The Constrained Disorder Principle Accounts for the Variability That Characterizes Breathing: A Method for Treating Chronic Respiratory Diseases and Improving Mechanical Ventilation
Abstract
Highlights
- The constrained disorder principle (CDP) defines systems by their inherent disorder bounded by variable boundaries.
- The present paper describes the mechanisms of breathing and cellular respiration, focusing on their inherent variability and how the CDP accounts for the variability in breathing and respiration.
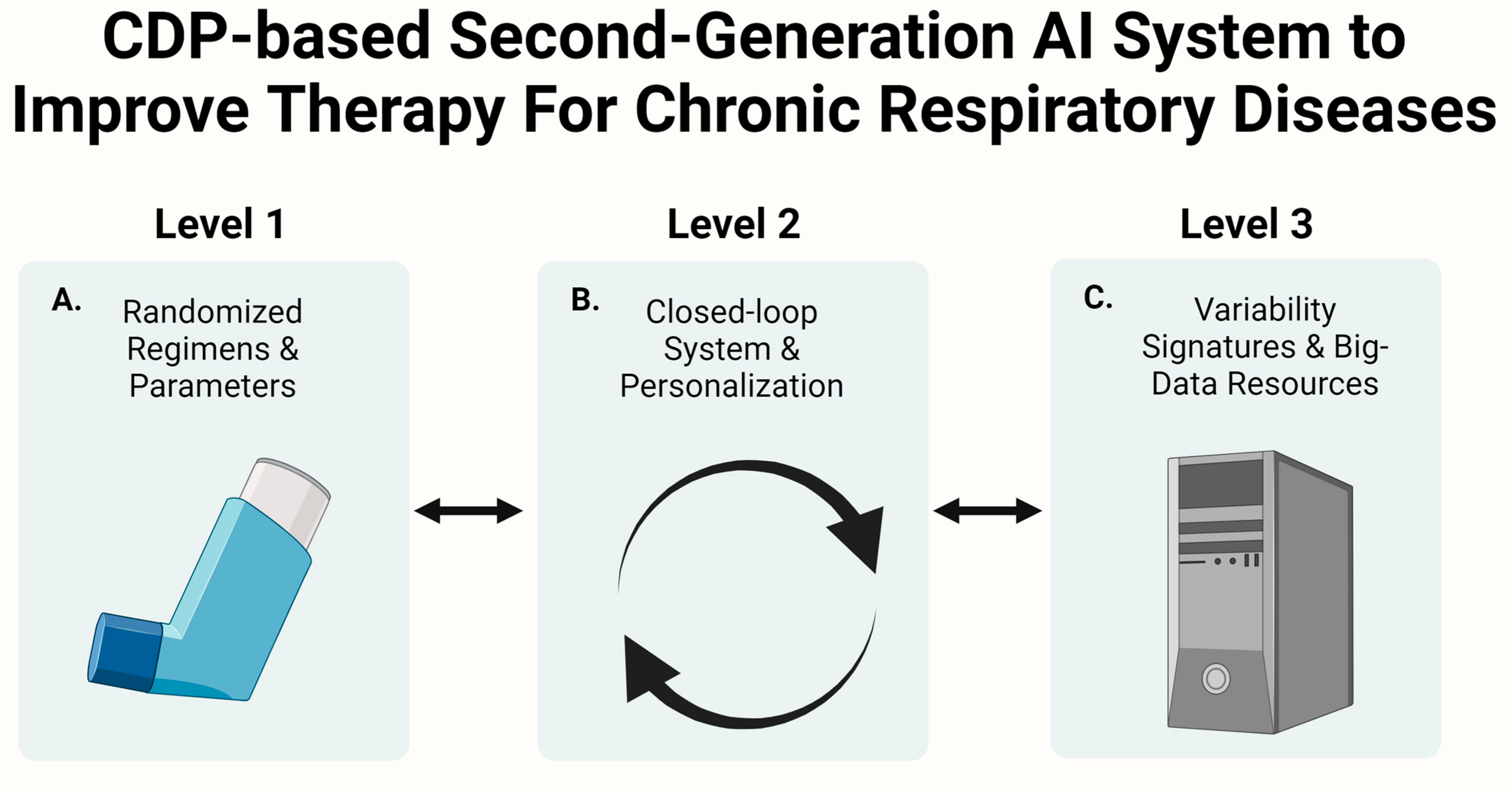
- The article describes using CDP-based artificial intelligence platforms to augment the respiratory process’s efficiency and treat respiratory diseases.
Abstract
1. Introduction
1.1. The Constrained Disorder Principle Defines Biological Processes
1.2. The Constrained Disorder Principle Accounts for the Stochasticity in Respiration
1.3. The Regulation of Cellular Respiration and Electrons Transport
1.4. The Constrained Disorder Principle Accounts for Variability in Cellular Respiration
1.5. Tunneling in Redox Reactions Implies That Variability Underlies Respiration at the Atomic Level and Is a Manifestation of Quantum Effects
1.6. Altered Variability in Lung Diseases
1.7. Using the Constrained Disorder Principle-Based Platform for Augmenting Cellular Respiration and Improving Therapies for Chronic Respiratory Diseases
1.8. The CDP-Based Platform for Improving Mechanical Ventilation and Assessment of Extubation Readiness
2. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Patel, H.; Kerndt, C.C.; Bhardwaj, A. Physiology, Respiratory Quotient; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Dellaca, R.L.; Aliverti, A.; Mauro, A.L.; Lutchen, K.R.; Pedotti, A.; Suki, B. Correlated Variability in the Breathing Pattern and End-Expiratory Lung Volumes in Conscious Humans. PLoS ONE 2015, 10, e0116317. [Google Scholar] [CrossRef]
- Calvillo, L.; Redaelli, V.; Ludwig, N.; Qaswal, A.B.; Ghidoni, A.; Faini, A.; Rosa, D.; Lombardi, C.; Pengo, M.; Bossolasco, P.; et al. Quantum Biology Research Meets Pathophysiology and Therapeutic Mechanisms: A Biomedical Perspective. Quantum Rep. 2022, 4, 148–172. [Google Scholar] [CrossRef]
- Ilan, Y. The constrained disorder principle defines living organisms and provides a method for correcting disturbed biological systems. Comput. Struct. Biotechnol. J. 2022, 20, 6087–6096. [Google Scholar] [CrossRef]
- Finn, E.H.; Misteli, T. Molecular basis and biological function of variability in spatial genome organization. Science 2019, 365, eaaw9498. [Google Scholar] [CrossRef]
- Chiera, M.; Cerritelli, F.; Casini, A.; Barsotti, N.; Boschiero, D.; Cavigioli, F.; Corti, C.G.; Manzotti, A. Heart Rate Variability in the Perinatal Period: A Critical and Conceptual Review. Front. Neurosci. 2020, 14, 561186. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Kirschner, M.W.; Mitchison, T. Microtubule dynamics. Nature 1986, 324, 621. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Overcoming randomness does not rule out the importance of inherent randomness for functionality. J. Biosci. 2019, 44, 1–12. [Google Scholar] [CrossRef]
- Ilan, Y. Generating randomness: Making the most out of disordering a false order into a real one. J. Transl. Med. 2019, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Advanced Tailored Randomness: A Novel Approach for Improving the Efficacy of Biological Systems. J. Comput. Biol. 2020, 27, 20–29. [Google Scholar] [CrossRef]
- Ilan, Y. Order Through Disorder: The Characteristic Variability of Systems. Front. Cell Dev. Biol. 2020, 8, 186. [Google Scholar] [CrossRef]
- El-Haj, M.; Kanovitch, D.; Ilan, Y. Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: A novel platform for designing personalized immunotherapies. Immunol. Res. 2019, 67, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Randomness in microtubule dynamics: An error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol. Int. 2019, 43, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Microtubules: From understanding their dynamics to using them as potential therapeutic targets. J. Cell. Physiol. 2018, 234, 7923–7937. [Google Scholar] [CrossRef] [PubMed]
- Ilan-Ber, T.; Ilan, Y. The role of microtubules in the immune system and as potential targets for gut-based immunotherapy. Mol. Immunol. 2019, 111, 73–82. [Google Scholar] [CrossRef]
- Forkosh, E.; Kenig, A.; Ilan, Y. Introducing variability in targeting the microtubules: Review of current mechanisms and future directions in colchicine therapy. Pharmacol. Res. Perspect. 2020, 8, e00616. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. β-Glycosphingolipids as Mediators of Both Inflammation and Immune Tolerance: A Manifestation of Randomness in Biological Systems. Front. Immunol. 2019, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Microtubules as a potential platform for energy transfer in biological systems: A target for implementing individualized, dynamic variability patterns to improve organ function. Mol. Cell. Biochem. 2022, 478, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Overcoming Compensatory Mechanisms toward Chronic Drug Administration to Ensure Long-Term, Sustainable Beneficial Effects. Mol. Ther.-Methods Clin. Dev. 2020, 18, 335–344. [Google Scholar] [CrossRef]
- Ilan, Y. Second-Generation Digital Health Platforms: Placing the Patient at the Center and Focusing on Clinical Outcomes. Front. Digit. Heal. 2020, 2, 569178. [Google Scholar] [CrossRef]
- Ilan, Y. Improving Global Healthcare and Reducing Costs Using Second-Generation Artificial Intelligence-Based Digital Pills: A Market Disruptor. Int. J. Environ. Res. Public Heal. 2021, 18, 811. [Google Scholar] [CrossRef]
- Ilan, Y. Enhancing the plasticity, proper function and efficient use of energy of the Sun, genes and microtubules using variability. Clin. Transl. Discov. 2022, 2, e103. [Google Scholar] [CrossRef]
- Tobin, M.J.; Mador, M.J.; Guenther, S.M.; Lodato, R.F.; Sackner, M.A.; Gustafsson, P.M.; Bengtsson, L.; Lindblad, A.; Robinson, P.D.; Suki, B.; et al. Variability of resting respiratory drive and timing in healthy subjects. J. Appl. Physiol. 1988, 65, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Benchetrit, G. Breathing pattern in humans: Diversity and individuality. Respir. Physiol. 2000, 122, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.J.; Rodrigues, V.R.; Davenport, P.W. Characterizing and Modeling Breathing Dynamics: Flow Rate, Rhythm, Period, and Frequency. Front. Physiol. 2022, 12, 772295. [Google Scholar] [CrossRef] [PubMed]
- Brack, T.; Jubran, A.; Tobin, M.J. Effect of Resistive Loading on Variational Activity of Breathing. Am. J. Respir. Crit. Care Med. 1998, 157, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Brack, T.; Jubran, A.; Tobin, M.J. Effect of elastic loading on variational activity of breathing. Am. J. Respir. Crit. Care Med. 1997, 155, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Jubran, A.; Grant, B.J.B.; Tobin, M.J. Effect of Hyperoxic Hypercapnia on Variational Activity of Breathing. Am. J. Respir. Crit. Care Med. 1997, 156, 1129–1139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bosch, O.F.C.v.D.; Alvarez-Jimenez, R.; de Grooth, H.-J.; Girbes, A.R.J.; Loer, S.A. Breathing variability—Implications for anaesthesiology and intensive care. Crit. Care 2021, 25, 1–13. [Google Scholar] [CrossRef]
- Engoren, M. Approximate entropy of respiratory rate and tidal volume during weaning from mechanical ventilation. Crit. Care Med. 1998, 26, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.; Jacono, F.J.; Park, S.; Jamasebi, R.; Thungtong, A.; Loparo, K.A.; Dick, T.E. A method for analyzing temporal patterns of variability of a time series from Poincaré plots. J. Appl. Physiol. 2012, 113, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kamen, P.W.; Tonkin, A.M. Application of the Poincaré plot to heart rate variability: A new measure of functional status in heart failure. Aust. N. Z. J. Med. 1995, 25, 18–26. [Google Scholar] [CrossRef]
- Fadel, P.J.; Barman, S.M.; Phillips, S.W.; Gebber, G.L. Fractal fluctuations in human respiration. J. Appl. Physiol. 2004, 97, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.; Owens, R.; Butler, J.; Suki, B.; Malhotra, A. Variability of respiratory mechanics during sleep in overweight and obese subjects with and without asthma. Respir. Physiol. Neurobiol. 2013, 186, 290–5. [Google Scholar] [CrossRef]
- Glass, L. Synchronization and rhythmic processes in physiology. Nature 2001, 410, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Cernelc, M.; Suki, B.; Reinmann, B.; Hall, G.L.; Frey, U. Correlation properties of tidal volume and end-tidal O2 and CO2 concentrations in healthy infants. J. Appl. Physiol. 2002, 92, 1817–1827. [Google Scholar] [CrossRef]
- Botros, S.M.; Brace, E.N. Neural network implementation of a three-phase model of respiratory rhythm generation. Biol. Cybern. 1990, 63, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J. Oscillators and Oscillations in the Basal Ganglia. Neurosci. 2014, 21, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Betka, S.; Adler, D.; Similowski, T.; Blanke, O. Breathing control, brain, and bodily self-consciousness: Toward immersive digiceuticals to alleviate respiratory suffering. Biol. Psychol. 2022, 171, 108329. [Google Scholar] [CrossRef]
- Güldner, A.; Huhle, R.; Beda, A.; Kiss, T.; Bluth, T.; Rentzsch, I.; Kerber, S.; Carvalho, N.C.; Kasper, M.; Pelosi, P.; et al. Periodic Fluctuation of Tidal Volumes Further Improves Variable Ventilation in Experimental Acute Respiratory Distress Syndrome. Front. Physiol. 2018, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, S.; Badr, M.S. Control of Ventilation in Health and Disease. Chest 2016, 151, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Emond, L.; Nattie, E. Brainstem Catecholaminergic Neurons Modulate both Respiratory and Cardiovascular Function. In Integration in Respiratory Control; Springer: New York, NY, USA, 2008; Volume 605, pp. 371–376. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Bayliss, D.A. Neural Control of Breathing and CO2 Homeostasis. Neuron 2015, 87, 946–961. [Google Scholar] [CrossRef]
- Li, A.; Nattie, E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J. Physiol. 2006, 570, 385–396. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; von Saint André-von Arnim, A.; McLaughlin, J. Cellular Respiration. In Pediatric Critical Care, 4th ed.; Fuhrman, B.P., Zimmerman, J.J., Eds.; Mosby: Saint Louis, MO, USA, 2011; Chapter 74; pp. 1058–1072. [Google Scholar]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sauve, A.A. NAD + metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. et Biophys. Acta (BBA)-Proteins Proteom. 2016, 1864, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.M. Aerobic Respiration: Criticism of the Proton-centric Explanation Involving Rotary Adenosine Triphosphate Synthesis, Chemiosmosis Principle, Proton Pumps and Electron Transport Chain. Biochem. Insights 2018, 11. [Google Scholar] [CrossRef]
- Milshteyn, D.; Cooper, G.; Deamer, D. Chemiosmotic energy for primitive cellular life: Proton gradients are generated across lipid membranes by redox reactions coupled to meteoritic quinones. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Allen, A.; Stanley, C.A.; Smith, T.J. The structure and allosteric regulation of glutamate dehydrogenase. Neurochem. Int. 2011, 59, 445–455. [Google Scholar] [CrossRef]
- McGresham, M.S.; Lovingshimer, M.; Reinhart, G.D. Allosteric Regulation in Phosphofructokinase from the Extreme Thermophile Thermus thermophilus. Biochemistry 2013, 53, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sugden, M.C.; Holness, M.J. The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: Going round in circles? Islets 2011, 3, 302–319. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Loscalzo, J. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.N.; Lemasters, J.J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 2014, 19, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Jouaville, L.S.; Pinton, P.; Bastianutto, C.; Rutter, G.A.; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 1999, 96, 13807–13812. [Google Scholar] [CrossRef]
- Jacobson, J.; Duchen, M.R. Interplay between mitochondria and cellular calcium signalling. Mol. Cell. Biochem. 2004, 256, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Osellame, L.D.; Stojanovski, D.; Ryan, M.T. The regulation of mitochondrial morphology: Intricate mechanisms and dynamic machinery. Cell. Signal. 2011, 23, 1534–1545. [Google Scholar] [CrossRef]
- O’Leary, B.M.; Asao, S.; Millar, A.H.; Atkin, O.K. Core principles which explain variation in respiration across biological scales. New Phytol. 2018, 222, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Jubrias, S.A.; Distefano, G.; Amati, F.; Mackey, D.C.; Glynn, N.W.; Manini, T.M.; Wohlgemuth, S.E.; Leeuwenburgh, C.; Cummings, S.R.; et al. Skeletal Muscle Mitochondrial Energetics Are Associated With Maximal Aerobic Capacity and Walking Speed in Older Adults. J. Gerontol. Ser. A 2012, 68, 447–455. [Google Scholar] [CrossRef]
- Berg, S.A.v.D.; Nabben, M.; Bijland, S.; Voshol, P.J.; van Klinken, J.B.; Havekes, L.M.; Romijn, J.A.; Hoeks, J.; Hesselink, M.K.; Schrauwen, P.; et al. High levels of whole-body energy expenditure are associated with a lower coupling of skeletal muscle mitochondria in C57Bl/6 mice. Metabolism 2010, 59, 1612–1618. [Google Scholar] [CrossRef]
- Monternier, P.-A.; Marmillot, V.; Rouanet, J.-L.; Roussel, D. Mitochondrial phenotypic flexibility enhances energy savings during winter fast in king penguin chicks. J. Exp. Biol. 2014, 217, 2691–7. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.A.; Siebenmann, C.; Hug, M.; Toigo, M.; Lundby, C.; Meinild, A.-K. Twenty-eight days at 3454-m altitude diminishes respiratory capacity but enhances efficiency in human skeletal muscle mitochondria. FASEB J. 2012, 26, 5192–5200. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Turner, N.; Hinde, J.; Else, P.; Guderley, H. How might you compare mitochondria from different tissues and different species? J. Comp. Physiol. B 2006, 176, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Salin, K.; Luquet, E.; Rey, B.; Roussel, D.; Voituron, Y. Alteration of mitochondrial efficiency affects oxidative balance, development and growth in frog (Rana temporaria) tadpoles. J. Exp. Biol. 2012, 215, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Brand The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 2005, 33, 897–904. [CrossRef] [PubMed]
- Park, S.-Y.; Gifford, J.R.; Andtbacka, R.H.I.; Trinity, J.D.; Hyngstrom, J.R.; Garten, R.S.; Diakos, N.A.; Ives, S.J.; Dela, F.; Larsen, S.; et al. Cardiac, skeletal, and smooth muscle mitochondrial respiration: Are all mitochondria created equal? Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H346–H352. [Google Scholar] [CrossRef]
- Holmström, M.H.; Iglesias-Gutierrez, E.; Zierath, J.R.; Garcia-Roves, P.M. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am. J. Physiol. Metab. 2012, 302, E731–E739. [Google Scholar] [CrossRef] [PubMed]
- Salin, K.; Auer, S.K.; Rey, B.; Selman, C.; Metcalfe, N.B. Variation in the link between oxygen consumption and ATP production, and its relevance for animal performance. Proc. R. Soc. B: Boil. Sci. 2015, 282, 20151028. [Google Scholar] [CrossRef]
- Burton, T.; Killen, S.S.; Armstrong, J.D.; Metcalfe, N.B. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B Boil. Sci. 2011, 278, 3465–3473. [Google Scholar] [CrossRef] [PubMed]
- Padalko, V.I. Uncoupler of Oxidative Phosphorylation Prolongs the Lifespan of Drosophila. Biochemistry 2005, 70, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Blount, J.D.; Vitikainen, E.I.K.; Stott, I.; Cant, M.A. Oxidative shielding and the cost of reproduction. Biol. Rev. 2015, 91, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.K.; Brand, M.D. Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature 1993, 362, 628–630. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Chandel, N.S.; Chihanga, T.; Ruby, H.N.; Ma, Q.; Bashir, S.; Devarajan, P.; Kennedy, M.A.; Bernard, O.; Jeny, F.; et al. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Physiol. 2011, 300, C385–C393. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.A.; Dickinson, K.; Brand, M.D. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes. Rev. 2001, 2, 255–265. [Google Scholar] [CrossRef]
- Hinkle, P.C. P/O ratios of mitochondrial oxidative phosphorylation. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2005, 1706, 1–11. [Google Scholar] [CrossRef]
- Sommer, A.M.; Pörtner, H.O. Mitochondrial Function in Seasonal Acclimatization versus Latitudinal Adaptation to Cold in the Lugworm Arenicola marina(L.). Physiol. Biochem. Zoöl. 2004, 77, 174–186. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J.; Škop, V.; Liu, N.; Guo, J.; Gavrilova, O.; Reitman, M.L.; Ono-Moore, K.D.; Rutkowsky, J.M.; A Pearson, N.; et al. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Teulier, L.; Rouanet, J.-L.; Letexier, D.; Romestaing, C.; Belouze, M.; Rey, B.; Duchamp, C.; Roussel, D. Cold-acclimation-induced non-shivering thermogenesis in birds is associated with upregulation of avian UCP but not with innate uncoupling or altered ATP efficiency. J. Exp. Biol. 2010, 213, 2476–2482. [Google Scholar] [CrossRef]
- Meyer, E.H.; Tomaz, T.; Carroll, A.J.; Estavillo, G.; Delannoy, E.; Tanz, S.K.; Small, I.D.; Pogson, B.J.; Millar, A.H. Remodeled Respiration in ndufs4 with Low Phosphorylation Efficiency Suppresses Arabidopsis Germination and Growth and Alters Control of Metabolism at Night. Plant Physiol. 2009, 151, 603–619. [Google Scholar] [CrossRef]
- Rasmusson, A.G.; Geisler, D.A.; Møller, I.M. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion 2008, 8, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Garvin, M.R.; Thorgaard, G.H.; Narum, S.R. Differential Expression of Genes that Control Respiration Contribute to Thermal Adaptation in Redband Trout ( Oncorhynchus mykiss gairdneri ). Genome Biol. Evol. 2015, 7, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, L.; Ramsey, J.J.; Hagopian, K.; Weindruch, R.; Harper, M.-E. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am. J. Physiol. Metab. 2005, 289, E429–E438. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fink, B.D.; Herlein, J.A.; Oltman, C.L.; Lamping, K.G.; Sivitz, W.I. Dietary fat, fatty acid saturation and mitochondrial bioenergetics. J. Bioenerg. Biomembr. 2013, 46, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.D.; Tse, H.M. Targeting Mitochondrial-Derived Reactive Oxygen Species in T Cell-Mediated Autoimmune Diseases. Front. Immunol. 2021, 12, 703972. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Keipert, S.; Voigt, A.; Klaus, S. Dietary effects on body composition, glucose metabolism, and longevity are modulated by skeletal muscle mitochondrial uncoupling in mice. Aging Cell 2010, 10, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Mitochondrial Oxygen Consumption and Reactive Oxygen Species Production are Independently Modulated: Implications for Aging Studies. Rejuvenation Res. 2007, 10, 215–224. [Google Scholar] [CrossRef]
- Salin, K.; Villasevil, E.M.; Anderson, G.J.; Selman, C.; Chinopoulos, C.; Metcalfe, N.B. The RCR and ATP/O Indices Can Give Contradictory Messages about Mitochondrial Efficiency. Integr. Comp. Biol. 2018, 58, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Toyomizu, M.; Kikusato, M.; Kawabata, Y.; Azad, A.K.; Inui, E.; Amo, T. Meat-type chickens have a higher efficiency of mitochondrial oxidative phosphorylation than laying-type chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, N.; Iqbal, M.; Pumford, N.R.; Lassiter, K.; Ojano-Dirain, C.; Wing, T.; Bottje, W. Investigation of mitochondrial protein expression and oxidation in heart muscle in low and high feed efficient male broilers in a single genetic line. Poult. Sci. 2010, 89, 349–352. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E. A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Atkin, O.K.; Menz, R.I.; Henry, B.; Farquhar, G.; Day, D.A. Analysis of Respiratory Chain Regulation in Roots of Soybean Seedlings1. Plant Physiol. 1998, 117, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Whelan, J.; Soole, K.L.; Day, D.A. Organization and Regulation of Mitochondrial Respiration in Plants. Annu. Rev. Plant Biol. 2011, 62, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Amthor, J.S. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Ann. Bot. 2000, 86, 1–20. [Google Scholar] [CrossRef]
- Lucia, U. Bioengineering thermodynamics of biological cells. Theor. Biol. Med Model. 2015, 12, 29. [Google Scholar] [CrossRef]
- Nunn, A.V.; Guy, G.W.; Bell, J.D. The quantum mitochondrion and optimal health. Biochem. Soc. Trans. 2016, 44, 1101–1110. [Google Scholar] [CrossRef]
- Thomsen, K. Timelessness Strictly inside the Quantum Realm. Entropy 2021, 23, 772. [Google Scholar] [CrossRef] [PubMed]
- Bera, M.N.; Acín, A.; Kuś, M.; Mitchell, M.W.; Lewenstein, M. Randomness in quantum mechanics: Philosophy, physics and technology. Rep. Prog. Phys. 2017, 80, 124001. [Google Scholar] [CrossRef] [PubMed]
- Kennett, E.; Kuchel, P. Redox Reactions and Electron Transfer Across the Red Cell Membrane. IUBMB Life 2003, 55, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P.; Fernie, A.R. Metabolic Control of Redox and Redox Control of Metabolism in Plants. Antioxid. Redox Signal. 2014, 21, 1389–1421. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J.; Md; Zhao, Y.; Zhang, Z.; Zou, Y.; Yang, Y.; Fessel, J.P.; et al. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Jin, X.; Willmore, W.G. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2013, 2, 123–139. [Google Scholar] [CrossRef]
- Sutcliffe, M.J.; Scrutton, N.S. Enzymology takes a quantum leap forward. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2000, 358, 367–386. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Xin, H.; Sim, W.J.; Namgung, B.; Choi, Y.; Li, B.; Lee, L.P. Quantum biological tunnel junction for electron transfer imaging in live cells. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Tyburski, R.; Liu, T.; Glover, S.D.; Hammarström, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef]
- Usselman, R.J.; Chavarriaga, C.; Castello, P.R.; Procopio, M.; Ritz, T.; Dratz, E.A.; Singel, D.J.; Martino, C.F. The Quantum Biology of Reactive Oxygen Species Partitioning Impacts Cellular Bioenergetics. Sci. Rep. 2016, 6, 38543. [Google Scholar] [CrossRef] [PubMed]
- Canfield, J.; Belford, R.; Debrunner, P.; Schulten, K. A perturbation theory treatment of oscillating magnetic fields in the radical pair mechanism. Chem. Phys. 1994, 182, 1–18. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Genet. 2013, 11, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Usselman, R.J.; Hill, I.; Singel, D.J.; Martino, C.F. Spin Biochemistry Modulates Reactive Oxygen Species (ROS) Production by Radio Frequency Magnetic Fields. PLoS ONE 2014, 9, e93065. [Google Scholar] [CrossRef] [PubMed]
- Hogben, H.J.; Efimova, O.; Wagner-Rundell, N.; Timmel, C.R.; Hore, P. Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: Origin of Zeeman resonances observed by in vivo EPR spectroscopy. Chem. Phys. Lett. 2009, 480, 118–122. [Google Scholar] [CrossRef]
- Brand, K.A.; Hermfisse, U. Aerobic glycolysis by proliferating cells: A protective strategy against reactive oxygen species. FASEB J. 1997, 11, 388–395. [Google Scholar] [CrossRef]
- Liemburg-Apers, D.C.; Willems, P.H.G.M.; Koopman, W.J.H.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef]
- Willems, P.H.; Rossignol, R.; Dieteren, C.E.; Murphy, M.P.; Koopman, W.J. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free. Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef]
- Marais, A.; Adams, B.; Ringsmuth, A.K.; Ferretti, M.; Gruber, J.M.; Hendrikx, R.; Schuld, M.; Smith, S.L.; Sinayskiy, I.; Krüger, T.P.J.; et al. The future of quantum biology. J. R. Soc. Interface 2018, 15, 20180640. [Google Scholar] [CrossRef]
- Brack, T.; Jubran, A.; Tobin, M.J. Dyspnea and Decreased Variability of Breathing in Patients with Restrictive Lung Disease. Am. J. Respir. Crit. Care Med. 2002, 165, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Loveridge, B.; West, P.; Anthonisen, N.R.; Kryger, M.H. Breathing patterns in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1984, 130. [Google Scholar] [CrossRef]
- Schechtman, V.L.; Lee, M.Y.; Wilson, A.J.; Harper, R.M. Dynamics of Respiratory Patterning in Normal Infants and Infants Who Subsequently Died of the Sudden Infant Death Syndrome. Pediatr. Res. 1996, 40, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Thamrin, C.; Frey, U.; Kaminsky, D.A.; Reddel, H.K.; Seely, A.J.E.; Suki, B.; Sterk, P.J. Systems Biology and Clinical Practice in Respiratory Medicine. The Twain Shall Meet. Am. J. Respir. Crit. Care Med. 2016, 194, 1053–1061. [Google Scholar] [CrossRef]
- Yentes, J.M.; Fallahtafti, F.; Denton, W.; Rennard, S.I. COPD Patients Have a Restricted Breathing Pattern That Persists with Increased Metabolic Demands. COPD: J. Chronic Obstr. Pulm. Dis. 2020, 17, 245–252. [Google Scholar] [CrossRef]
- Usemann, J.; Suter, A.; Zannin, E.; Proietti, E.; Fouzas, S.; Schulzke, S.; Latzin, P.; Frey, U.; Fuchs, O.; Korten, I.; et al. Variability of Tidal Breathing Parameters in Preterm Infants and Associations with Respiratory Morbidity during Infancy: A Cohort Study. J. Pediatr. 2019, 205, 61–69.e1. [Google Scholar] [CrossRef]
- Hmeidi, H.; Motamedi-Fakhr, S.; Chadwick, E.; Gilchrist, F.J.; Lenney, W.; Iles, R.; Wilson, R.C.; Alexander, J. Tidal breathing parameters measured using structured light plethysmography in healthy children and those with asthma before and after bronchodilator. Physiol. Rep. 2017, 5, e13168. [Google Scholar] [CrossRef]
- Thamrin, C.; Taylor, D.R.; Jones, S.L.; Suki, B.; Frey, U. Variability of lung function predicts loss of asthma control following withdrawal of inhaled corticosteroid treatment. Thorax 2010, 65, 403–408. [Google Scholar] [CrossRef]
- Thamrin, C.; Stern, G.; Strippoli, M.-P.F.; Kuehni, C.E.; Suki, B.; Taylor, D.R.; Frey, U. Fluctuation analysis of lung function as a predictor of long-term response to 2-agonists. Eur. Respir. J. 2008, 33, 486–493. [Google Scholar] [CrossRef]
- Frey, U.; Brodbeck, T.; Majumdar, A.; Taylor, D.R.; Town, G.I.; Silverman, M.; Suki, B. Risk of severe asthma episodes predicted from fluctuation analysis of airway function. Nature 2005, 438, 667–670. [Google Scholar] [CrossRef]
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farre, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Dellacá, R.L.; King, G.; Thamrin, C. Toward Predicting Individual Risk in Asthma Using Daily Home Monitoring of Resistance. Am. J. Respir. Crit. Care Med. 2017, 195, 265–267. [Google Scholar] [CrossRef]
- Thamrin, C.; Zindel, J.; Nydegger, R.; Reddel, H.K.; Chanez, P.; Wenzel, S.E.; FitzPatrick, S.; Watt, R.A.; Suki, B.; Frey, U. Predicting future risk of asthma exacerbations using individual conditional probabilities. J. Allergy Clin. Immunol. 2011, 127, 1494–1502.e3. [Google Scholar] [CrossRef] [PubMed]
- Timmins, S.C.; Coatsworth, N.; Palnitkar, G.; Thamrin, C.; Farrow, C.E.; Schoeffel, R.E.; Berend, N.; Diba, C.; Salome, C.M.; King, G.G. Day-to-day variability of oscillatory impedance and spirometry in asthma and COPD. Respir. Physiol. Neurobiol. 2013, 185, 416–424. [Google Scholar] [CrossRef]
- Zimmermann, S.C.; Huvanandana, J.; Nguyen, C.D.; Bertolin, A.; Watts, J.C.; Gobbi, A.; Farah, C.S.; Peters, M.J.; Dellacà, R.L.; King, G.G.; et al. Day-to-day variability of forced oscillatory mechanics for early detection of acute exacerbations in COPD. Eur. Respir. J. 2020, 56, 1901739. [Google Scholar] [CrossRef]
- Veit, T.; Barnikel, M.; Crispin, A.; Kneidinger, N.; Ceelen, F.; Arnold, P.; Munker, D.; Schmitzer, M.; Barton, J.; Schiopu, S.; et al. Variability of forced vital capacity in progressive interstitial lung disease: A prospective observational study. Respir. Res. 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Askanazi, J.; Silverberg, P.A.; Hyman, A.I.; Rosenbaum, S.H.; Foster, R.; Kinney, J.M. Patterns of ventilation in postoperative and acutely ill patients. Crit. Care Med. 1979, 7, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Preas, H.L.; Jubran, A.; Vandivier, R.W.; Reda, D.; Godin, P.; Banks, S.M.; Tobin, M.J.; Suffredini, F. Effect of endotoxin on ventilation and breath variability: Role of cyclooxygenase pathway. Am. J. Respir. Crit. Care Med. 2001, 164, 620–626. [Google Scholar] [CrossRef]
- Bien, M.-Y.; Hseu, S.-S.; Yien, H.-W.; Kuo, B.I.-T.; Lin, Y.-T.; Wang, J.-H.; Kou, Y.R. Breathing pattern variability: A weaning predictor in postoperative patients recovering from systemic inflammatory response syndrome. Intensiv. Care Med. 2004, 30, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Brigham, K.; Meyrick, B. Endotoxin and lung injury. Am. Rev. Respir. Dis. 1986, 133, 913–927. [Google Scholar]
- Ilan, Y. Making use of noise in biological systems. Prog. Biophys. Mol. Biol. 2023, 178, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Haney, S.; Hancox, R.J. Rapid onset of tolerance to beta-agonist bronchodilation. Respir. Med. 2005, 99, 566–571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haney, S.; Hancox, R.J. Overcoming beta-agonist tolerance: High dose salbutamol and ipratropium bromide. Two randomised controlled trials. Respir. Res. 2007, 8, 19. [Google Scholar] [CrossRef]
- Wraight, J.; Hancox, R.; Herbison, G.; Cowan, J.; Flannery, E.; Taylor, D. Bronchodilator tolerance: The impact of increasing bronchoconstriction. Eur. Respir. J. 2003, 21, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Weksler-Zangen, S.; Ilan, Y. Role of the Immune System and the Circadian Rhythm in the Pathogenesis of Chronic Pancreatitis. Pancreas 2020, 49, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Ishay, Y.; Kolben, Y.; Kessler, A.; Ilan, Y. Role of circadian rhythm and autonomic nervous system in liver function: A hypothetical basis for improving the management of hepatic encephalopathy. Am. J. Physiol. Liver Physiol. 2021, 321, G400–G412. [Google Scholar] [CrossRef] [PubMed]
- Kolben, Y.; Weksler-Zangen, S.; Ilan, Y. Adropin as a potential mediator of the metabolic system-autonomic nervous system-chronobiology axis: Implementing a personalized signature-based platform for chronotherapy. Obes. Rev. 2020, 22, e13108. [Google Scholar] [CrossRef]
- Kenig, A.; Kolben, Y.; Asleh, R.; Amir, O.; Ilan, Y. Improving Diuretic Response in Heart Failure by Implementing a Patient-Tailored Variability and Chronotherapy-Guided Algorithm. Front. Cardiovasc. Med. 2021, 8, 695547. [Google Scholar] [CrossRef]
- Azmanov, H.; Ross, E.L.; Ilan, Y. Establishment of an Individualized Chronotherapy, Autonomic Nervous System, and Variability-Based Dynamic Platform for Overcoming the Loss of Response to Analgesics. Pain Physician 2021, 24, 243–252. [Google Scholar] [PubMed]
- Potruch, A.; Khoury, S.T.; Ilan, Y. The role of chronobiology in drug-resistance epilepsy: The potential use of a variability and chronotherapy-based individualized platform for improving the response to anti-seizure drugs. Seizure 2020, 80, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Isahy, Y.; Ilan, Y. Improving the long-term response to antidepressants by establishing an individualized platform based on variability and chronotherapy. Int. J. Clin. Pharmacol. Ther. 2021, 59, 768–774. [Google Scholar] [CrossRef]
- Khoury, T.; Ilan, Y. Introducing Patterns of Variability for Overcoming Compensatory Adaptation of the Immune System to Immunomodulatory Agents: A Novel Method for Improving Clinical Response to Anti-TNF Therapies. Front. Immunol. 2019, 10, 2726. [Google Scholar] [CrossRef] [PubMed]
- Khoury, T.; Ilan, Y. Platform introducing individually tailored variability in nerve stimulations and dietary regimen to prevent weight regain following weight loss in patients with obesity. Obes. Res. Clin. Pract. 2021, 15, 114–123. [Google Scholar] [CrossRef]
- Kenig, A.; Ilan, Y. A Personalized Signature and Chronotherapy-Based Platform for Improving the Efficacy of Sepsis Treatment. Front. Physiol. 2019, 10, 1542. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Why targeting the microbiome is not so successful: Can randomness overcome the adaptation that occurs following gut manipulation? Clin. Exp. Gastroenterol. 2019, 12, 209–217. [Google Scholar] [CrossRef]
- Gelman, R.; Bayatra, A.; Kessler, A.; Schwartz, A.; Ilan, Y. Targeting SARS-CoV-2 receptors as a means for reducing infectivity and improving antiviral and immune response: An algorithm-based method for overcoming resistance to antiviral agents. Emerg. Microbes Infect. 2020, 9, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Ishay, Y.; Potruch, A.; Schwartz, A.; Berg, M.; Jamil, K.; Agus, S.; Ilan, Y. A digital health platform for assisting the diagnosis and monitoring of COVID-19 progression: An adjuvant approach for augmenting the antiviral response and mitigating the immune-mediated target organ damage. BioMedicine 2021, 143, 112228. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y.; Spigelman, Z. Establishing patient-tailored variability-based paradigms for anti-cancer therapy: Using the inherent trajectories which underlie cancer for overcoming drug resistance. Cancer Treat. Res. Commun. 2020, 25, 100240. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, N.; Azmanov, H.; Kesler, A.; Ilan, Y. Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur. J. Hum. Genet. 2021, 29, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Digital Medical Cannabis as Market Differentiator: Second-Generation Artificial Intelligence Systems to Improve Response. Front. Med. 2022, 8, 788777. [Google Scholar] [CrossRef] [PubMed]
- Gelman, R.; Berg, M.; Ilan, Y. A Subject-Tailored Variability-Based Platform for Overcoming the Plateau Effect in Sports Training: A Narrative Review. Int. J. Environ. Res. Public Heal. 2022, 19, 1722. [Google Scholar] [CrossRef] [PubMed]
- Azmanov, H.; Bayatra, A.; Ilan, Y. Digital Analgesic Comprising a Second-Generation Digital Health System: Increasing Effectiveness by Optimizing the Dosing and Minimizing Side Effects. J. Pain Res. 2022, 15, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Kolben, Y.; Azmanov, H.; Gelman, R.; Dror, D.; Ilan, Y. Using chronobiology-based second-generation artificial intelligence digital system for overcoming antimicrobial drug resistance in chronic infections. Ann. Med. 2023, 55, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, N.; Elkhateeb, N.; Sigawi, T.; Rinsky-Halivni, L.; Ilan, Y. Improving the effectiveness of anti-aging modalities by using the constrained disorder principle-based management algorithms. Front. Aging 2022, 3, 1044038. [Google Scholar] [CrossRef]
- Ilan, Y. Next-Generation Personalized Medicine: Implementation of Variability Patterns for Overcoming Drug Resistance in Chronic Diseases. J. Pers. Med. 2022, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, N.; Ilan, Y. The Constrained-Disorder Principle Assists in Overcoming Significant Challenges in Digital Health: Moving from “Nice to Have” to Mandatory Systems. Clin. Pract. 2023, 13, 994–1014. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Constrained disorder principle-based variability is fundamental for biological processes: Beyond biological relativity and physiological regulatory networks. Prog. Biophys. Mol. Biol. 2023, 180–181, 37–48. [Google Scholar] [CrossRef]
- Sigawi, T.; Lehmann, H.; Hurvitz, N.; Ilan, Y. Constrained disorder principle-based second-generation algorithms implement quantified variability signatures to improve the function of complex systems. J. Bioinform. Syst. Biol. 2023, 06, 82–89. [Google Scholar] [CrossRef]
- Gelman, R.; Hurvitz, N.; Nesserat, R.; Kolben, Y.; Nachman, D.; Jamil, K.; Agus, S.; Asleh, R.; Amir, O.; Berg, M.; et al. A second-generation artificial intelligence-based therapeutic regimen improves diuretic resistance in heart failure: Results of a feasibility open-labeled clinical trial. BioMedicine 2023, 161, 114334. [Google Scholar] [CrossRef]
- Suki, B.; Alencar, A.M.; Sujeer, M.K.; Lutchen, K.R.; Collins, J.J.; Andrade, J.S.; Ingenito, E.P.; Zapperi, S.; Stanley, H.E. Life-support system benefits from noise. Nature 1998, 393, 127–128. [Google Scholar] [CrossRef]
- Brewster, J.F.; Graham, M.R.; Mutch, W.A.C. Convexity, Jensen’s inequality and benefits of noisy mechanical ventilation. J. R. Soc. Interface 2005, 2, 393–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Abreu, M.G.; Spieth, P.M.; Pelosi, P.; Carvalho, A.R.; Walter, C.; Schreiber-Ferstl, A.; Aikele, P.; Neykova, B.; Hübler, M.; Koch, T. Noisy pressure support ventilation: A pilot study on a new assisted ventilation mode in experimental lung injury*. Crit. Care Med. 2008, 36, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, G.R.; E Kowalski, S.; Girling, L.G.; Thiessen, D.B.; A Mutch, W. Improved arterial oxygenation after oleic acid lung injury in the pig using a computer-controlled mechanical ventilator. Am. J. Respir. Crit. Care Med. 1996, 154, 1567–1572. [Google Scholar] [CrossRef]
- Arold, S.P.; Suki, B.; Alencar, A.M.; Lutchen, K.R.; Ingenito, E.P. Variable ventilation induces endogenous surfactant release in normal guinea pigs. Am. J. Physiol. Cell. Mol. Physiol. 2003, 285, L370–L375. [Google Scholar] [CrossRef]
- Graham, M.R.; Goertzen, A.L.; Girling, L.G.; Friedman, T.; Pauls, R.J.; Dickson, T.; Espenell, A.E.G.; Mutch, W.A.C. Quantitative computed tomography in porcine lung injury with variable versus conventional ventilation: Recruitment and surfactant replacement*. Crit. Care Med. 2011, 39, 1721–1730. [Google Scholar] [CrossRef]
- Bellardine, C.L.; Hoffman, A.M.; Tsai, L.; Ingenito, E.P.; Arold, S.P.; Lutchen, K.R.; Suki, B. Comparison of variable and conventional ventilation in a sheep saline lavage lung injury model*. Crit. Care Med. 2006, 34, 439–445. [Google Scholar] [CrossRef]
- Mutch, W.A.C.; Harms, S.; Lefevre, G.R.; Graham, M.R.; Girling, L.G.; Kowalski, S.E. Biologically variable ventilation increases arterial oxygenation over that seen with positive end-expiratory pressure alone in a porcine model of acute respiratory distress syndrome. Crit. Care Med. 2000, 28, 2457–2464. [Google Scholar] [CrossRef]
- Arold, S.P.; Mora, R.; Lutchen, K.R.; Ingenito, E.P.; Suki, B. Variable Tidal Volume Ventilation Improves Lung Mechanics and Gas Exchange in a Rodent Model of Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2002, 165, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Spieth, P.M.; Carvalho, A.R.; Pelosi, P.; Hoehn, C.; Meissner, C.; Kasper, M.; Hübler, M.; von Neindorff, M.; Dassow, C.; Barrenschee, M.; et al. Variable Tidal Volumes Improve Lung Protective Ventilation Strategies in Experimental Lung Injury. Am. J. Respir. Crit. Care Med. 2009, 179, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.R.; Gulati, H.; Kha, L.; Girling, L.G.; Goertzen, A.; Mutch, W.A.C. Resolution of pulmonary edema with variable mechanical ventilation in a porcine model of acute lung injury. Can. J. Anaesth. 2011, 58, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Boker, A.; Haberman, C.J.; Girling, L.; Guzman, R.P.; Louridas, G.; Tanner, J.R.; Cheang, M.; Maycher, B.W.; Bell, D.D.; Doak, G.J. Variable Ventilation Improves Perioperative Lung Function in Patients Undergoing Abdominal Aortic Aneurysmectomy. Anesthesiology 2004, 100, 608–616. [Google Scholar] [CrossRef]
- Mutch, W.A.C.; Buchman, T.G.; Girling, L.G.; Walker, E.K.-Y.; McManus, B.M.; Graham, M.R. Biologically variable ventilation improves gas exchange and respiratory mechanics in a model of severe bronchospasm*. Crit. Care Med. 2007, 35, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.K. Extubation failure: An outcome to be avoided. Crit. Care 2004, 8, 310–2. [Google Scholar] [CrossRef] [PubMed]
- Krasinkiewicz, J.M.; Friedman, M.L.; E Slaven, J.; Lutfi, R.; Abu-Sultaneh, S.; Tori, A.J. Extubation Readiness Practices and Barriers to Extubation in Pediatric Subjects. Respir. Care 2020, 66, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhang, H.; Jiang, M.; Ning, G.; Fang, L.; Ge, H. Comprehensive breathing variability indices enhance the prediction of extubation failure in patients on mechanical ventilation. Comput. Biol. Med. 2023, 153, 106459. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Cracco, C.; Teixeira, A.; Mercat, A.; Diehl, J.-L.; Lefort, Y.; Derenne, J.-P.; Similowski, T. Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation*. Crit. Care Med. 2006, 34, 2076–2083. [Google Scholar] [CrossRef]
- Seely, A.J.; Bravi, A.; Herry, C.; Green, G.; Longtin, A.; Ramsay, T.; Fergusson, D.; McIntyre, L.; Kubelik, D.; E Maziak, D.; et al. Do heart and respiratory rate variability improve prediction of extubation outcomes in critically ill patients? Crit. Care 2014, 18, R65. [Google Scholar] [CrossRef]
- I Naik, B.; Lynch, C.; Durbin, C.G. Variability in Mechanical Ventilation: What’s All the Noise About? Respir. Care 2015, 60, 1203–1210. [Google Scholar] [CrossRef]
- Mauri, T.; Foti, G.; Fornari, C.; Grasselli, G.; Pinciroli, R.; Lovisari, F.; Tubiolo, D.; Volta, C.A.; Spadaro, S.; Rona, R.; et al. Sigh in Patients With Acute Hypoxemic Respiratory Failure and ARDS. Chest 2021, 159, 1426–1436. [Google Scholar] [CrossRef]
- Mauri, T.; for the Protection Study Group; Foti, G.; Fornari, C.; Constantin, J.-M.; Guerin, C.; Pelosi, P.; Ranieri, M.; Conti, S.; Tubiolo, D.; et al. Pressure support ventilation + sigh in acute hypoxemic respiratory failure patients: Study protocol for a pilot randomized controlled trial, the PROTECTION trial. Trials 2018, 19, 1–10. [Google Scholar] [CrossRef]
- El-Khatib, M.; Jamaleddine, G.; Soubra, R.; Muallem, M. Pattern of spontaneous breathing: Potential marker for weaning outcome. Intensive Care Med. 2001, 27, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zein, H.; Baratloo, A.; Negida, A.; Safari, S. Ventilator Weaning and Spontaneous Breathing Trials; an Educational Review. Emergency 2016, 4, 65–71. [Google Scholar] [PubMed]
- Knox, K.E.; Nava-Guerra, L.; Hotz, J.C.R.; Newth, C.J.L.; Khoo, M.C.K.; Khemani, R.G. High Breath-by-Breath Variability Is Associated With Extubation Failure in Children. Crit. Care Med. 2020, 48, 1165–1174. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adar, O.; Hollander, A.; Ilan, Y. The Constrained Disorder Principle Accounts for the Variability That Characterizes Breathing: A Method for Treating Chronic Respiratory Diseases and Improving Mechanical Ventilation. Adv. Respir. Med. 2023, 91, 350-367. https://doi.org/10.3390/arm91050028
Adar O, Hollander A, Ilan Y. The Constrained Disorder Principle Accounts for the Variability That Characterizes Breathing: A Method for Treating Chronic Respiratory Diseases and Improving Mechanical Ventilation. Advances in Respiratory Medicine. 2023; 91(5):350-367. https://doi.org/10.3390/arm91050028
Chicago/Turabian StyleAdar, Ofek, Adi Hollander, and Yaron Ilan. 2023. "The Constrained Disorder Principle Accounts for the Variability That Characterizes Breathing: A Method for Treating Chronic Respiratory Diseases and Improving Mechanical Ventilation" Advances in Respiratory Medicine 91, no. 5: 350-367. https://doi.org/10.3390/arm91050028
APA StyleAdar, O., Hollander, A., & Ilan, Y. (2023). The Constrained Disorder Principle Accounts for the Variability That Characterizes Breathing: A Method for Treating Chronic Respiratory Diseases and Improving Mechanical Ventilation. Advances in Respiratory Medicine, 91(5), 350-367. https://doi.org/10.3390/arm91050028







