Effect of Hydration on Pulmonary Function and Development of Exercise-Induced Bronchoconstriction among Professional Male Cyclists
Abstract
:Highlights
- Exercise-induced bronchoconstriction (EIB) is a common problem in elite athletes. This study aimed to investigate the effects of systemic hydration on pulmonary function and to establish whether it can reverse dehydration-induced alterations in pulmonary function.
- Systemic hydration had a positive effect on both pulmonary function and exercise capacity (VO2 max).
- Hydration potentially plays a regulatory role in stabilizing the airway in elite athletes, protecting them from airway hyper-responsiveness.
- Of particular interest are the small airways, which appear to be affected independently or in combination with a decrease in FEV1 and proper hydration can protect them from further injury.
Abstract
1. Introduction
1.1. Exercise-Induced Bronchoconstriction
1.2. Hydration and Pulmonary Function
1.3. Cycling
2. Materials and Methods
2.1. Procedures
2.2. Spirometry
2.3. Airway Inflammation
2.4. Body Hydration Status
2.5. Anthropometric Characteristics
2.6. Systemic Hydration Protocol
2.7. Cardiopulmonary Exercise Test (CPET)
2.8. Statistical Analysis
3. Results
3.1. Phase A of the Study (before Hydration)
3.2. Phase B of the Study (Hydration Prior to Exercise)
4. Discussion
4.1. Effects of Exercise on Pulmonary Function
4.2. Effects of Hydration on Pulmonary Function
4.3. Effects of Hydration on Exercise Capacity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiler, J.M.; Bonini, S.; Coifman, R.; Craig, T.; Delgado, L.; Capao-Filipe, M.; Passali, D.; Randolph, C.; Storms, W.; Ad Hoc Committee of Sports Medicine Committee of American Academy of Allergy, Asthma & Immunology. American Academy of Allergy, Asthma & Immunology Work Group report: Exercise-induced asthma. J. Allergy Clin. Immunol. 2007, 119, 1349–1358. [Google Scholar] [PubMed]
- Pigakis, K.M.; Stavrou, V.T.; Pantazopoulos, I.; Daniil, Z.; Kontopodi, A.K.; Gourgoulianis, K.; Kontopodi, A. Exercise-Induced Bronchospasm in Elite Athletes. Cureus 2022, 14, e20898. [Google Scholar] [CrossRef] [PubMed]
- Holzer, K.; Douglass, J.A. Exercise-Induced bronchoconstriction in elite athletes: Measuring the fall. Thorax 2006, 61, 94–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiwe, J.; Cooke, A.; Nanda, A.; Epstein, S.Z.; Wasan, A.N.; Shepard, K.V., 2nd; Capao-Filipe, M.; Nish, A.; Rubin, M.; Gregory, K.L.; et al. Work Group Report: Perspectives in Diagnosis and Management of Exercise-Induced Bronchoconstriction in Athletes. J. Allergy Clin. Immunol. Pr. 2020, 8, 2542–2555. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.S.; Buston, M.H.; Wharton, M.J. The effect of exercise on ventilatory function in the child with asthma. Br. J. Dis. Chest 1962, 56, 78–86. [Google Scholar] [CrossRef]
- Couto, M.; Kurowski, M.; Moreira, A.; Bullens, D.M.A.; Carlsen, K.H.; Delgodo, L.; Kowalski, M.L.; Seys, S.F. Mechanism of exercise-induced bronchoconstriction in athletes: Current perspectives and future challenges. Allergy 2018, 73, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, K.H.; Anderson, S.D.; Bjermer, L.; Bonini, S.; Brusasco, V.; Canonica, W.; Cummiskey, J.; Delgado, L.; Del Giacco, S.R.; Drobnic, F.; et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: Epidemiology, mechanisms, and diagnosis: Part I of the report from the joint task force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy 2008, 63, 387–403. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.P.; Hallstrand, T.S.; Mastronarde, J.G.; Kaminsky, D.A.; Rundel, K.W.; Hull, J.H.; Storms, W.W.; Welier, J.M.; Cheek, F.M.; Wilson, K.C.; et al. An Official American Thoracic Society Clinical Practice Guideline: Exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2013, 197, 1016–1027. [Google Scholar] [CrossRef]
- Boulet, L.P.; O’Byrne, P.M. Asthma and exercise-induced bronchoconstriction in athletes. N. Engl. J. Med. 2015, 372, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Price, O.J.; Ansley, L.; Menzies-Gow, A.; Cullinan, P.; Hull, J.H. Airway dysfunction in elite athletes—An occupational lung disease? Allergy 2013, 68, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Bonini, M.; Palange, P. Exercise-induced bronchoconstriction: New evidence in pathogenesis, diagnosis, and treatment. Asthma Res. Pract. 2015, 1, 2. [Google Scholar] [CrossRef]
- Fitch, K.D. An overview of asthma and airway hyperresponsiveness on Olympic athletes. Br. J. Sport. Med. 2012, 46, 413–416. [Google Scholar] [CrossRef]
- Hallstrand, T.S.; Moody, M.W.; Wurfel, M.M.; Schwartz, L.B.; Henderson, W.R., Jr.; Aitken, M.L. Inflammatory basis of exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2005, 172, 679–686. [Google Scholar] [CrossRef]
- Anderson, S.D.; Holzer, K. Exercise-induced asthma: Is the right diagnosis in elite athletes. J. Allergy Clin. Immunol. 2000, 106, 419–428. [Google Scholar] [CrossRef]
- Kippelen, P.; Anderson, S.D. Airway injury during high–level exercise. Br. J. Sport. Med. 2012, 46, 385–390. [Google Scholar] [CrossRef]
- Zarqa, A.; Norsk, P.; Ulrik, C.S. Mechanism and Management of Exercise Induced Asthma in Elite Athletes. J. Asthma 2012, 49, 480–486. [Google Scholar]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, J.S.; Stachenfeld, N.S. Exercise and fluid replacement. Med. Sci. Sport. Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, assessment, and performance effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Govindaraj, M. The effect of dehydration on the ventilatory capacity in normal subjects. Am. Rev. Respir. Dis. 1972, 105, 842–844. [Google Scholar] [CrossRef]
- Simpson, A.J.; Romer, L.M.; Kippelen, P. Exercise-induced dehydration alters pulmonary function but does not modify airway responsiveness to dry air in athletes with mild asthma. J. Appl. Physiol. 2017, 122, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Bosken, C.H.; Lim, S.P.; Dohn, M.N.; Greene, N.B.; Baughman, R.P. Effects of hypohydration on lung functions in humans. Am. Rev. Respir. Dis. 1987, 135, 597–599. [Google Scholar] [PubMed]
- Baile, E.M. The anatomy and physiology of the bronchial circulation. J. Aerosol. Med. 1996, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Boucher, R.C. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003, 445, 495–498. [Google Scholar] [CrossRef]
- Anderson, S.D.; Daviskas, E. Pathophysiology of exercise-induced asthma: Role of respiratory water loss. In Allergic and Respiratory Disease in Sports Medicine; Weiler, J., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 87–114. [Google Scholar]
- Macklem, P.T.; Proctor, D.F.; Hogg, J.C. The stability of peripheral airways. Respir. Physiol. 1970, 8, 191–203. [Google Scholar] [CrossRef]
- Allen, H.; Price, O.J.; Greenwell, J.; Hull, J.H. Respiratory impact of a grand tour: Insight from professional cycling. Eur. J. Appl. Physiol. 2021, 121, 1027–1036. [Google Scholar] [CrossRef]
- Bell, P.G.; Furber, M.J.; Van Someren, K.A.; Anton-Solanas, A.; Swart, J. The Physiological Profile of a Multiple Tour de France Winning Cyclist. Med. Sci. Sport. Exerc. 2017, 49, 115–123. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Katayama, K.; Vermeulen, T.D.; Stuckless, T.J.R.; Brown, C.V.; Foster, G.E.; Shell, A.W. Work of breathing influences muscle sympathetic nerve activity during semi-recumbent cycle exercise. Acta Physiol. 2019, 225, e13212. [Google Scholar] [CrossRef]
- Allen, J.R.; Satiroglu, R.; Fico, B.; Tanaka, H.; Vardarli, E.; Luci, J.; Coyle, E.F. Inertial Load Power Cycling Training Increases Muscle Mass and Aerobic Power in Older Adults. Med. Sci. Sport. Exerc. 2021, 53, 1188–1193. [Google Scholar] [CrossRef]
- Lucia, A.; Hoyos, J.; Pardo, J.; Chicharro, J.L. Regular Papers Metabolic and Neuromuscular Adaptations to Endurance Training in Professional Cyclists: A Longitudinal Study. J. Physiol. 2000, 50, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, J.W.; Whyte, G.P.; McConnell, A.K.; Harries, M.G. Impact of changes in the IOC-MC asthma criteria: A British perspective. Thorax 2005, 60, 629–632. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, J.W.; Whyte, G.P.; McConnell, A.K.; Nevill, A.M.; Harries, M.G. Mid-expiratory flow versus FEV1 measurements in the diagnosis of exercise-induced asthma in elite athletes. Thorax 2006, 61, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Stensrud, T.; Rossvoll, Ø.; Mathiassen, M.; Melau, J.; Illidi, C.; Ostgaard, H.N.; Hisdal, J.; Stang, J. Lung function and oxygen saturation after participation in Norseman Xtreme Triathlon. Scand. J. Med. Sci. Sport. 2020, 30, 1008–1016. [Google Scholar] [CrossRef]
- Kwi, B.-K.; Kwak, Y.-S. Dehydration affects exercise-induced asthma and anaphylaxis. J. Exerc. Rehabil. 2019, 15, 647–650. [Google Scholar]
- Norton, K.; Olds, T. Morphological evolution of athletes over the 20th century: Causes and consequences. Sport. Med. 2001, 31, 763–783. [Google Scholar] [CrossRef]
- Norton, k.; Olds, T.; Olive, S.; Craig, N.P. Measurement techniques in anthropometry. In Anthropometrica; Norton, K., Olds, T., Eds.; University of New South Wales Press: Sydney, Australia, 1996; pp. 44–53. [Google Scholar]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and the stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Halstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.; Sue, D.; Stringer, W.; Whipp, B. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Application, 4th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Bonini, M.; Di Paolo, M.; Bagnasco, D.; Balardini, I.; Braido, F.; Caminati, M.; Capragnana, E.; Contoli, M.; Corsico, A.; Del Giacco, S. Minimal clinically important difference for asthma endpoints: An expert consensus report. Eur. Respir. Rev. 2020, 29, 190137. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Ivrin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykkon, G.I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Chicago, IL, USA, 1988. [Google Scholar]
- Evans, G.H.; James, L.J.; Shirreffs, S.M.; Maughan, R. Optimizing the restoration and maintenance of fluid balance after exercise-induced dehydration. J. Appl. Physiol. 2017, 122, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Heart 2007, 93, 1285–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavorsky, G.S.; Zimmerman, R.D.; Shendell, D.G.; Goodfellow, L.T. Acute Reduction in Spirometry Values After Prolonged Exercise Among Recreational Runners. Respir. Care 2019, 64, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bekos, C.; Zimmermann, M.; Unger, L.; Janik, S.; Mitterbauer, A.; Koller, M.; Fritz, R.; Gabler, C.; Didcock, J.; Kliman, J. Exercise-induced bronchoconstriction, temperature regulation and the role of heat shock proteins in non-asthmatic recreational marathon and half-marathon runners. Sci. Rep. 2019, 9, 4168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helenius, I.J.; Tikkanen, H.O.; Haahtela, T. Occurrence of exercise-induced bronchospasm in elite runners: Dependence on atopy and exposure to cold air and pollen. Br. J. Sport. Med. 1998, 32, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Cheuvront, S.N.; Kenefick, R.W.; Charkoudian, N.; Sawka, M.N. Physiologic basis for understanding quantitative dehydration assessment. Am. J. Clin. Nutr. 2013, 97, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Marshall, H.; Gibson, O.R.; Romer, L.M.; Illidi, C.; Hull, J.H.; Kippelen, P. Systemic but not local rehydration restores dehydration-induced changes in pulmonary function in healthy adults. J. Appl. Physiol. 2021, 130, 517–527. [Google Scholar] [CrossRef]
- Pogson, Z.E.; McKeever, T.M.; Fogarty, A. The association between serum osmolality and lung function among adults. Eur. Respir. J. 2008, 32, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Du, S.M.; Zhang, J.F.; Ma, G.S. Effects of Dehydration and Rehydration on Cognitive Performance and Mood among Male College Students in Cangzhou, China: A Self-Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 1891. [Google Scholar] [CrossRef] [Green Version]
- McKechnie, J.K.; Leary, W.P.; Noakes, T.D.; Kallmeyer, J.C.; MacSearraigh, E.T.; Olivier, L.R. Acute pulmonary edema in two athletes during a 90-km running race. S. Afr. Med. J. 1979, 56, 261–265. [Google Scholar]
- Rowlands, D.S.; Kopetschny, B.H.; Badenhorst, C.E. The Hydrating Effects of Hypertonic, Isotonic, and Hypotonic Sports Drinks and Waters on Central Hydration During Continuous Exercise: A Systematic Meta-Analysis and Perspective. Sport. Med. 2022, 52, 349–375. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casubari, R.; Coates, A. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [Green Version]
- Ciprandi, G.; Cirillo, I.; Klersy, C.; Barnes, P.; Palange, P.; Usmani, O. Role of FEF25-75 as an early marker of bronchial impairment in patients with seasonal allergic rhinitis. Am. J. Rhinol. 2006, 20, 641–647. [Google Scholar] [CrossRef]
- Bonini, M.; James, H.; Meah, S.; Barnes, P.; Palange, P.; Usmani, O. Small airways and exercise-induced bronchoconstriction (EIB) in elite athletes. Eur. Respir. J. 2014, 44, P2117. [Google Scholar]
- Couto, M.; Moreira, A. The athlete “out of breath”. Eur. Ann. Allergy Clin. Immunol. 2016, 48, 36–45. [Google Scholar]
- Racinais, S.; Ihsan, M.; Taylor, L.; Cardinale, M.; Adami, P.E.; Alonso, J.M.; Bouscaren, N.; Buitrago, S.; Esh, C.J.; Gomez-Ezeira, J. Hydration and cooling in elite athletes: Relationship with performance, body max loss and body temperatures during the Doha 2019 IAAF World Athletics Championships. Sport. Med. 2021, 55, 1335–1341. [Google Scholar] [CrossRef]
- Greenhaff, P.L. Cardiovascular fitness and thermoregulation during prolonged exercise in man. Br. J. Sport. Med. 1989, 23, 109–114. [Google Scholar] [CrossRef]
- Watso, J.; Farquhar, W. Hydration status and cardiovascular function. Nutrients 2019, 11, 1866. [Google Scholar] [CrossRef] [Green Version]
- Schierbauer, J.; Hoffmeister, T.; Treff, G.; Wachsmuth, N.B.; Schimidt, W.F. Effect of Exercise-Induced Reductions in Blood Volume on Cardiac Output and Oxygen Transport Capacity. Front. Physiol. 2021, 12, 679232. [Google Scholar] [CrossRef]
- Tikhomirova, I. The effect of dehydration on macro- and microrheological blood properties. Clin. Hemorheol. Microcirc. 2002, 26, 85–90. [Google Scholar]
- Young, H.; Cousins, A.; Johnston, S.; Fletcher, J.M.; Benton, D. Autonomic adaptations mediate the effect of hydration on brain functioning and mood: Evidence from two randomized controlled trials. Sci. Rep. 2019, 9, 16412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charkoudian, N.; Halliwill, J.R.; Morgan, B.J.; Eisenach, J.H.; Joyner, M.J. Influences of hydration on post-exercise cardiovascular control in humans. J. Physiol. 2003, 552 Pt 2, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Fehling, P.; Holler, J.; Lefferts, W.; Hultquist, E.M.; Wharton, M.; Rowland, T.M.; Smith, D.L.l. Effect of exercise, heat stress and dehydration on myocardial performance. Occup. Med. 2015, 65, 317–323. [Google Scholar] [CrossRef] [Green Version]

| Mean ± SD | m (IQR) | Min–Max | ||
|---|---|---|---|---|
| Age | years | 27.0 ± 5.0 | 30 (22–33) | 18–34 |
| Training age | years | 12.0 ± 5.0 | 14 (7–17) | 3–19 |
| Height | cm | 177 ± 5 | 178 (174–182) | 167–187 |
| Body mass | kg | 74.7 ± 5.2 | 74.6 (72–78) | 64–85 |
| BMI | kg/m2 | 23.8 ± 1.4 | 23.6 (22.6–24.4) | 21.5–26.8 |
| Body fat | % | 11.6 ± 1.0 | 11.7 (11–12.4) | 11.3–13.1 |
| Hydration | % | 53.0 ± 7.0 | 53.0 (46.0–58.0) | 41.0–69.0 |
| Posm | mosm·kg−1 | 283 ± 2.4 | 281 (279–286) | 278–288 |
| Mean ± SD | m (IQR) | Min–Max | ||
|---|---|---|---|---|
| VO2 max | mL·kg−1·min−1 | 65 ± 4 | 62 (60–65) | 54–74 |
| HR max | bpm | 187 ± 6 | 186 (183–190) | 180–206 |
| Phase I | p Values | |||
|---|---|---|---|---|
| Before CPET | After CPET | |||
| Mean ± SD | Mean ± SD | |||
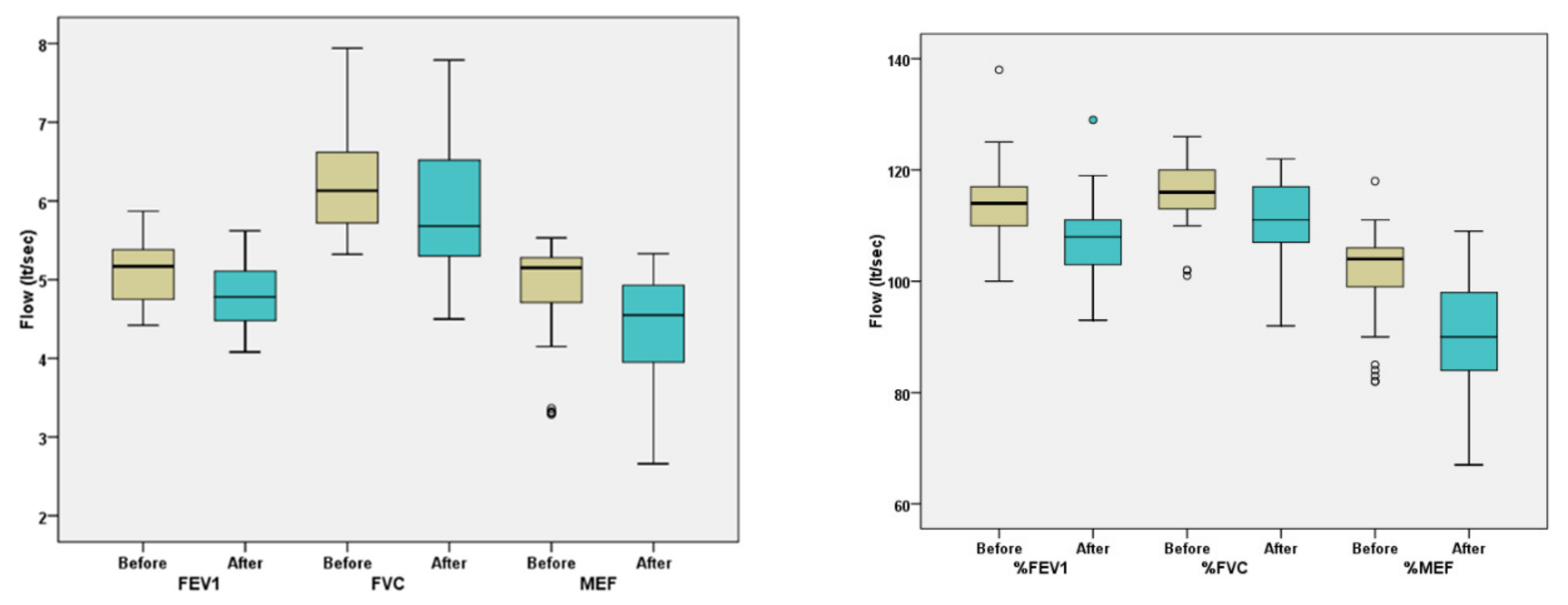
| FEV1 | L | 5.2 ± 0.4 | 4.9 ± 0.5 | <0.001 |
| % of pred. | 114.0 ± 6.9 | 108.5 ± 8.4 | <0.001 | |
| FVC | L | 6.4 ± 0.6 | 6.1 ± 0.7 | <0.001 |
| % of pred. | 117.1 ± 6.7 | 112.4 ± 8.9 | <0.001 | |
| MEF25–75 | L | 5.0 ± 1.1 | 4.4 ± 1.2 | <0.001 |
| % of pred. | 103.1 ± 8.3 | 90.8 ± 11.5 | <0.001 | |
| Differences in Percentages | n | % | |
|---|---|---|---|
| FEV1 | <10% | 70 | 70 |
| ≥10% | 30 | 30 | |
| MEF25–75 | <20% | 67 | 67 |
| ≥20% | 33 | 33 | |
| Both criteria | 20 | 20 | |
| One of these criteria | 43 | 43 | |
| Phase B | 41 | 41 |
| Reduction in Lung Function | ||||
|---|---|---|---|---|
| No (n = 59) | Yes (n = 41) | p Value | ||
| Mean ± SD | Mean ± SD | |||
| Age | Year | 29.0 ± 5.0 | 24.0 ± 6.0 | <0.001 |
| Training age | years | 14.0 ± 5.0 | 10.0 ± 6.0 | <0.001 |
| Height | cm | 179 ± 4.0 | 175 ± 6.0 | 0.001 |
| Body mass | kg | 75.6 ± 4.1 | 73.5 ± 6.4 | 0.047 |
| Body mass index | kg/m2 | 23.6 ± 1.0 | 23.9 ± 1.9 | 0.346 |
| Body fat | % | 11.7 ± 1.0 | 12.5 ± 0.9 | 0.352 |
| Hydration | % | 59.0 ± 4.7 | 46.0 ± 2.2 | <0.001 |
| Posm | mosm·kg−1 | 282.08 ± 1.3 | 286.07 ± 1.2 | 0.016 |
| Phase A | Phase Β | |||
|---|---|---|---|---|
| Mean ± Sd | Mean ± Sd | p Values | ||
| BMI | kg/m2 | 23.9 ± 1.9 | 23.9 ± 1.9 | 0.130 |
| Body fat | % | 21.5 ± 0.9 | 21.6 ± 0.9 | 0.210 |
| Hydration | % | 46.0 ± 2.2 | 49.4 ± 2.3 | <0.001 |
| VO2 max | mL·kg−1·min−1 | 61.8 ± 4.3 | 64.1 ± 4.4 | <0.001 |
| Posm | mosm·kg−1 | 286.07 ± 1.2 | 280.1 ± 1.2 | <0.001 |
| Phase B | ||||
|---|---|---|---|---|
| Before CPET | After CPET | |||
| Mean ± SD | Mean ± SD | p Values | ||
| FEV1 | L | 5.1 ± 0.4 | 4.8 ± 0.4 | <0.001 |
| % of pred. | 114.1 ± 7.6 | 107.2 ± 7.7 | <0.001 | |
| FVC | L | 6.2 ± 0.7 | 5.9 ± 0.8 | <0.001 |
| % of pred. | 116.2 ± 6.2 | 111 ± 7.9 | <0.001 | |
| MEF25–75 | L | 4.9 ± 0.7 | 4.4 ± 0.7 | <0.001 |
| % of pred. | 101.7 ± 8.2 | 90.4 ± 11 | <0.001 | |
| Phase A | Phase B | p Values | |
|---|---|---|---|
| % Differences | Mean ± SD | Mean ± SD | |
| FEV1 | −10.8 ± 2.7 | −6.9 ± 3 | <0.001 |
| FVC | −8.3 ± 4.6 | −5.2 ± 4.6 | <0.001 |
| MEF | −20.7 ± 4.6 | −11.3 ± 7.6 | <0.001 |
| FEV1 | VO2 max | ||
|---|---|---|---|
| HYD% | Pearson’s R correlation | 0.449 | 0.338 |
| Sig. (2-taled) | 0.003 | 0.031 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigakis, K.M.; Stavrou, V.T.; Pantazopoulos, I.; Daniil, Z.; Kontopodi-Pigaki, A.K.; Gourgoulianis, K. Effect of Hydration on Pulmonary Function and Development of Exercise-Induced Bronchoconstriction among Professional Male Cyclists. Adv. Respir. Med. 2023, 91, 239-253. https://doi.org/10.3390/arm91030019
Pigakis KM, Stavrou VT, Pantazopoulos I, Daniil Z, Kontopodi-Pigaki AK, Gourgoulianis K. Effect of Hydration on Pulmonary Function and Development of Exercise-Induced Bronchoconstriction among Professional Male Cyclists. Advances in Respiratory Medicine. 2023; 91(3):239-253. https://doi.org/10.3390/arm91030019
Chicago/Turabian StylePigakis, Konstantinos M., Vasileios T. Stavrou, Ioannis Pantazopoulos, Zoe Daniil, Aggeliki K. Kontopodi-Pigaki, and Konstantinos Gourgoulianis. 2023. "Effect of Hydration on Pulmonary Function and Development of Exercise-Induced Bronchoconstriction among Professional Male Cyclists" Advances in Respiratory Medicine 91, no. 3: 239-253. https://doi.org/10.3390/arm91030019
APA StylePigakis, K. M., Stavrou, V. T., Pantazopoulos, I., Daniil, Z., Kontopodi-Pigaki, A. K., & Gourgoulianis, K. (2023). Effect of Hydration on Pulmonary Function and Development of Exercise-Induced Bronchoconstriction among Professional Male Cyclists. Advances in Respiratory Medicine, 91(3), 239-253. https://doi.org/10.3390/arm91030019









