Cystic Fibrosis: Understanding Cystic Fibrosis Transmembrane Regulator Mutation Classification and Modulator Therapies
Abstract
:Highlights
- The study identifies and categorizes the six main classes of CFTR mutations based on their functional effects.
- The study explores the emerging field of CFTR modulators, which are designed to restore CFTR function or mitigate its consequences. These modulators are characterized by their mode of action and the specific mutation class they target.
- By understanding the broad range of CFTR mutations and their impacts on disease pathophysiology, it is possible to establish tailored treatment strategies for CF patients.
- The study highlights the potential for precision medicine methods in CF therapy.
Abstract
1. Introduction
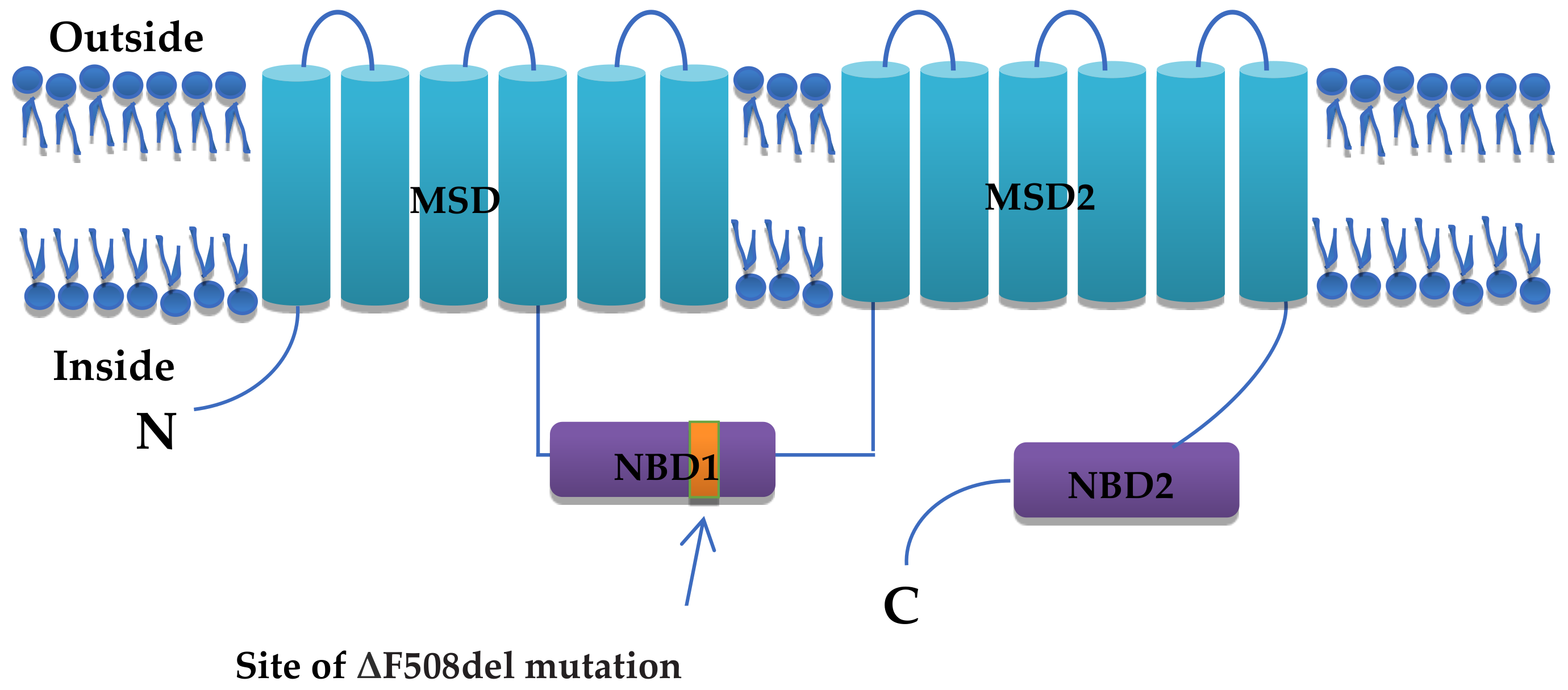
2. Structure and Function of CFTR
3. Mechanism of Action of CFTR
4. CFTR Mutations and Cystic Fibrosis
5. Classification of CFTR Mutations
5.1. Class I
5.2. Class II
5.3. Class III
5.4. Class IV
5.5. Class V
5.6. Class VI
| Classes | Class I | Class II | Class III | Class IV | Class V | Class VI | |
|---|---|---|---|---|---|---|---|
| IA | IB | ||||||
| CFTR Defect | No mRNA synthesis | No Synthesis (Blocked Protein Production) | Misprocessing/Misfolding/Defective Trafficking | Regulation/Gating | Reduced Channel Conductance | Reduced Synthesis | Reduced Stability |
| Mutation | Frameshift, splicing, nonsense mutation | Canonical splices (mis-splice), frameshift, premature stop codon, nonsense (null) | Amino acid deletion, missense. | Missense, Amino acid change. | Missense, Amino acid change. | Missense, mis-splice (other splicing defects) | Missense, Amino acid change, nonsense, frameshift |
| Mechansim | Premature termination/ stop codon ↓ Unstable mRNA ↓ Degraded by Nonsense mRNA decay (NMD) surveillance system | Premature termination/stop codon in mRNA ↓ Formation of small quantities or severely defective or truncated CFTR protein ↓ Rapidly degraded by the ER ↓ Near complete absence of functional protein in the apical cell membrane | Synthesis of a misprocessed/immature/misfolded protein that is only partially glycosylated ↓ Not released from the ER and when prematurely released, rapidly degraded by the ubiquitin–proteasomal pathway ↓ The absence of mature CFTR and a small quantity of immature protein is transported to the apical membrane of the epithelial cell which does not function properly as a chloride channel | Normal, correctly folded CFTR is synthesized and trafficked to the apical membrane ↓ Activation/regulation by cAMP and ATP are disturbed ↓ Opening time of the channel is significantly reduced | CFTR is properly synthesized and transported, responds to cAMP and ATP ↓ Channel conductance is greatly reduced. | Slightly improper splicing ↓ Defective/inefficient trafficking ↓ Decreased synthesis of fully active CFTR | Properly synthesized CFTR ↓ Increased endocytosis or abnormal recycling ↓ Reduced Stability/Destabilized Protein |
| Examples | Dele2,3 (21 kb), 1717-1G→A | 3950delT, G542X, R553X W1282X, | Arg560Thr, Asn1303Lys, Ile507del, Phe508del | G551D, Gly178Arg, Gly551Ser, Ser549Asn | R117H, R334W, G314E,R347P, D1152H, R117C | 3849 + 10 kb C>T, 3272-26 A>G, 3849–10 kb CT, A455E | 1811 + 1.6 kb A>G, 4326delTC, Gln1412X, 4279insA |
| References | [32,33] | [19,29,31,38] | [19,38,48] | [19,38,48] | [19,29,48] | [19,29,38,48] | [19,29,31] |
6. CFTR Modulators
6.1. Class I Defects
6.2. Class II Defects
6.3. Class III Defects
6.4. Class IV Defects
6.5. Class V and VI Defects
7. Monotherapy to Triple Combination Therapy (ETI)
| Study | Study Population | Problem Targeted | Conclusions |
|---|---|---|---|
| [86] | 34 adults with CF and at least one F508del. 3–12 months. 23 patient completed the study. | Cystic fibrosis related glycemia (CFRG). | Improvement in CGM derived measure of hyperglycemia and glycemic variability. No significant effect on hypoglycemia. |
| [87] | 30 PW homozygous for F508del or at least one F508del. | Airway and systemic inflammation. | Reduced circulating concentration of IL-6, sTNFR1 and CRP shows effect on systemic inflammation. Sputum production diminished in two-thirds of the population after 1 year of therapy. |
| [65] | 19 patient heterozygous for F508del and modulator naïve. | Systemic and immune cell-derived inflammatory cytokines level response to ETI. | Downregulation of routine serum inflammatory markers, reduced sweat chloride concentrations, as well as a significant reduction in both CF serum and stimulated PBMCs IL-18, IL-1β, TNF, and IL-6 levels. |
| [88] | 21 PwCF. | Changes in sputum proteome. | Mean FEV1 value improved by 13.7% after ETI therapy. Sputum proteome shifted towards intermediate state different from both CF and normal control. |
| [89] | 127 PwCF. | Iron status in PwCf. | Significant improvement in mean iron level (increased by 20.24 μg/dL), and ferratin level increased by 31.4%. |
| [90] | 25 PwCF, 13 homozygous for F508del, 12 heterzygous for F508del. | Nasal nitric oxide level. | Nasal NO level showed significant increase after several month even reached normal level. |
| [91] | 29 PwCF, 15 homozygous for F508del, 14 heterzygous for F508del. | Nutritional status and digestive function. | Data showed improvements in nutritional parameters, improved defecation, lower pancreatic substitution requirements, and improvements in exocrine pancreatic function (mutation specific). |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rohlfs, E.M.; Zhou, Z.; Heim, R.A.; Nagan, N.; Rosenblum, L.S.; Flynn, K.; Scholl, T.; Akmaev, V.R.; Sirko-Osadsa, D.A.; Allitto, B.A. Cystic fibrosis carrier testing in an ethnically diverse US population. Clin. Chem. 2011, 57, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Fanen, P.; Wohlhuter-Haddad, A.; Hinzpeter, A. Genetics of cystic fibrosis: CFTR mutation classifications toward genotype-based CF therapies. Int. J. Biochem. Cell Biol. 2014, 52, 94–102. [Google Scholar] [CrossRef]
- Ong, T.; Ramsey, B.W. Cystic fibrosis: A review. JAMA 2023, 329, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Amaral, M.D. Progress in therapies for cystic fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Sheema; Bashir, K.; Fiaz, S.; Khan, A.W.; Haqqani, S.; Bibi, A.; Nawaz, K.; Khan, M.A.; Ullah, A. Molecular identification of HCV genotypes among injecting drug users having HCV and HIV co-infection. Bull. Biol. Allied Sci. Res. 2024, 2024, 71. [Google Scholar] [CrossRef]
- Ullah, I.; Ullah, A.; Rehman, S.; Ullah, S.; Ullah, H.; Haqqni, S.; Amir, M.; Gul, F.; Bashir, K. Prevalence and risk factors of helicobacter pylori infection among individuals with tobacco consumption habits in district Peshawar: A cross-sectional study. Bull. Biol. Allied Sci. Res. 2023, 2023, 42. [Google Scholar] [CrossRef]
- Green, D.M.; Lahiri, T.; Raraigh, K.S.; Ruiz, F.; Spano, J.; Antos, N.; Bonitz, L.; Christon, L.; Gregoire-Bottex, M.; Hale, J.E. Cystic Fibrosis Foundation Evidence-Based Guideline for the Management of CRMS/CFSPID. Pediatrics 2024, 153, e2023064657. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.P.; Cotton, C.U.; Donaldson, S.H.; Solomon, G.M.; VanDevanter, D.R.; Boyle, M.P.; Gentzsch, M.; Nick, J.A.; Illek, B.; Wallenburg, J.C. CFTR modulator theratyping: Current status, gaps and future directions. J. Cyst. Fibros. 2019, 18, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-C.; Yeh, J.-T.; Zhang, J.; Yu, Y.-C.; Yeh, H.-I.; Destefano, S. Structural mechanisms of CFTR function and dysfunction. J. Gen. Physiol. 2018, 150, 539–570. [Google Scholar] [CrossRef]
- Csanády, L.; Vergani, P.; Gadsby, D.C. Structure, gating, and regulation of the CFTR anion channel. Physiol. Rev. 2019, 99, 707–738. [Google Scholar] [CrossRef]
- Ramananda, Y.; Naren, A.P.; Arora, K. Functional Consequences of CFTR Interactions in Cystic Fibrosis. Int. J. Mol. Sci. 2024, 25, 3384. [Google Scholar] [CrossRef] [PubMed]
- Levring, J.; Chen, J. Structural identification of a selectivity filter in CFTR. Proc. Natl. Acad. Sci. USA 2024, 121, e2316673121. [Google Scholar] [CrossRef] [PubMed]
- Robert, R.; Norez, C.; Becq, F. Disruption of CFTR chloride channel alters mechanical properties and cAMP-dependent Cl− transport of mouse aortic smooth muscle cells. J. Physiol. 2005, 568, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Decherf, G.; Bouyer, G.; Egée, S.; Thomas, S.L. Chloride channels in normal and cystic fibrosis human erythrocyte membrane. Blood Cells Mol. Dis. 2007, 39, 24–34. [Google Scholar] [CrossRef]
- Lamhonwah, A.M.; Bear, C.E.; Huan, L.J.; Chiaw, P.K.; Ackerley, C.A.; Tein, I. Cystic fibrosis transmembrane conductance regulator in human muscle: Dysfunction causes abnormal metabolic recovery in exercise. Ann. Neurol. 2010, 67, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Linsdell, P. Mechanism of chloride permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. Exp. Physiol. 2006, 91, 123–129. [Google Scholar] [CrossRef]
- Nawaz, K.; Khan, S.; Bibi, A. Insights into scabies prevalence and risk factors. Bull. Biol. Allied Sci. Res. 2024, 2024, 68. [Google Scholar] [CrossRef]
- Ullah, A.; Bibi, A.; Ullah, I.; Kayani, R.E.Z.; Asim, M.; Munawar, N.; Amjad, M.; Siraj, M.; Gohar, M.; Khan, M.A. An overview of hepatitis C virus and liver cirrhosis in Pakistan. Bull. Biol. Allied Sci. Res. 2024, 2024, 64. [Google Scholar] [CrossRef]
- Lubamba, B.; Dhooghe, B.; Noel, S.; Leal, T. Cystic fibrosis: Insight into CFTR pathophysiology and pharmacotherapy. Clin. Biochem. 2012, 45, 1132–1144. [Google Scholar] [CrossRef]
- Ishiguro, H.; Steward, M.C.; Naruse, S.; Ko, S.B.; Goto, H.; Case, R.M.; Kondo, T.; Yamamoto, A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J. Gen. Physiol. 2009, 133, 315–326. [Google Scholar] [CrossRef]
- Tang, L.; Fatehi, M.; Linsdell, P. Mechanism of direct bicarbonate transport by the CFTR anion channel. J. Cyst. Fibros. 2009, 8, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.S.; Yang, N.; Quinton, P.M. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator–dependent bicarbonate secretion. J. Clin. Investig. 2009, 119, 3497. [Google Scholar] [CrossRef]
- O’Riordan, T.G.; Donn, K.H.; Hodsman, P.; Ansede, J.H.; Newcomb, T.; Lewis, S.A.; Flitter, W.D.; White, V.S.; Johnson, M.R.; Montgomery, A.B. Acute hyperkalemia associated with inhalation of a potent ENaC antagonist: Phase 1 trial of GS-9411. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 200–208. [Google Scholar] [CrossRef]
- Borowitz, D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr. Pulmonol. 2015, 50, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Hermans, C.; Dom, G.; Terryn, S.; Leal, T.; Lebecque, P.; Cassiman, J.-J.; Scholte, B.J.; de Jonge, H.R.; Courtoy, P.J. Cystic fibrosis is associated with a defect in apical receptor–mediated endocytosis in mouse and human kidney. J. Am. Soc. Nephrol. 2007, 18, 707–718. [Google Scholar]
- Raggi, C.; Fujiwara, K.; Leal, T.; Jouret, F.; Devuyst, O.; Terryn, S. Decreased renal accumulation of aminoglycoside reflects defective receptor-mediated endocytosis in cystic fibrosis and Dent’s disease. Pflügers Arch. Eur. J. Physiol. 2011, 462, 851–860. [Google Scholar] [CrossRef]
- Moran, O. The gating of the CFTR channel. Cell. Mol. Life Sci. 2017, 74, 85–92. [Google Scholar] [CrossRef]
- Wang, W.; El Hiani, Y.; Linsdell, P. Alignment of transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J. Gen. Physiol. 2011, 138, 165–178. [Google Scholar] [CrossRef]
- Bergeron, C.; Cantin, A.M. Cystic fibrosis: Pathophysiology of lung disease. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: Leipzig, Germany, 2019; pp. 715–726. [Google Scholar]
- Wang, X.R.; Li, C. Decoding F508del misfolding in cystic fibrosis. Biomolecules 2014, 4, 498–509. [Google Scholar] [CrossRef]
- Espel, J.C.; Palac, H.L.; Bharat, A.; Cullina, J.; Prickett, M.; Sala, M.; McColley, S.A.; Jain, M. The relationship between sweat chloride levels and mortality in cystic fibrosis varies by individual genotype. J. Cyst. Fibros. 2018, 17, 34–42. [Google Scholar] [CrossRef]
- Bergeron, C.; Cantin, A.M. New Therapies to Correct the Cystic Fibrosis Basic Defect. Int. J. Mol. Sci. 2021, 22, 6193. [Google Scholar] [CrossRef] [PubMed]
- Marson, F.A.L.; Bertuzzo, C.S.; Ribeiro, J.D. Classification of CFTR mutation classes. Lancet Respir. Med. 2016, 4, e37–e38. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, A.; Fu, L.; Bali, V.; Bartoszewski, R.; Rab, A.; Havasi, V.; Keiles, S.; Kappes, J.; Kumar, R.; Lefkowitz, E. The silent codon change I507-ATC→ ATT contributes to the severity of the ΔF508 CFTR channel dysfunction. FASEB J. 2013, 27, 4630. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R.; Engelhardt, J.; Zeitlin, P.L. Genetics and pathophysiology of cystic fibrosis. In Kendig’s Disorders of the Respiratory Tract in Children; Elsevier: Amsterdam, The Netherlands, 2019; pp. 757–768.e756. [Google Scholar]
- Yu, Y.C.; Sohma, Y.; Hwang, T.C. On the mechanism of gating defects caused by the R117H mutation in cystic fibrosis transmembrane conductance regulator. J. Physiol. 2016, 594, 3227–3244. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; L’hostis, C.; Férec, C. The changing epidemiology of cystic fibrosis: Incidence, survival and impact of the CFTR gene discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Assael, B.M. Cystic fibrosis: A clinical view. Cell. Mol. Life Sci. 2017, 74, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, I.; Pique, L.; Graham, S.; Pearl, M.; Cherry, A.; Kharrazi, M. The Spectrum of CFTR Variants in Nonwhite Cystic Fibrosis Patients: Implications for Molecular Diagnostic Testing. J. Mol. Diagn. JMD 2016, 18, 39–50. [Google Scholar] [CrossRef]
- Sugarman, E.A.; Rohlfs, E.M.; Silverman, L.M.; Allitto, B.A. CFTR mutation distribution among U.S. Hispanic and African American individuals: Evaluation in cystic fibrosis patient and carrier screening populations. Genet. Med. Off. J. Am. Coll. Med. Genet. 2004, 6, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Estabrooks, S.; Brodsky, J.L. Regulation of CFTR biogenesis by the proteostatic network and pharmacological modulators. Int. J. Mol. Sci. 2020, 21, 452. [Google Scholar] [CrossRef]
- Bobadilla, J.L.; Macek, M., Jr.; Fine, J.P.; Farrell, P.M. Cystic fibrosis: A worldwide analysis of CFTR mutations—Correlation with incidence data and application to screening. Hum. Mutat. 2002, 19, 575–606. [Google Scholar] [CrossRef]
- Laselva, O.; Bartlett, C.; Gunawardena, T.N.; Ouyang, H.; Eckford, P.D.; Moraes, T.J.; Bear, C.E.; Gonska, T. Rescue of multiple class II CFTR mutations by elexacaftor+ tezacaftor+ ivacaftor mediated in part by the dual activities of elexacaftor as both corrector and potentiator. Eur. Respir. J. 2021, 57, 2002774. [Google Scholar] [CrossRef] [PubMed]
- Accurso, F.J.; Rowe, S.M.; Clancy, J.; Boyle, M.P.; Dunitz, J.M.; Durie, P.R.; Sagel, S.D.; Hornick, D.B.; Konstan, M.W.; Donaldson, S.H. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 2010, 363, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.; Dupuis, A.; Griffin, K.; Castellani, C.; Tullis, E.; Gonska, T. Phenotypic spectrum of patients with cystic fibrosis and cystic fibrosis-related disease carrying p. Arg117His. J. Cyst. Fibros. 2019, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Bareil, C.; Bergougnoux, A. CFTR gene variants, epidemiology and molecular pathology. Arch. Pédiatr. 2020, 27, eS8–eS12. [Google Scholar] [CrossRef]
- Yeh, J.T.; Yu, Y.C.; Hwang, T.C. Structural mechanisms for defective CFTR gating caused by the Q1412X mutation, a severe Class VI pathogenic mutation in cystic fibrosis. J. Physiol. 2019, 597, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.P.; De Boeck, K. A new era in the treatment of cystic fibrosis: Correction of the underlying CFTR defect. Lancet Respir. Med. 2013, 1, 158–163. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2020, 10, 1662. [Google Scholar] [CrossRef]
- Bell, S.C.; De Boeck, K.; Amaral, M.D. New pharmacological approaches for cystic fibrosis: Promises, progress, pitfalls. Pharmacol. Ther. 2015, 145, 19–34. [Google Scholar] [CrossRef]
- Lukacs, G.L.; Verkman, A. CFTR: Folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol. Med. 2012, 18, 81–91. [Google Scholar] [CrossRef]
- Derichs, N. Targeting a genetic defect: Cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis. Eur. Respir. Rev. 2013, 22, 58–65. [Google Scholar] [CrossRef]
- Kerem, E.; Hirawat, S.; Armoni, S.; Yaakov, Y.; Shoseyov, D.; Cohen, M.; Nissim-Rafinia, M.; Blau, H.; Rivlin, J.; Aviram, M. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: A prospective phase II trial. Lancet 2008, 372, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Gaudelus, I.; Renouil, M.; Fajac, A.; Bidou, L.; Parbaille, B.; Pierrot, S.; Davy, N.; Bismuth, E.; Reinert, P.; Lenoir, G. In vitro prediction of stop-codon suppression by intravenous gentamicin in patients with cystic fibrosis: A pilot study. BMC Med. 2007, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Gaudelus, I.; Boeck, K.D.; Casimir, G.J.; Vermeulen, F.; Leal, T.; Mogenet, A.; Roussel, D.; Fritsch, J.; Hanssens, L.; Hirawat, S. Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Welch, E.M.; Barton, E.R.; Zhuo, J.; Tomizawa, Y.; Friesen, W.J.; Trifillis, P.; Paushkin, S.; Patel, M.; Trotta, C.R.; Hwang, S. PTC124 targets genetic disorders caused by nonsense mutations. Nature 2007, 447, 87–91. [Google Scholar] [CrossRef]
- Pettit, R.S.; Fellner, C. CFTR Modulators for the Treatment of Cystic Fibrosis. P&T Peer-Rev. J. Formul. Manag. 2014, 39, 500–511. [Google Scholar]
- Aslam, A.A.; Sinha, I.P.; Southern, K.W. Ataluren and similar compounds (specific therapies for premature termination codon class I mutations) for cystic fibrosis. Cochrane Database Syst. Rev. 2023, 3, CD012040. [Google Scholar] [CrossRef] [PubMed]
- Dormer, R.L.; Harris, C.M.; Clark, Z.; Pereira, M.M.C.; Doull, I.J.M.; Norez, C.; Becq, F.; McPherson, M.A. Sildenafil (Viagra) corrects ΔF508-CFTR location in nasal epithelial cells from patients with cystic fibrosis. Thorax 2005, 60, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Villella, V.R.; Esposito, S.; Brunetti-Pierri, N.; Medina, D.; Settembre, C.; Gavina, M.; Pulze, L.; Giardino, I.; Pettoello-Mantovani, M. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 2010, 12, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Caraher, E. Current and emerging therapies for the treatment of cystic fibrosis or mitigation of its symptoms. Drugs R&D 2016, 16, 1–17. [Google Scholar]
- Ferreira, F.C.; Amaral, M.D.; Bacalhau, M.; Lopes-Pacheco, M. PTI-801 (posenacaftor) shares a common mechanism with VX-445 (elexacaftor) to rescue p. Phe508del-CFTR. Eur. J. Pharmacol. 2024, 967, 176390. [Google Scholar] [CrossRef]
- Aluma, B.E.B.; Sarouk, I.; Senderowitz, H.; Cohen-Cymberknoh, M.; Khazanov, N.; Dagan, A.; Bezalel, Y.; Ashkenazi, M.; Keler, S.; Efrati, O. Phenotypic and molecular characteristics of CF patients carrying the I1234V mutation. Respir. Med. 2020, 170, 106027. [Google Scholar] [CrossRef] [PubMed]
- Aluma, B.E.B.; Reiter, J.; Efrati, O.; Bezalel, Y.; Keler, S.; Ashkenazi, M.; Dagan, A.; Buchnik, Y.; Sadras, I.; Cohen-Cymberknoh, M. Clinical efficacy of CFTR modulator therapy in people with cystic fibrosis carrying the I1234V mutation. J. Cyst. Fibros. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Griffiths, H.H.; Gillgrass, L.; Caley, L.R.; Spoletini, G.; Clifton, I.J.; Etherington, C.; Savic, S.; McDermott, M.F.; Peckham, D. Anti-inflammatory effects of elexacaftor/tezacaftor/ivacaftor in adults with cystic fibrosis heterozygous for F508del. PLoS ONE 2024, 19, e0304555. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, A.; Gelzo, M.; Iacotucci, P.; Longobardi, A.; Taccetti, G.; Terlizzi, V.; Carnovale, V. One year of treatment with elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis homozygous for the F508del mutation causes a significant increase in liver biochemical indexes. Front. Mol. Biosci. 2024, 10, 1327958. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Munck, A.; Walker, S.; Faro, A.; Hiatt, P.; Gilmartin, G.; Higgins, M. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J. Cyst. Fibros. 2014, 13, 674–680. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef]
- Pyle, L.C.; Ehrhardt, A.; Mitchell, L.H.; Fan, L.; Ren, A.; Naren, A.P.; Li, Y.; Clancy, J.; Bolger, G.B.; Sorscher, E.J. Regulatory domain phosphorylation to distinguish the mechanistic basis underlying acute CFTR modulators. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L587–L597. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Burton, B.; Huang, C.-J.; Worley, J.; Cao, D.; Johnson, J.P., Jr.; Urrutia, A.; Joubran, J.; Seepersaud, S.; Sussky, K. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J. Cyst. Fibros. 2012, 11, 237–245. [Google Scholar] [CrossRef]
- Cai, Z.; Taddei, A.; Sheppard, D.N. Differential sensitivity of the cystic fibrosis (CF)-associated mutants G551D and G1349D to potentiators of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl–channel. J. Biol. Chem. 2006, 281, 1970–1977. [Google Scholar] [CrossRef]
- Esposito, S.; Villella, V.R.; Ferrari, E.; Monzani, R.; Tosco, A.; Rossin, F.; D’Eletto, M.; Castaldo, A.; Luciani, A.; Silano, M. Genistein antagonizes gliadin-induced CFTR malfunction in models of celiac disease. Aging 2019, 11, 2003. [Google Scholar] [CrossRef]
- Latorre, R.V.; Calicchia, M.; Bigliardi, M.; Conti, J.; Kleinfelder, K.; Melotti, P.; Sorio, C. Functional rescue of CFTR in rectal organoids from patients carrying R334W variant by CFTR modulators and PDE4 inhibitor Roflumilast. Respir. Investig. 2024, 62, 455–461. [Google Scholar] [CrossRef]
- Stastna, N.; Pokojova, E. Case report of two adults with F508del/3849+ 10 kb C> T genotype regaining exocrine pancreatic function following treatment with elexacaftor/tezacaftor/ivacaftor. J. Cyst. Fibros. 2023; in press. [Google Scholar] [CrossRef]
- Tomati, V.; Costa, S.; Capurro, V.; Pesce, E.; Pastorino, C.; Lena, M.; Sondo, E.; Di Duca, M.; Cresta, F.; Cristadoro, S. Rescue by elexacaftor-tezacaftor-ivacaftor of the G1244E cystic fibrosis mutation’s stability and gating defects are dependent on cell background. J. Cyst. Fibros. 2023, 22, 525–537. [Google Scholar] [CrossRef]
- Bacalhau, M.; Ferreira, F.C.; Silva, I.A.; Buarque, C.D.; Amaral, M.D.; Lopes-Pacheco, M. Additive potentiation of R334W-CFTR function by novel small molecules. J. Pers. Med. 2023, 13, 102. [Google Scholar] [CrossRef]
- Hassan, N.; Amin, F.; Bashir, K.; Irshad, M.; Jamil, S.; Munawar, N.; Haqqani, H.; Shabir, H.; Khan, M. Antiviral response of drugs used against hbv patients of Khyber Pakhtunkhwa, Pakistan. Bull. Biol. Allied Sci. Res. 2023, 2023, 49. [Google Scholar] [CrossRef]
- Gohar, M.; Rehman, I.; Ahmad, J.; Ahmad, F.; Bashir, K.; Ikram, S.; Hassan, N.; Khan, M.; Ullah, A. Prevalence of hepatitis b virus and genotypes in the region of khyber pakhtunkhwa Pakistan. Bull. Biol. Allied Sci. Res. 2023, 2023, 53. [Google Scholar] [CrossRef]
- Awan, S.J.; Fatima, Z.; Kamran, S.; Khan, A.S.; Fatima, T.; Imran, S.; Shabbir, M.; Nadeem, S.I. GUAR Gum in therapeutics: A succinct exploration. Bull. Biol. Allied Sci. Res. 2024, 2024, 60. [Google Scholar] [CrossRef]
- Costa, E.; Girotti, S.; Pauro, F.; Leufkens, H.G.; Cipolli, M. The impact of FDA and EMA regulatory decision-making process on the access to CFTR modulators for the treatment of cystic fibrosis. Orphanet J. Rare Dis. 2022, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Jones, I.; Dransfield, M.T.; Haque, N.; Gleason, S.; Hayes, K.A.; Kulmatycki, K.; Yates, D.P.; Danahay, H.; Gosling, M. Efficacy and safety of the CFTR potentiator icenticaftor (QBW251) in COPD: Results from a phase 2 randomized trial. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Cousar, J.L.; Mall, M.A.; Ramsey, B.W.; McKone, E.F.; Tullis, E.; Marigowda, G.; McKee, C.M.; Waltz, D.; Moskowitz, S.M.; Savage, J. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res. 2019, 5, 00082. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.C.; Van de Steen, O.; van Koningsbruggen-Rietschel, S.; Drevinek, P.; Derichs, N.; McKone, E.F.; Kanters, D.; Allamassey, L.; Namour, F.; de Kock, H. GLPG1837, a CFTR potentiator, in p. Gly551Asp (G551D)-CF patients: An open-label, single-arm, phase 2a study (SAPHIRA1). J. Cyst. Fibros. 2019, 18, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E. VX-445–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Bacalhau, M.; Camargo, M.; Magalhães-Ghiotto, G.A.V.; Drumond, S.; Castelletti, C.H.M.; Lopes-Pacheco, M. Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis. Pharmaceuticals 2023, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Scully, K.J.; Marchetti, P.; Sawicki, G.S.; Uluer, A.; Cernadas, M.; Cagnina, R.E.; Kennedy, J.C.; Putman, M.S. The effect of elexacaftor/tezacaftor/ivacaftor (ETI) on glycemia in adults with cystic fibrosis. J. Cyst. Fibros. 2022, 21, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Casey, M.; Gabillard-Lefort, C.; McElvaney, O.F.; McElvaney, O.J.; Carroll, T.; Heeney, R.C.; Gunaratnam, C.; Reeves, E.P.; Murphy, M.P.; McElvaney, N.G. Effect of elexacaftor/tezacaftor/ivacaftor on airway and systemic inflammation in cystic fibrosis. Thorax 2023, 78, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Maher, R.E.; Barry, P.J.; Emmott, E.; Jones, A.M.; Lin, L.; McNamara, P.S.; Smith, J.A.; Lord, R.W. Influence of highly effective modulator therapy on the sputum proteome in cystic fibrosis. J. Cyst. Fibros. 2024, 23, 269–277. [Google Scholar] [CrossRef]
- James, A.; Li, G.; List, R.; Lonabaugh, K.; Smith, A.D.; Barros, A.; Somerville, L.; Albon, D. Analysis of iron status after initiation of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis. Pediatr. Pulmonol. 2024, 59, 669–678. [Google Scholar] [CrossRef]
- Pioch, C.O.; Ziegahn, N.; Allomba, C.; Busack, L.M.; Schnorr, A.N.; Tosolini, A.; Fuhlrott, B.R.; Zagkla, S.; Othmer, T.; Syunyaeva, Z. Elexacaftor/tezacaftor/ivacaftor improves nasal nitric oxide in patients with cystic fibrosis. J. Cyst. Fibros. 2024; in press. [Google Scholar] [CrossRef]
- Stastna, N.; Kunovsky, L.; Svoboda, M.; Pokojova, E.; Homola, L.; Mala, M.; Gracova, Z.; Jerabkova, B.; Skrickova, J.; Trna, J. Improved nutritional outcomes and gastrointestinal symptoms in adult cystic fibrosis patients treated with elexacaftor/tezacaftor/ivacaftor. Dig. Dis. 2024, 42, 361–368. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, S.; Peng, J.-L.; Zahid, K.R.; Zhou, Y.-M.; Ali, Q.; Qiu, C.-R. Cystic Fibrosis: Understanding Cystic Fibrosis Transmembrane Regulator Mutation Classification and Modulator Therapies. Adv. Respir. Med. 2024, 92, 263-277. https://doi.org/10.3390/arm92040026
Anwar S, Peng J-L, Zahid KR, Zhou Y-M, Ali Q, Qiu C-R. Cystic Fibrosis: Understanding Cystic Fibrosis Transmembrane Regulator Mutation Classification and Modulator Therapies. Advances in Respiratory Medicine. 2024; 92(4):263-277. https://doi.org/10.3390/arm92040026
Chicago/Turabian StyleAnwar, Saba, Jin-Liang Peng, Kashif Rafiq Zahid, Yu-Ming Zhou, Qurban Ali, and Chong-Rong Qiu. 2024. "Cystic Fibrosis: Understanding Cystic Fibrosis Transmembrane Regulator Mutation Classification and Modulator Therapies" Advances in Respiratory Medicine 92, no. 4: 263-277. https://doi.org/10.3390/arm92040026
APA StyleAnwar, S., Peng, J.-L., Zahid, K. R., Zhou, Y.-M., Ali, Q., & Qiu, C.-R. (2024). Cystic Fibrosis: Understanding Cystic Fibrosis Transmembrane Regulator Mutation Classification and Modulator Therapies. Advances in Respiratory Medicine, 92(4), 263-277. https://doi.org/10.3390/arm92040026






