]
- Sila, D.; Lenski, M.; Vojtkova, M.; Elgharbawy, M.; Charvat, F.; Rath, S. Efficacy of Mechanical Thrombectomy using Penumbra ACE(TM) Aspiration Catheter Compared to Stent Retriever Solitaire(TM) FR in Patients with Acute Ischemic Stroke. Brain Sci. 2021, 11, 504. [Google Scholar] [CrossRef]
- Woo, H.G.; Jung, C.; Sunwoo, L.; Bae, Y.J.; Choi, B.S.; Kim, J.H.; Kim, B.J.; Han, M.K.; Bae, H.J.; Jung, S.; et al. Dichotomizing Level of Pial Collaterals on Multiphase CT Angiography for Endovascular Treatment in Acute Ischemic Stroke: Should It Be Refined for 6-Hour Time Window? Neurointervention 2019, 14, 99–106. [Google Scholar] [CrossRef]
- Krajickova, D.; Krajina, A.; Herzig, R.; Lojik, M.; Chovanec, V.; Raupach, J.; Vitkova, E.; Waishaupt, J.; Vysata, O.; Valis, M. Mechanical recanalization in ischemic anterior circulation stroke within an 8-hour time window: A real-world experience. Diagn. Interv. Radiol 2017, 23, 465–471. [Google Scholar] [CrossRef]
- Daroff, R.B.; Jankovic, J.; Mazziotta, J.C.; Pomeroy, S.L. Bradley’s Neurology in Clinical Practice; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2. [Google Scholar]
- Saver, J.L. Time is brain—Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Mistry, E.A.; Mistry, A.M.; Nakawah, M.O.; Chitale, R.V.; James, R.F.; Volpi, J.J.; Fusco, M.R. Mechanical Thrombectomy Outcomes With and Without Intravenous Thrombolysis in Stroke Patients: A Meta-Analysis. Stroke 2017, 48, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Vidale, S.; Romoli, M.; Consoli, D.; Agostoni, E.C. Bridging versus Direct Mechanical Thrombectomy in Acute Ischemic Stroke: A Subgroup Pooled Meta-Analysis for Time of Intervention, Eligibility, and Study Design. Cerebrovasc. Dis. 2020, 49, 223–232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).