In Silico Design of a New Epitope-Based Vaccine against Grass Group 1 Allergens
Abstract
:Highlights
- A peptide consisting of 20 amino acids was designed in silico as a grass pollen allergy vaccine.
- The epitope-based vaccine has been validated by molecular docking analysis and ex vivo T-cell stimulation assay. It covers more than 80% of the European and global population.
- The immunogenic peptide can be used for treatment of patients with grass pollen allergy by triggering the T-cell response and production of competitive IgG antibodies.
- Additional studies are needed before the clinical application of the epitope-based vaccine for immunotherapy.
Abstract
1. Introduction
2. Materials and Methods
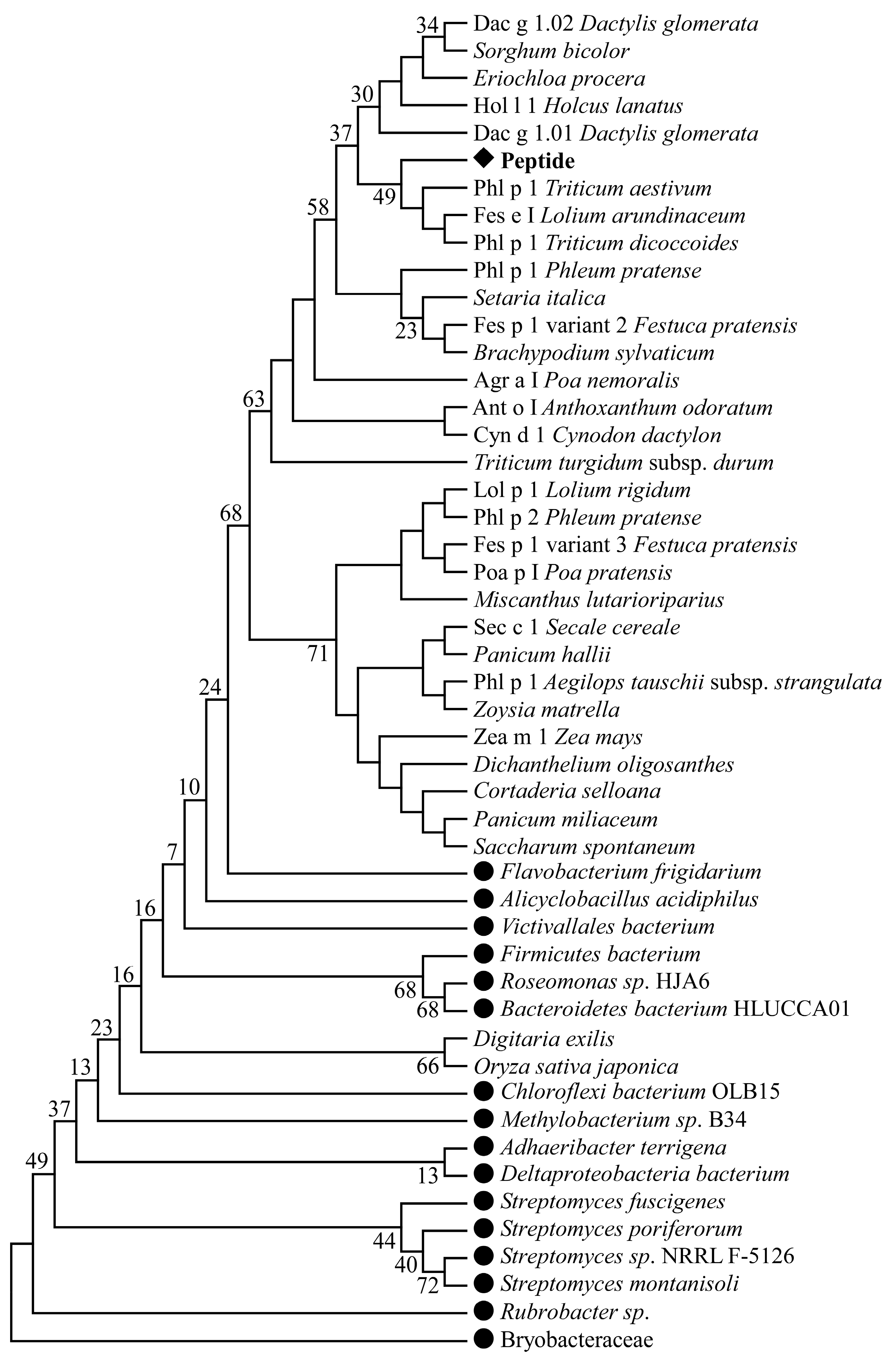
2.1. Pollen T-Cell and B-Cell Epitope Sequence Retrieval, Multiple Sequence Alignment, and Phylogenetic Analysis
2.2. Prediction of CD8+ T-Cell Epitopes and Their MHC-I-Binding Alleles
2.3. Prediction of CD4+ T-Cell Epitopes and Their MHC-II-Binding Alleles
2.4. Population Coverage Prediction for CD8+ and CD4+ T-Cell Epitopes and Their Alleles
2.5. Identification of B-Cell Epitopes
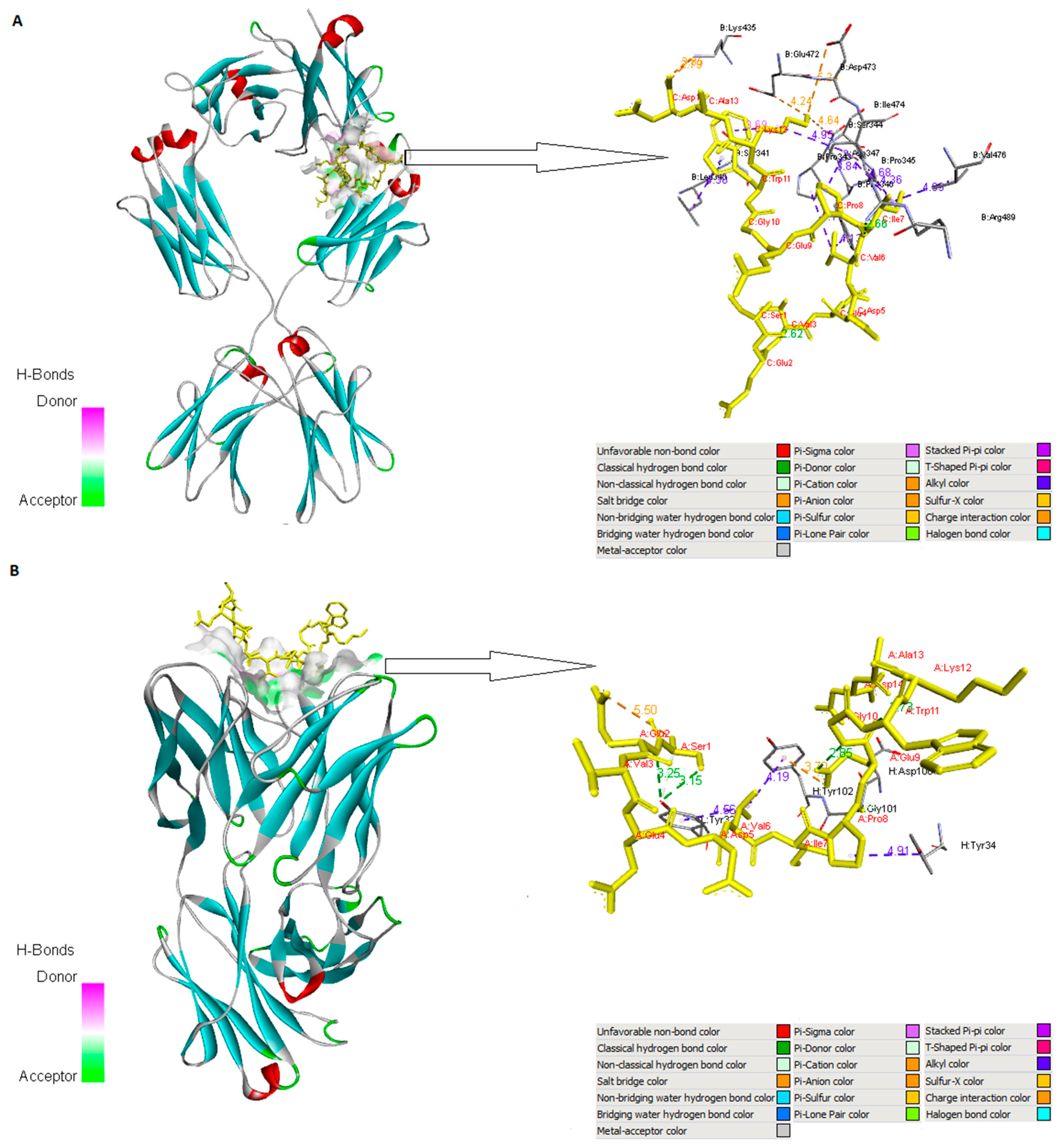
2.6. Molecular Interaction Analysis
2.7. Participants, Functional Validation of T-Cell Reactivity, and Antibody Recognition
2.8. Statistics
3. Results
3.1. Pollen T-Cell and B-Cell Epitope Sequence Retrieval, Multiple Sequence Alignment, and Phylogenetic Analysis
3.2. Prediction of CD8+ T-Cell Epitopes and Their MHC-I-Binding Alleles
3.3. Prediction of CD4+ T-Cell Epitopes and Their MHC-II-Binding Alleles
3.4. Population Coverage Analysis of the Selected CD8+ and CD4+ T-Cell Epitopes
3.5. Identification of B-Cell Epitopes
3.6. Molecular Interaction Analysis
3.7. Ex Vivo Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woodfolk, J.A.; Commins, S.P.; Schuyler, A.J.; Erwin, E.A.; Platts-Mills, T.A.E. Allergens, sources, particles, and molecules: Why do we make IgE responses? Allergol. Int. 2015, 64, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Calderón, M.A.; Rodríguez del Río, P.; Vidal, C.; Just, J.; Pfaar, O.; Linneberg, A.; Demoly, P. An EAACI “European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI)”: The methodology. Clin. Transl. Allergy 2014, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Cichocka-Jarosz, E.; Lis, G.; Pietrzyk, J.J. [Pollen allergy. I. Pathophysiology and clinic]. Przegl. Lek. 1997, 54, 614–619. [Google Scholar] [PubMed]
- Vizzardelli, C.; Zimmann, F.; Nagl, B.; Kitzmüller, C.; Vollmann, U.; Gindl, M.; Tangermann, S.; Jahn-Schmid, B.; Kenner, L.; Bohle, B. NSG mice humanized with allergen-specific T-cell lines as in vivo model of respiratory allergy. Allergy 2020, 75, 2081–2084. [Google Scholar] [CrossRef]
- Gangl, K.; Niederberger, V.; Valenta, R. Multiple grass mixes as opposed to single grasses for allergen immunotherapy in allergic rhinitis. Clin. Exp. Allergy 2013, 43, 1202–1216. [Google Scholar] [CrossRef]
- Werfel, T.; Heratizadeh, A.; Niebuhr, M.; Kapp, A.; Roesner, L.M.; Karch, A.; Erpenbeck, V.J.; Lösche, C.; Jung, T.; Krug, N.; et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J. Allergy Clin. Immunol. 2015, 136, 96–103.e109. [Google Scholar] [CrossRef]
- García-Mozo, H. Poaceae pollen as the leading aeroallergen worldwide: A review. Allergy 2017, 72, 1849–1858. [Google Scholar] [CrossRef]
- Andersson, K.; Lidholm, J. Characteristics and Immunobiology of Grass Pollen Allergens. Int. Arch. Allergy Immunol. 2003, 130, 87–107. [Google Scholar] [CrossRef]
- Valenta, R.; Vrtala, S.; Ebner, C.; Kraft, D.; Scheiner, O. Diagnosis of Grass Pollen Allergy with Recombinant Timothy Grass (Phleum pratense) Pollen Allergens. Int. Arch. Allergy Immunol. 1992, 97, 287–294. [Google Scholar] [CrossRef]
- Chabre, H.; Gouyon, B.; Huet, A.; Boran-Bodo, V.; Nony, E.; Hrabina, M.; Fenaille, F.; Lautrette, A.; Bonvalet, M.; Maillère, B.; et al. Molecular variability of group 1 and 5 grass pollen allergens between Pooideae species: Implications for immunotherapy. Clin. Exp. Allergy 2010, 40, 505–519. [Google Scholar] [CrossRef]
- Marcucci, F.; Sensi, L.; Incorvaia, C.; Dell’Albani, I.; Di Cara, G.; Frati, F. Specific IgE response to different grass pollen allergen components in children undergoing sublingual immunotherapy. Clin. Mol. Allergy 2012, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J.; Bedinger, P.; Durachko, D.M. Group I allergens of grass pollen as cell wall-loosening agents. Proc. Natl. Acad. Sci. USA 1997, 94, 6559–6564. [Google Scholar] [CrossRef] [PubMed]
- Flicker, S.; Steinberger, P.; Ball, T.; Krauth, M.; Verdino, P.; Valent, P.; Almo, S.; Valenta, R. Spatial clustering of the IgE epitopes on the major timothy grass pollen allergen Phl p 1: Importance for allergenic activity. J. Allergy Clin. Immunol. 2006, 117, 1336–1343. [Google Scholar] [CrossRef]
- Basu, A. Immunoinformatics Based Study of T Cell Epitopes in Zea m 1 Pollen Allergen. Medicina 2019, 55, 236. [Google Scholar] [CrossRef]
- Von Bubnoff, D.; Geiger, E.; Bieber, T. Antigen-presenting cells in allergy. J. Allergy Clin. Immunol. 2001, 108, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Jardetzky, T.S.; Brown, J.H.; Gorga, J.C.; Stern, L.J.; Urban, R.G.; Strominger, J.L.; Wiley, D.C. Crystallographic analysis of endogenous peptides associated with HLA-DR1 suggests a common, polyproline II-like conformation for bound peptides. Proc. Natl. Acad. Sci. USA 1996, 93, 734–738. [Google Scholar] [CrossRef]
- O’Hehir, R.E.; Prickett, S.R.; Rolland, J.M. T Cell Epitope Peptide Therapy for Allergic Diseases. Curr. Allergy Asthma Rep. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Suleman, M.; ul Qamar, M.T.; Kiran; Rasool, S.; Rasool, A.; Albutti, A.; Alsowayeh, N.; Alwashmi, A.S.S.; Aljasir, M.A.; Ahmad, S.; et al. Immunoinformatics and Immunogenetics-Based Design of Immunogenic Peptides Vaccine against the Emerging Tick-Borne Encephalitis Virus (TBEV) and Its Validation through In Silico Cloning and Immune Simulation. Vaccines 2021, 9, 1210. [Google Scholar] [CrossRef]
- Alharbi, M.; Alshammari, A.; Alasmari, A.F.; Alharbi, S.; Tahir ul Qamar, M.; Abbasi, S.W.; Shaker, B.; Ahmad, S. Whole Proteome-Based Therapeutic Targets Annotation and Designing of Multi-Epitope-Based Vaccines against the Gram-Negative XDR-Alcaligenes faecalis Bacterium. Vaccines 2022, 10, 462. [Google Scholar] [CrossRef]
- Alharbi, M.; Alshammari, A.; Alasmari, A.F.; Alharbi, S.M.; Tahir ul Qamar, M.; Ullah, A.; Ahmad, S.; Irfan, M.; Khalil, A.A.K. Designing of a Recombinant Multi-Epitopes Based Vaccine against Enterococcus mundtii Using Bioinformatics and Immunoinformatics Approaches. Int. J. Environ. Res. Public Health 2022, 19, 3729. [Google Scholar] [CrossRef] [PubMed]
- Tourani, M.; Karkhah, A.; Najafi, A. Development of an epitope-based vaccine inhibiting immune cells rolling and migration against atherosclerosis using in silico approaches. Comput. Biol. Chem. 2017, 70, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Oli, A.N.; Obialor, W.O.; Ifeanyichukwu, M.O.; Odimegwu, D.C.; Okoyeh, J.N.; Emechebe, G.O.; Adejumo, S.A.; Ibeanu, G.C. Immunoinformatics and Vaccine Development: An Overview. ImmunoTargets Ther. 2020, 9, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, W.L.G.; Larché, M.; Kay, A.B. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: A randomised controlled trial. Lancet 2002, 360, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bauchau, V. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur. Respir. J. 2004, 24, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Linhart, B.; Valenta, R. Vaccines for allergy. Curr. Opin. Immunol. 2012, 24, 354–360. [Google Scholar] [CrossRef]
- Lehtonen, S.; Collins, T. Phylogeny Estimation and Alignment via POY versus Clustal + PAUP*: A Response to Ogden and Rosenberg (2007). Syst. Biol. 2008, 57, 653–657. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K.; Battistuzzi, F.U. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- Peters, B.; Sette, A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinform. 2005, 6, 132. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Lund, O. Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinform. 2007, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, M.; Karosiene, E.; Rasmussen, M.; Stryhn, A.; Buus, S.; Nielsen, M. Accurate pan-specific prediction of peptide-MHC class II binding affinity with improved binding core identification. Immunogenetics 2015, 67, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.; Nielsen, M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Peters, B.; Paul, S.; Alvarez, B.; Reynisson, B. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef]
- Kaabinejadian, S.; Barra, C.; Alvarez, B.; Yari, H.; Hildebrand, W.H.; Nielsen, M. Accurate MHC Motif Deconvolution of Immunopeptidomics Data Reveals a Significant Contribution of DRB3, 4 and 5 to the Total DR Immunopeptidome. Front. Immunol. 2022, 13, 835454. [Google Scholar] [CrossRef]
- Bui, H.-H.; Sidney, J.; Dinh, K.; Southwood, S.; Newman, M.J.; Sette, A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinform. 2006, 7, 153. [Google Scholar] [CrossRef]
- Parker, J.M.R.; Guo, D.; Hodges, R.S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: Correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry 2002, 25, 5425–5432. [Google Scholar] [CrossRef]
- Larsen, J.; Lund, O.; Nielsen, M. Improved method for predicting linear B-cell epitopes. Immunome Res. 2006, 2, 2. [Google Scholar] [CrossRef]
- Emini, E.A.; Hughes, J.V.; Perlow, D.S.; Boger, J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 1985, 55, 836–839. [Google Scholar] [CrossRef]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef]
- Karplus, P.A.; Schulz, G.E. Prediction of chain flexibility in proteins. Naturwissenschaften 1985, 72, 212–213. [Google Scholar] [CrossRef]
- Yang, J.T. Prediction of protein secondary structure from amino acid sequence. J. Protein Chem. 1996, 15, 185–191. [Google Scholar] [CrossRef]
- Gao, Y.D.; Huang, J.F. [An extension strategy of Discovery Studio 2.0 for non-bonded interaction energy automatic calculation at the residue level]. Dongwuxue Yanjiu 2011, 32, 262–266. [Google Scholar] [CrossRef]
- Yang, Z.; Lasker, K.; Schneidman-Duhovny, D.; Webb, B.; Huang, C.C.; Pettersen, E.F.; Goddard, T.D.; Meng, E.C.; Sali, A.; Ferrin, T.E. UCSF Chimera, MODELLER, and IMP: An integrated modeling system. J. Struct. Biol. 2012, 179, 269–278. [Google Scholar] [CrossRef]
- Blaszczyk, M.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. Protein–peptide docking using CABS-dock and contact information. Brief. Bioinform. 2019, 20, 2299–2305. [Google Scholar] [CrossRef]
- Mashiach, E.; Schneidman-Duhovny, D.; Andrusier, N.; Nussinov, R.; Wolfson, H.J. FireDock: A web server for fast interaction refinement in molecular docking. Nucleic Acids Res. 2008, 36, W229–W232. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, H.C.S.; Filipek, S.; Vogel, H. PyMOL and Inkscape Bridge the Data and the Data Visualization. Structure 2016, 24, 2041–2042. [Google Scholar] [CrossRef]
- Seneviratne, S.L.; Jones, L.; King, A.S.; Black, A.; Powell, S.; McMichael, A.J.; Ogg, G.S. Allergen-specific CD8+ T cells and atopic disease. J. Clin. Investig. 2002, 110, 1283–1291. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Sibuyi, N.R.S.; Martin, D.R.; Goboza, M.; Klein, A.; Madiehe, A.M.; Meyer, M. Immunoinformatics design of a novel epitope-based vaccine candidate against dengue virus. Sci. Rep. 2021, 11, 19707. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, L.; Gong, W. In silico Analysis of Peptide-Based Biomarkers for the Diagnosis and Prevention of Latent Tuberculosis Infection. Front. Microbiol. 2022, 13, 947852. [Google Scholar] [CrossRef]
- Ghandadi, M. An Immunoinformatic Strategy to Develop New Mycobacterium tuberculosis Multi-epitope Vaccine. Int. J. Pept. Res. Ther. 2022, 28, 99. [Google Scholar] [CrossRef] [PubMed]
- Moten, D.; Kolchakova, D.; Todorov, K.; Mladenova, T.; Dzhambazov, B. Design of an Epitope-Based Peptide Vaccine Against the Major Allergen Amb a 11 Using Immunoinformatic Approaches. Protein J. 2022, 41, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Larché, M.; Wraith, D.C. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat. Med. 2005, 11, S69–S76. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Bohle, B.; Jahn-Schmid, B.; Wiedermann, U.; Daser, A.; Renz, H.; Kraft, D.; Ebner, C. Modulation of the allergic immune response in BALB/c mice by subcutaneous injection of high doses of the dominant T cell epitope from the major birch pollen allergen Bet v 1. Clin. Exp. Immunol. 1997, 107, 536–541. [Google Scholar] [CrossRef]
- Akinfenwa, O.; Huang, H.-J.; Linhart, B.; Focke-Tejkl, M.; Vrtala, S.; Poroshina, A.; Nikonova, A.; Khaitov, M.; Campion, N.J.; Eckl-Dorna, J.; et al. Preventive Administration of Non-Allergenic Bet v 1 Peptides Reduces Allergic Sensitization to Major Birch Pollen Allergen, Bet v 1. Front. Immunol. 2021, 12, 744544. [Google Scholar] [CrossRef]
- Ferreira, F.; Hawranek, T.; Gruber, P.; Wopfner, N.; Mari, A. Allergic cross-reactivity: From gene to the clinic. Allergy 2004, 59, 243–267. [Google Scholar] [CrossRef]
- Davies, J.M. Grass pollen allergens globally: The contribution of subtropical grasses to burden of allergic respiratory diseases. Clin. Exp. Allergy 2014, 44, 790–801. [Google Scholar] [CrossRef]
- Yin, D.; Li, L.; Song, X.; Li, H.; Wang, J.; Ju, W.; Qu, X.; Song, D.; Liu, Y.; Meng, X.; et al. A novel multi-epitope recombined protein for diagnosis of human brucellosis. BMC Infect. Dis. 2016, 16, 219. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Nezafat, N.; Eslami, M.; Ghoshoon, M.B.; Shoolian, E.; Najafipour, S.; Morowvat, M.H.; Dehshahri, A.; Erfani, N.; Ghasemi, Y. Structural vaccinology considerations for in silico designing of a multi-epitope vaccine. Infect. Genet. Evol. 2018, 58, 96–109. [Google Scholar] [CrossRef]
- Onile, O.S.; Ojo, G.J.; Oyeyemi, B.F.; Agbowuro, G.O.; Fadahunsi, A.I. Development of multiepitope subunit protein vaccines against Toxoplasma gondii using an immunoinformatics approach. NAR Genom. Bioinform. 2020, 2, lqaa048. [Google Scholar] [CrossRef]
- Yurina, V.; Adianingsih, O.R. Predicting epitopes for vaccine development using bioinformatics tools. Ther. Adv. Vaccines Immunother. 2022, 10, 25151355221100218. [Google Scholar] [CrossRef]
- Zieglmayer, P.; Focke-Tejkl, M.; Schmutz, R.; Lemell, P.; Zieglmayer, R.; Weber, M.; Kiss, R.; Blatt, K.; Valent, P.; Stolz, F.; et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine 2016, 11, 43–57. [Google Scholar] [CrossRef]
- Berg, L. Exploring Non-Covalent Interactions between Drug-Like Molecules and the Protein Acetylcholinesterase; Umeå University, VMC-KBC Umeå: Umeå, Sweden, 2017. [Google Scholar]
- Alagumuthu, M.; Rajpoot, S.; Baig, M.S. Structure-Based Design of Novel Peptidomimetics Targeting the SARS-CoV-2 Spike Protein. Cell. Mol. Bioeng. 2020, 14, 177–185. [Google Scholar] [CrossRef]
- Scheiner, S.; Kar, T.; Gu, Y. Strength of the CαH··O Hydrogen Bond of Amino Acid Residues. J. Biol. Chem. 2001, 276, 9832–9837. [Google Scholar] [CrossRef]


| T-Cell Epitopes | Interacting MHC I Alleles | Start Position | End Position | Length of Epitope | Percentile Rank |
|---|---|---|---|---|---|
| SEVEDVIPEGWK | HLA-B*44:03 HLA-B*44:02 HLA-A*68:01 HLA-B*18:01 HLA-B*40:01 HLA-A*11:01 HLA-B*53:01 | 4 | 15 | 12 | 0.9 1.1 7.7 9.2 11.0 17.0 18.0 |
| KSEVEDVIPEGW | HLA-B*44:03 HLA-B*58:01 HLA-B*57:01 HLA-B*44:02 HLA-B*18:01 HLA-B*53:01 HLA-B*40:01 | 3 | 14 | 12 | 0.39 0.5 0.57 0.58 1.3 4.2 5.6 |
| SEVEDVIPEGWKA | HLA-B*44:03 HLA-B*44:02 HLA-B*18:01 HLA-B*40:01 HLA-B*40:02 HLA-B*53:01 HLA-A*68:01 HLA-A*25:01 HLA-A*68:02 | 4 | 16 | 13 | 1.2 2.0 2.5 2.9 3.1 3.3 3.7 5.4 7.5 |
| TKSEVEDVIPEGW | HLA-B*44:03 HLA-B*58:01 HLA-B*44:02 HLA-B*57:01 HLA-B*18:01 HLA-B*53:01 HLA-B*38:01 | 1 | 14 | 14 | 0.76 0.94 4.9 6.1 8.6 9.0 11.0 |
| VEDVIPEGW | HLA-B*44:02 HLA-B*44:03 HLA-B*40:01 HLA-C*08:02 HLA-B*57:01 HLA-A*23:01 HLA-A*24:02 HLA-C*04:01 HLA-A*01:01 HLA-B*51:01 | 6 | 14 | 9 | 0.02 0.03 1.0 1.3 1.5 2.26 2.8 3.1 3.2 6.7 |
| T-Cell Epitopes | Interacting MHC II Alleles | Start Position | End Position | Length | Percentile Rank |
|---|---|---|---|---|---|
| GTKSEVEDVIPEGWK | HLA-DQA1*05:01/DQB1*02:01 HLA-DQA1*03:01/DQB1*03:02 HLA-DQA1*01:01/DQB1*05:01 HLA-DQA1*04:01/DQB1*04:02 HLA-DPA1*01/DPB1*04:01 HLA-DRB1*07:03/DRB3*01:01 | 5 | 19 | 15 | 0.34 2.70 40.00 53.00 71.00 73.00 |
| KSEVEDVIPEGWKAD | HLA-DQA1*05:01/DQB1*02:01 HLA-DQA1*03:01/DQB1*03:02 HLA-DQA1*01:01/DQB1*05:01 HLA-DQA1*04:01/DQB1*04:02 HLA-DRB1*04:21 | 4 | 18 | 15 | 0.11 2.40 23.00 26.00 39.00 |
| TKSEVEDVIPEGWKA | HLA- DQA1*03:01/DQB1*02:01 HLA-DQA1*05:01/DQB1*02:01 HLA-DQA1*03:01/DQB1*03:02 HLA-DRB3*01:01 HLA-DQA1*01:01/DQB1*05:01 HLA-DQA1*04:01/DQB1*04:02 HLA-DPA1*01/DPB1*04:01 HLA-DPA1*01:03/DPB1*02:01 | 3 | 17 | 15 | 0.10 0.24 4.10 32.00 33.00 41.00 57.53 69.00 |
| SEVEDVIPEGWKADT | HLA-DQA1*05:01/DQB1*02:01 HLA-DQA1*03:01/DQB1*03:02 HLA-DQA1*05:01/DQB1*03:01 HLA-DQA1*01:01/DQB1*05:01 HLA-DRB1*04:21 | 6 | 20 | 15 | 0.66 8.05 22.00 58.00 59.00 |
| VEDVIPEGWKADTSY | HLA-DQA1*01:01/DQB1*05:01 HLA-DQA1*05:01/DQB1*03:01 HLA-DQA1*05:01/DQB1*02:01 HLA-DQA1*03:01/DQB1*03:02 HLA-DRB1*04:21 HLA-DRB1*04:26 | 2 | 16 | 15 | 15.00 26.00 30.00 30.00 43.00 49.00 |
| Predicted T-Cell Epitopes | Population Coverage | ||
|---|---|---|---|
| World | Europe | ||
| CD8+ CD4+ | SEVEDVIPEGWK GTKSEVEDVIPEGWK | 44.25% 92.11% | 47.68% 94.25% |
| CD8+ CD4+ | KSEVEDVIPEGW KSEVEDVIPEGWKAD | 35.60% 88.51% | 42.89% 86.39% |
| CD8+ CD4+ | SEVEDVIPEGWKA TKSEVEDVIPEGWKA | 43.85% 98.20% | 49.70% 99.86% |
| CD8+ CD4+ | TKSEVEDVIPEGW SEVEDVIPEGWKADT | 57.73% 94.13% | 69.73% 92.45% |
| CD8+ CD4+ | VEDVIPEGW VEDVIPEGWKADTSY | 78.87% 91.53% | 84.57% 90.55% |
| B-Cell Epitope | Emini Surface Accessibility | Parker Hydrophilicity | Karplus and Schulz Flexibility | Chou and Fashman Beta Turn | Kolaskar and Tongaonkar Antigenicity |
|---|---|---|---|---|---|
| S | 1.69 | 5.0 | 1.084 | 0.991 | 0.973 |
| E | 1.956 | 5.614 | 1.064 | 0.977 | 0.972 |
| V | 1.726 | 4.343 | 1.042 | 0.986 | 1.039 |
| E | 1.38 | 2.386 | 1.021 | 0.834 | 1.071 |
| D | 1.339 | 2.757 | 1.077 | 0.847 | 1.079 |
| V | 0.791 | 2.757 | 0.998 | 0.847 | 1.079 |
| I | 1.452 | 3.1 | 1.003 | 0.999 | 1.006 |
| P | 1.285 | 1.557 | 1.016 | 1.03 | 1.012 |
| E | 1.767 | 1.057 | 1.023 | 0.966 | 1.021 |
| G | 1.105 | 1.771 | 1.028 | 0.989 | 0.975 |
| W | 1.194 | 3.343 | 1.028 | 1.13 | 0.935 |
| K | 0.995 | 3.786 | 1.035 | 1.05 | 0.912 |
| A | 1.347 | 3.6 | 1.043 | 1.149 | 0.975 |
| D | 2.007 | 3.629 | 1.053 | 1.089 | 0.976 |
| PDB ID | glob | aVdW | rVdW | ACE | inside | aElec | rElec | laElec | lrElec | HB | piS | catpiS | aliph |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4JQX (VEDVIPEGW) | −72.1 | −43.5 | 15.1 | −4.8 | 0.9 | −13.3 | 17.7 | −8.7 | 15.9 | −3.8 | −4.5 | −1.5 | −5.5 |
| 4D8P (TKSEVEDVIPEGWKA) | −89.6 | −53.3 | 10.2 | −0.1 | 1.6 | −50.9 | 23.2 | −23.5 | 19.5 | −4.3 | −5.0 | 0.0 | −6.5 |
| 4J4P (SEVEDVIPEGWKAD) | −34.4 | −31.4 | 9.7 | −4.4 | 1.9 | −69.4 | 14.9 | −23.1 | 7.3 | −1.9 | −2.0 | 0.0 | −5.5 |
| 2VXQ (SEVEDVIPEGWKAD) | −29.9 | −26.7 | 12.0 | −9.4 | 15.0 | 0.0 | 30.2 | 0.0 | 11.9 | −2.7 | −2.5 | 0.0 | −1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moten, D.; Batsalova, T.; Apostolova, D.; Mladenova, T.; Dzhambazov, B.; Teneva, I. In Silico Design of a New Epitope-Based Vaccine against Grass Group 1 Allergens. Adv. Respir. Med. 2023, 91, 486-503. https://doi.org/10.3390/arm91060036
Moten D, Batsalova T, Apostolova D, Mladenova T, Dzhambazov B, Teneva I. In Silico Design of a New Epitope-Based Vaccine against Grass Group 1 Allergens. Advances in Respiratory Medicine. 2023; 91(6):486-503. https://doi.org/10.3390/arm91060036
Chicago/Turabian StyleMoten, Dzhemal, Tsvetelina Batsalova, Desislava Apostolova, Tsvetelina Mladenova, Balik Dzhambazov, and Ivanka Teneva. 2023. "In Silico Design of a New Epitope-Based Vaccine against Grass Group 1 Allergens" Advances in Respiratory Medicine 91, no. 6: 486-503. https://doi.org/10.3390/arm91060036
APA StyleMoten, D., Batsalova, T., Apostolova, D., Mladenova, T., Dzhambazov, B., & Teneva, I. (2023). In Silico Design of a New Epitope-Based Vaccine against Grass Group 1 Allergens. Advances in Respiratory Medicine, 91(6), 486-503. https://doi.org/10.3390/arm91060036










