Impact of Androgen Deprivation Therapy on Cardiovascular Outcomes in Prostate Cancer
Abstract
Introduction
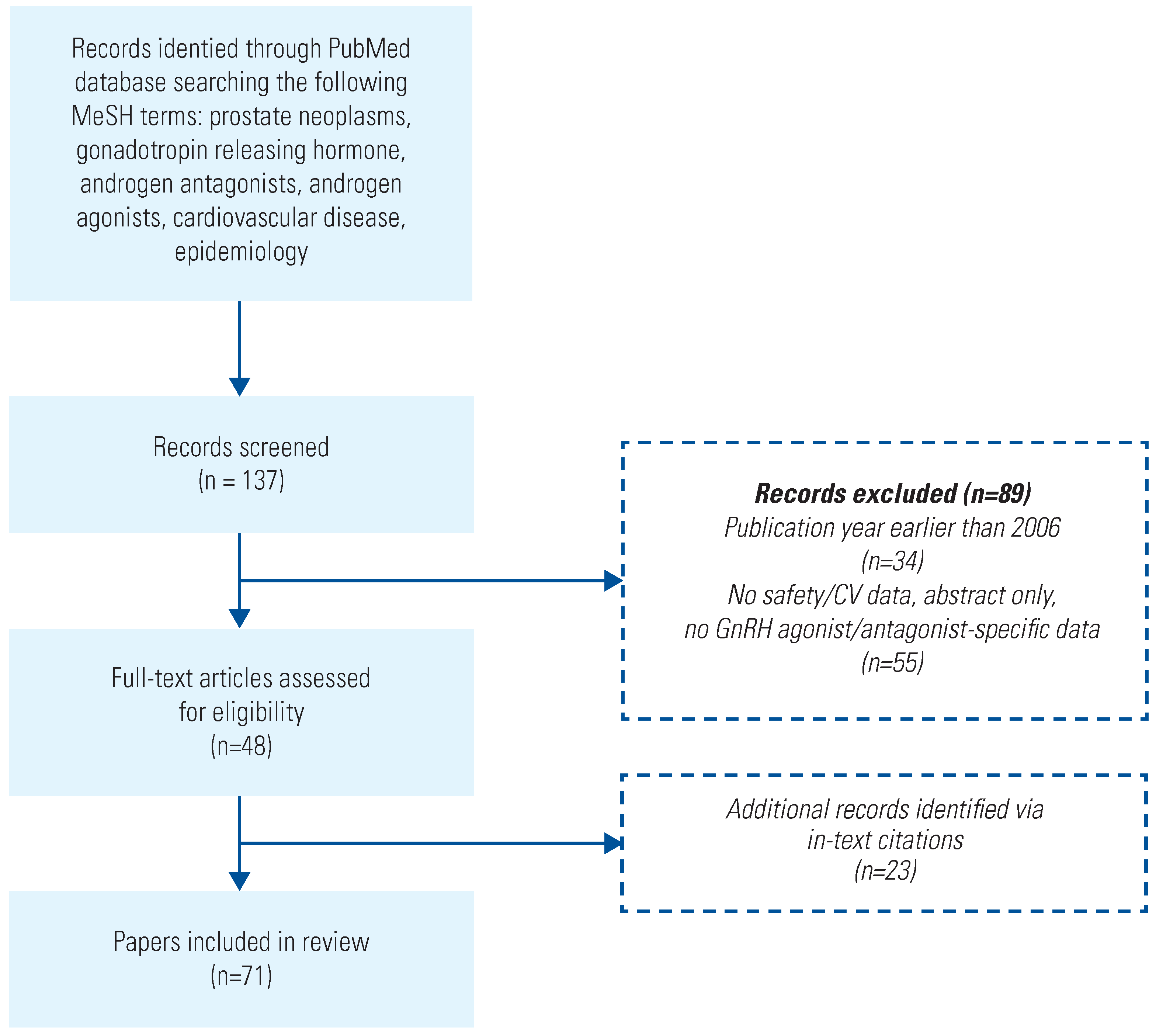
Search Method
CV Risks Reported With ADT
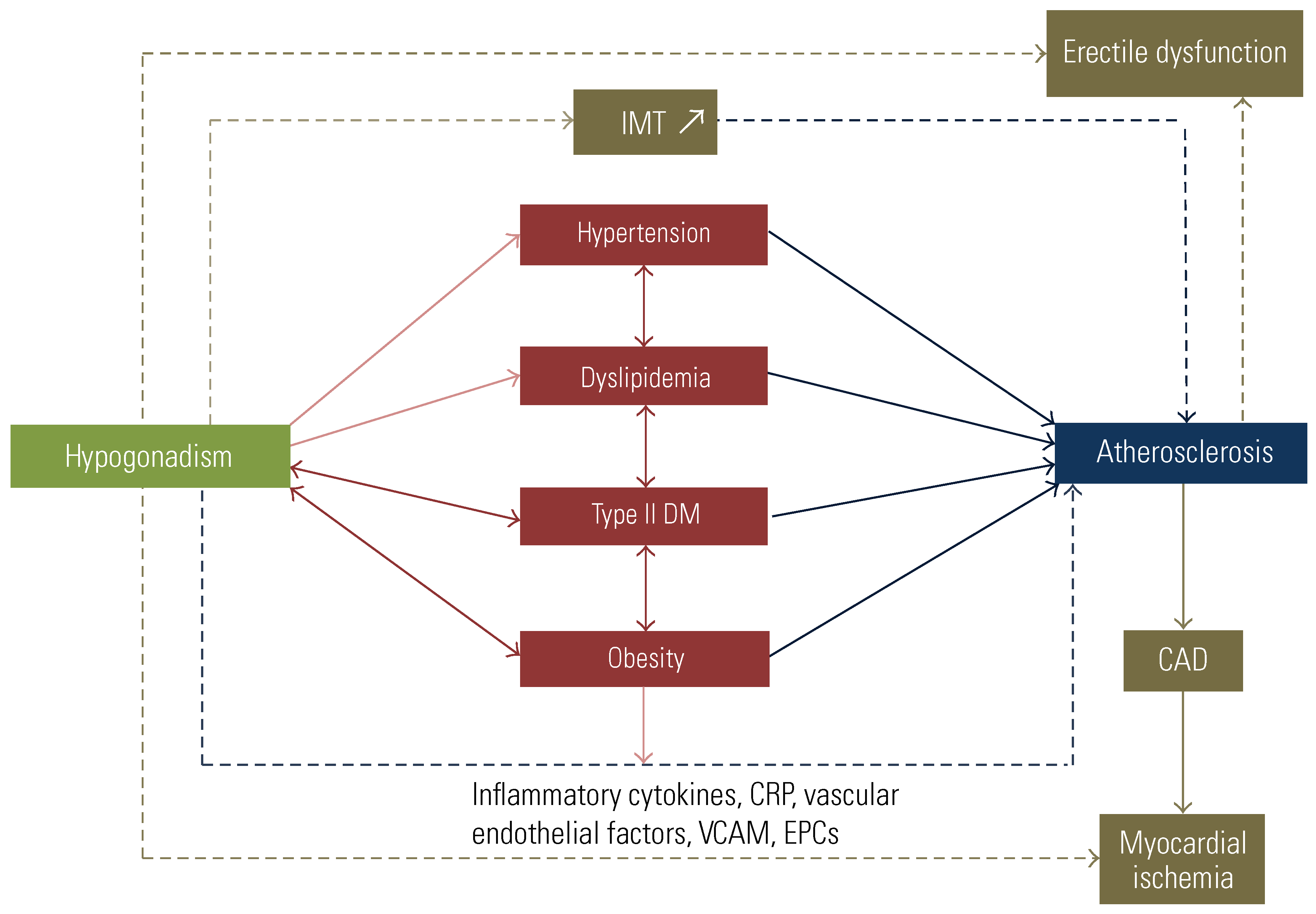
Quantifying the CV Risk of ADT and the Importance of Pre-Existing CVD
CV Risk with Orchiectomy
CV Risk with GnRH Antagonists and Agonists
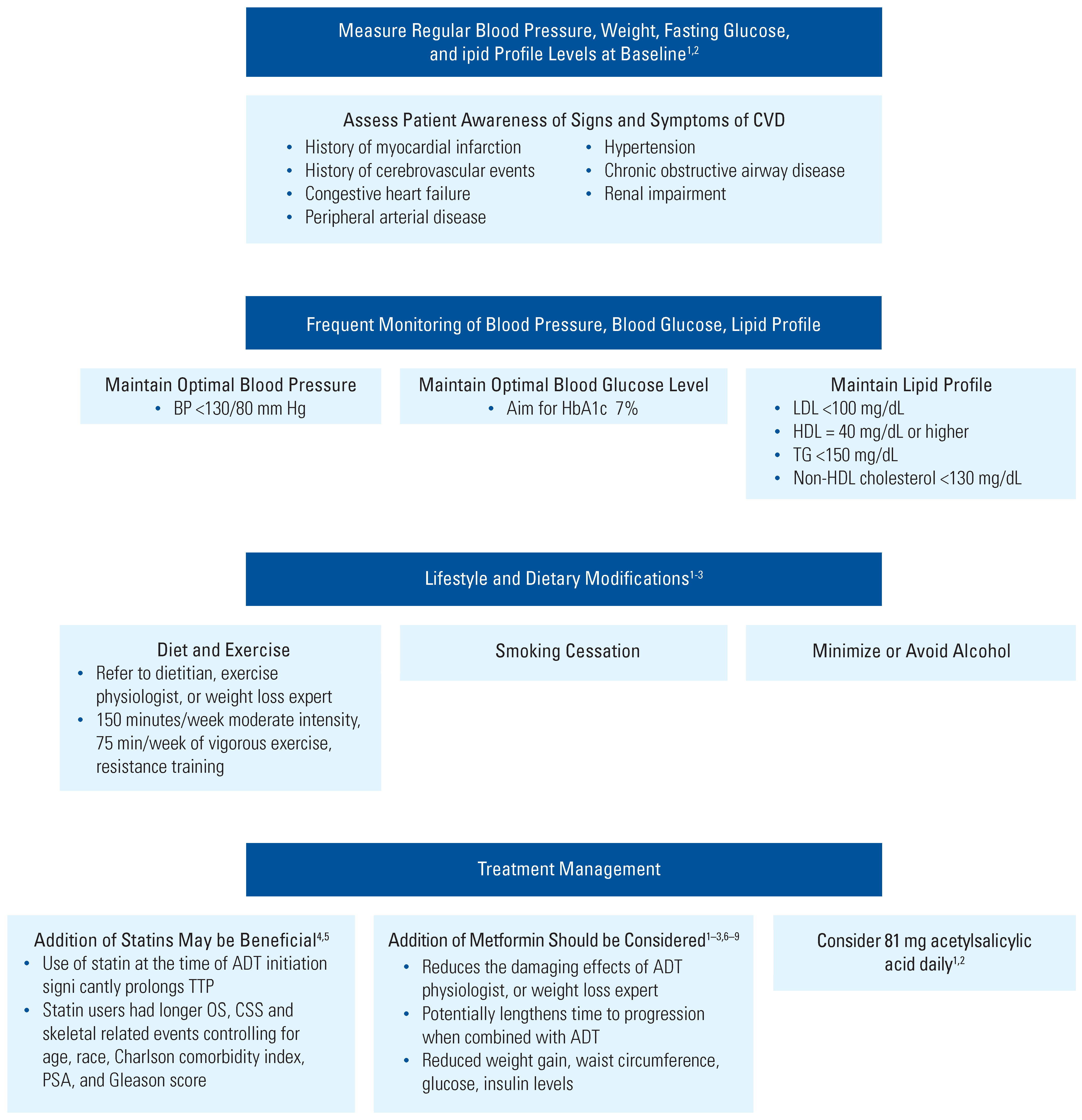
Management of CV Risks in Men With PCa Who Are Receiving ADT
Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | androgen deprivation therapy CVD cardiovascular disease |
| GnRH | gonadotropin-releasing hormone MI myocardial infarction |
| PCa | prostate cancer |
| SIR | standardized incidence ratio |
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Prostate Cancer. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 11 November 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, K.M.; Deng, L.; Bluethmann, S.M.; Zhou, S.; Trifiletti, D.M.; Jiang, C.; et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019, 40, 3889–3897. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Ingelsson, E.; Bratt, O.; Bill-Axelson, A.; et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: Results from the Population-Based PCBaSe Sweden. J. Clin. Oncol. 2010, 28, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Parikh, R.B.; Hubbard, R.A.; Cashy, J.; Takvorian, S.U.; Vaughn, D.J.; et al. Assessment and management of cardiovascular risk factors among US veterans with prostate cancer. JAMA Netw. Open. 2021, 4, e210070. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Santos, M.; Jones, L.W.; Beckman, J.A.; Penson, D.F.; Morgans, A.; et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation 2016, 133, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Punnen, S.; Cooperberg, M.R.; Sadetsky, N.; Carroll, P.R. Androgen deprivation therapy and cardiovascular risk. J. Clin. Oncol. 2011, 29, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Zareba, P.; Duivenvoorden, W.; Leong, D.P.; Pinthus, J.H. Androgen deprivation therapy and cardiovascular disease: What is the linking mechanism? Ther. Adv. Urol. 2016, 8, 118–129. [Google Scholar] [CrossRef]
- Ahmadi, H.; Daneshmand, S. Androgen deprivation therapy for prostate cancer: Long-term safety and patient outcomes. Patient Relat. Outcome Meas. 2014, 5, 63–70. [Google Scholar] [CrossRef]
- Lopes, R.D.; Higano, C.S.; Slovin, S.F.; Nelson, A.J.; Bigelow, R.; Sørensen, P.S.; et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: The primary results of the PRONOUNCE Randomized Trial. Circulation 2021, 144, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Wallach, J.D.; Deng, Y.; McCoy, R.G.; Dhruva, S.S.; Herrin, J.; Berkowitz, A.; et al. Real-world cardiovascular outcomes associated with degarelix vs. leuprolide for prostate cancer treatment. JAMA Netw. Open. 2021, 4, e2130587. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, P.C.; Klotz, L.; Tombal, B.; Grady, J.; Olesen, T.K.; Nilsson, J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur. Urol. 2014, 65, 565–573. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2006, 24, 4448–4456. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; D’Amico, A.V.; Berger, P.; Clark, P.E.; Eckel, R.H.; Keating, N.L.; et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: A science advisory from the American Heart Association, American Cancer Society, and American Urological Association: Endorsed by the American Society for Radiation Oncology. CA Cancer J. Clin. 2010, 60, 194–201. [Google Scholar] [CrossRef] [PubMed]
- AbbVie Inc. Lupron-Depot (Leuprolide Acetate for Depot Suspension): Prescribing Information Full Prescribing Information; AbbVie, Inc.: North Chicago, IL, USA, 2019. [Google Scholar]
- Foresee Pharmaceuticals. CAMCEVI (Leuprolide) Injectable Emulsion, for Subcutaneous Use (Prescribing Information). Available online: https://www. accessdata.fda.gov/drugsatfda_docs/label/2021/211488s000lbl.pdf (accessed on 10 October 2021).
- Shore, N.; Mincik, I.; DeGuenther, M.; Student VJr Jievaltas, M.; Patockova, J.; et al. A phase 3, open-label, multicenter study of a 6-month pre-mixed depot formulation of leuprolide mesylate in advanced prostate cancer patients. World J. Urol. 2020, 38, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tolmar Pharmaceuticals Inc. Eligard (Leuprolide Acetate) for Injectable Suspension, for Subcutaneous Use (Prescribing Information). Available online: https://eligardhcp.com/sites/default/files/pdfs/ELI_Full_PI.pdf (accessed on 13 July 2021).
- Gandaglia, G.; Sun, M.; Popa, I.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; et al. Cardiovascular mortality in patients with metastatic prostate cancer exposed to androgen deprivation therapy: A population-based study. Clin. Genitourin Cancer. 2015, 13, e123–e130. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Sun, M.; Popa, I.; Schiffmann, J.; Abdollah, F.; Trinh, Q.-D.; et al. The impact of androgen-deprivation therapy (ADT) on the risk of cardiovascular (CV) events in patients with non-metastatic prostate cancer: A population-based study. BJU Int. 2014, 114, E82–E89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, S.; Sun, L.; Meng, F.; Zhao, L.; Zhao, Y.; et al. Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: A meta-analysis of population-based observational studies. PLoS ONE 2014, 9, e107516. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Je, Y.; Schutz, F.A.; Hoffman, K.E.; Hu, J.C.; Parekh, A.; et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: A meta-analysis of randomized trials. JAMA 2011, 306, 2359–2366. [Google Scholar] [CrossRef]
- Melloni, C.; Slovin, S.F.; Blemings, A.; Goodman, S.G.; Evans, C.P.; Nilsson, J.; et al. Cardiovascular safety of degarelix versus leuprolide for advanced prostate cancer. JACC CardioOncology 2020, 2, 70–81. [Google Scholar] [CrossRef]
- A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease (PRONOUNCE) (NCT02663908). Available online: https://clinicaltrials.gov/ct2/show/NCT02663908?term=degarelix+leuprolide+acetate&cond=Prostate+Cancer&draw=2 (accessed on 13 July 2021).
- Rhee, H.; Gunter, J.H.; Heathcote, P.; Ho, K.; Stricker, P.; Corcoran, N.M.; et al. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int. 2015, 115 (Suppl. 5), 3–13. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 1941, 43, 209–223. [Google Scholar] [CrossRef]
- Klotz, L.; O’Callaghan, C.; Ding, K.; Toren, P.; Dearnaley, D.; Higano, C.S.; et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: A secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J. Clin. Oncol. 2015, 33, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, W.C.M.; Naeim, M.; Hopmans, S.N.; Yousef, S.; Werstuck, G.H.; Dason, S.; et al. Protective effect of pharmacological castration on metabolic perturbations and cardiovascular disease in the hyperglycemic male ApoE(-/-):Ins2(+/Akita) mouse model. Prostate Cancer Prostatic Dis. 2021, 24, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; See, L.C.; Liu, J.R.; Chuang, C.K.; Pang, S.T.; Hsieh, I.C.; et al. Risk of cardiovascular ischemic events after surgical castration and gonadotropin-releasing hormone agonist therapy for prostate cancer: A nationwide cohort study. J. Clin. Oncol. 2017, 35, 3697–3705. [Google Scholar] [CrossRef] [PubMed]
- Kan, W.C.; Hsieh, K.L.; Chen, Y.C.; Ho, C.H.; Hong, C.S.; Chiang, C.Y.; et al. Comparison of surgical or medical castration-related cardiotoxicity in patients with prostate cancer. J. Urol. 2022, 207, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, F.B.; Sandin, F.; Garmo, H.; Lissbrant, I.F.; Ahlgren, G.; Van Hemelrijck, M.; et al. Gonadotropin-releasing hormone agonists, orchiectomy, and risk of cardiovascular disease: Semi-ecologic, nationwide, population-based study. Eur. Urol. 2017, 72, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Scailteux, L.M.; Vincendeau, S.; Balusson, F.; Leclercq, C.; Happe, A.; Le Nautout, B.; et al. Androgen deprivation therapy and cardiovascular risk: No meaningful difference between GnRH antagonist and agonists-a nationwide population-based cohort study based on 2010-2013 French health insurance data. Eur. J. Cancer. 2017, 77, 99–108. [Google Scholar] [CrossRef]
- Cardwell, C.R.; O’Sullivan, J.M.; Jain, S.; Harbinson, M.T.; Cook, M.B.; Hicks, B.M.; et al. The risk of cardiovascular disease in prostate cancer patients receiving androgen deprivation therapies. Epidemiology 2020, 31, 432–440. [Google Scholar] [CrossRef]
- Ma, C.; Abeysekera, I.R.; Xu, W.; Wang, Y.; Peng, J.; Li, H. Comparing the risk of cardiovascular disease following GnRH agonist and GnRH antagonist therapy for patient with prostate cancer: A systematic review and meta-analysis. Minerva Urol. Nephrol. 2021, 73, 276–282. [Google Scholar] [CrossRef]
- Shore, N.D.; Saad, F.; Cookson, M.S.; George, D.J.; Saltzstein, D.R.; Tutrone, R.; et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N. Engl. J. Med. 2020, 382, 2187–2196. [Google Scholar] [CrossRef]
- Chong, W.H. Endocrinologic and Metabolic Drugs Advisory Committee meeting. In Proceedings of the FDA Advisory Committee Meeting, Silver Spring, MD, USA, 24–25 October 2018. [Google Scholar]
- AbbVie Inc. AndroGel (testosterone gel 1% ). In Full Prescribing Information; AbbVie Inc.: North Chicago, IL, USA, 2019. [Google Scholar]
- AbbVie Inc. Lupron-Depot (leuprolide acetate for depot suspension). In Full Prescribing Information; AbbVie Inc.: North Chicago, IL, USA, 2019. [Google Scholar]
- Conteduca, V.; Di Lorenzo, G.; Tartarone, A.; Aieta, M. The cardiovascular risk of gonadotropin releasing hormone agonists in men with prostate cancer: An unresolved controversy. Crit. Rev. Oncol. Hematol. 2013, 86, 42–51. [Google Scholar] [CrossRef]
- Chazenbalk, G.; Singh, P.; Irge, D.; Shah, A.; Abbott, D.H.; Dumesic, D.A. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 2013, 78, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Cassinello, J.; Arranz, J.A.; Piulats, J.M.; Sánchez, A.; Pérez-Valderrama, B.; Mellado, B.; et al. SEOM clinical guidelines for the treatment of metastatic prostate cancer (2017). Clin. Transl. Oncol. 2018, 20, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Morris, M.J.; Rumble, R.B.; Basch, E.; Hotte, S.J.; Loblaw, A.; Rathkopf, D.; et al. Optimizing anticancer therapy in metastatic non-castrate prostate cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1521–1539. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines Version 2. 2021 Prostate Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 3 March 2021).
- Anderson-Carter, I.; Posielski, N.; Liou, J.I.; Khemees, T.A.; Downs, T.M.; Abel, E.J.; et al. The impact of statins in combination with androgen deprivation therapy in patients with advanced prostate cancer: A large observational study. Urol. Oncol. 2019, 37, 130–137. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Ding, K.; Crook, J.M.; O’Callaghan, C.J.; Higano, C.S.; Dearnaley, D.P.; et al. The association between statin use and outcomes in patients initiating androgen deprivation therapy. Eur. Urol. 2021, 79, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Harshman, L.C.; Wang, X.; Nakabayashi, M.; Xie, W.; Valenca, L.; Werner, L.; et al. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol. 2015, 1, 495–504. [Google Scholar] [CrossRef]
- He, K.; Hu, H.; Ye, S.; Wang, H.; Cui, R.; Yi, L. The effect of metformin therapy on incidence and prognosis in prostate cancer: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 2218. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.A.; Liou, J.I.; Cryns, V.L.; Downs, T.M.; Abel, E.J.; Jarrard, D.F. Metformin use is associated with improved survival for patients with advanced prostate cancer on androgen deprivation therapy. J. Urol. 2018, 200, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Nobes, J.P.; Langley, S.E.; Klopper, T.; Russell-Jones, D.; Laing, R.W. A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2012, 109, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Chen, M.H.; Renshaw, A.A.; Loffredo, M.; Kantoff, P.W. Androgen suppression and radiation vs. radiation alone for prostate cancer: A randomized trial. JAMA 2008, 299, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.E.; Smith, M.R. Metabolic sequelae associated with androgen deprivation therapy for prostate cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 240–246. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, S.; Garmo, H.; Holmberg, L.; Adolfsson, J.; Stattin, P.; Van Hemelrijck, M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol. 2015, 33, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Bosco, C.; Bosnyak, Z.; Malmberg, A.; Adolfsson, J.; Keating, N.L.; Van Hemelrijck, M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: A meta-analysis. Eur. Urol. 2015, 68, 386–396. [Google Scholar] [CrossRef]
- Lester, J.F.; Mason, M.D. Cardiovascular effects of hormone therapy for prostate cancer. Drug Healthc. Patient Saf. 2015, 7, 129–138. [Google Scholar] [CrossRef]
- Meng, F.; Zhu, S.; Zhao, J.; Vados, L.; Wang, L.; Zhao, Y.; et al. Stroke related to androgen deprivation therapy for prostate cancer: A meta-analysis and systematic review. BMC Cancer 2016, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lee Chuy, K.; Yang, J.C.; Bates, M.; Lombardo, M.; Steingart, R.M. Cardiovascular and metabolic effects of androgen-deprivation therapy for prostate cancer. J. Oncol. Pract. 2018, 14, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Chen, M.H.; Braccioforte, M.H.; Moran, B.J.; D’Amico, A.V. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA 2009, 302, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Klotz, L.; Persson, B.E.; Olesen, T.K.; Wilde, A.A. Cardiovascular safety of degarelix: Results from a 12-month, comparative, randomized, open label, parallel group phase III trial in patients with prostate cancer. J. Urol. 2010, 184, 2313–2319. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Klotz, L.; van der Meulen, E.; Colli, E.; Tanko, L.B. Gonadotropin-releasing hormone blockers and cardiovascular disease risk: Analysis of prospective clinical trials of degarelix. J. Urol. 2011, 186, 1835–1842. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klotz, L.; Miller, K.; Crawford, E.D.; Shore, N.; Tombal, B.; Karup, C.; et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomized clinical trials of degarelix versus luteinizing hormone-releasing hormone agonists. Eur. Urol. 2014, 66, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; UlcickasYood, M.; Xu, X.; Cassidy-Bushrow, A.E.; Tsai, H.-T.; Keating, N.L.; et al. Cardiovascular disease risk and androgen deprivation therapy in patients with localized prostate cancer: A prospective cohort study. Br. J. Cancer 2017, 117, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Gholmieh, J.M.; Azar, S.T. Connecting the lines between hypogonadism and atherosclerosis. Int. J. Endocrinol. 2012, 2012, 793953. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Gardiner, R.A.; Taylor, R.; Risbridger, G.P.; et al. Enhancing active surveillance of prostate cancer: The potential of exercise medicine. Nat. Rev. Urol. 2016, 13, 258–265. [Google Scholar] [CrossRef]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Alghandour, R.; Ebrahim, M.A.; Elshal, A.M.; Ghobrial, F.; Elzaafarany, M.; Elbaiomy, M. 617MO Repurposing metformin as an anticancer drug: Preliminary results of randomized controlled trial in advanced prostate cancer (MANSMED). Ann. Oncol. 2020, 31, S511. [Google Scholar] [CrossRef]

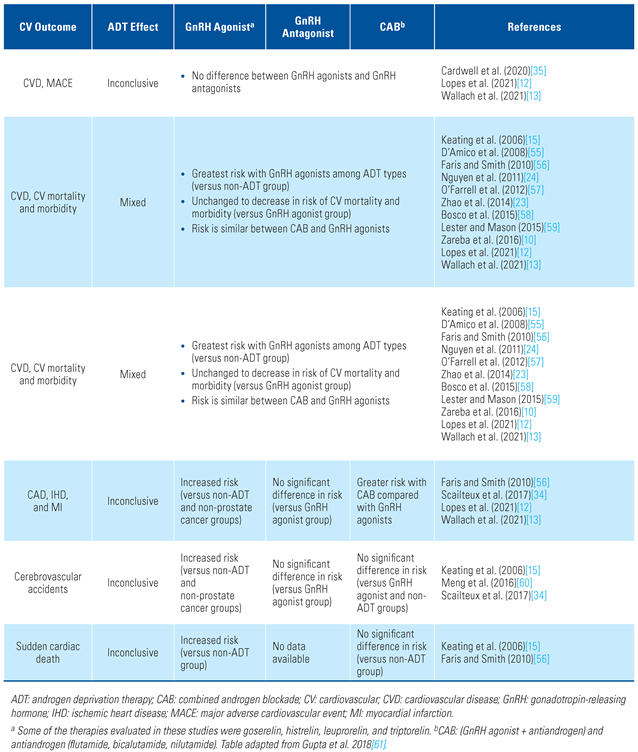
| Study Design | CV Results | CV Conclusions | Summary of CVD Risk GnRH Agonist Versus GnRH Antagonist/Control | Reference |
|---|---|---|---|---|
| Observational study Patients (n = 73 196) ≥66 years old first diagnosed with locoregional PCa from 1992–1999 | GnRH agonist use was associated with increased risk of the following:
| GnRH agonist use may be associated with an increased risk of CVD when compared to patients with untreated locoregional PCa | GnRH agonist > control | Keating et al. (2006) [15] |
| Retrospective study Single institution, pooled data (n = 5077) from 1997 to 2006 of patients with PCa clinical stage T1 to T3 N0 M0 | Agonist and antiandrogen use was not associated with an increased risk of CAD (aHR, 1.04; P = 0.82). For those with CAD-induced CHF or MI, use was associated with an increased risk of all-cause mortality (aHR, 1.96; P = 0.04) | No association with an increased risk of all-cause mortality in men with no comorbidity of CAD, but was increased in those with CAD morbidity | Inconclusive GnRH agonist = control | Nanda et al. (2009) [62] |
| Prospective study 1-year RCT of leuprolide acetate versus degarelix (n = 504) | The incidence of the most common event (ischemic heart disease) was lower in the degarelix group with 18 patients (4%) versus leuprolide group with 21 patients (10%) | No significant differences were found in either treatment group. Both have similar CV safety profiles | Inconclusive | Smith et al. (2010) [63] |
| Meta-analysis of randomized trials Pooled patient data (n = 141) from RCTs from 1966 to 2011 of men with unfavorable-risk, non-metastatic PCa | CV mortality in patients receiving GnRH agonists versus control cohort (non-ADT/delayed ADT) was not significantly different (11% versus 11.2%; 95% CI, 8.3%–15.0%, respectively; RR, 0.93, P = 0.41) | No association with GnRH agonist and increased risk of CVD | Inconclusive GnRH agonist = control | Nguyen et al. (2011) [24] |
| Retrospective study CaPSURE registry patients (n = 7248) | Agonist showed 2-fold greater likelihood of CV mortality (HR, 1.94); however, patients treated with WW/AS had >2-fold increased risk of CV mortality (HR, 2.46) | Patients matched on propensity to receive agonist did not show an association with CV mortality | Inconclusive GnRH agonist < WW/AS | Punnen et al. (2011) [9] |
| Pooled data from prospective trials Pooled analyses on CV incidence in patients (n = 1704) from 9 degarelix clinical trials | CV events were 5.5 and 6.1 eventsa per 100 person-years, before and after degarelix treatment, respectively (HR, 1.10, P = 0.45). In men without established CVD, the event rate was numerically lower after the initiation of degarelix treatment (5.6 versus 4.3 per 100 person-years; HR, 0.77; P = 0.11) | CVD rates were similar before and after degarelix treatment | Inconclusive GnRH antagonist = no GnRH antagonist treatment | Smith et al. (2011) [64] |
| Pooled data from prospective randomized trials Pooled patient data from 6 phase 3 prospective trials comparing GnRH agonists leuprolide or goserelin (n = 837) with antagonist degarelix (n = 1491) | Among patients with pre-existing CVD, the risk of CV events was significantly lower (56%) in the antagonist group versus the agonist group (HR, 0.44; P = 0.002). No difference in CV events for those without pre-existing CVD | These data suggest a greater risk of CV events for those using agonist with pre-existing CVD in patients with non-metastatic PCa | GnRH agonist > GnRH antagonist | Albertsen et al. (2014) [14] |
| Pooled data from prospective randomized trials Pooled patient data (n = 1925) from 5 trials in patients receiving degarelix and leuprolide or goserelin | Patients with underlying CVD at baseline (29.6%) showed a nonsignificant lower risk of death with degarelix versus GnRH agonist (HR, 0.40; P = 0.051) | Degarelix use may be associated with lower incidence of CVD in men with pre-existing CVD | GnRH agonist > GnRH antagonist | Klotz et al. (2014) [65] |
| Meta-analysis of population-based observational studies 9 studies comparing GnRH agonists (n = 119 625) versus control (n = 150 975) in patients with locoregional and non-metastatic PCa | GnRH agonists were associated with an increased risk of CVD (HR, 1.19; P = 0.01) compared with control cohort receiving non-ADT or WW/AS | ADT is associated with a 10% increased CV risk. Significantly increased risks of CV mortality were observed in GnRH cohorts | GnRH agonist > control | Zhao et al. (2014) [23] |
| Prospective study Large cohort (n = 7637) from healthcare records of patients who initially underwent active surveillance | GnRH agonist was associated with an increased risk of HF (aHR, 1.81) in men without pre-existing CVDb | GnRH agonist was associated with a greater risk of HF without any pre-existing CVD | GnRH agonist > control | Haque et al. (2017) [66] |
| Prospective study (HERO) Randomized, open-label, phase 3 trial. Compared patients with advanced PCa (n = 930) who received relugolix (GnRH antagonist) or leuprolide (GnRH agonist) for 48 weeks | The incidence of MACE was 2.9% in the relugolix group and 6.2% in the leuprolide group (HR, 0.46) | MedDRA query showed a lower risk of MACE with relugolix over leuprolide. Any-grade MACE incidence was 2.9% for relugolix versus 6.2% for leuprolide. Incidence of grade 3 or 4 MACE was 1.3% for both relugolix and leuprolide. Ischemic heart disease incidence was 4% for relugolix and 1.6% for leuprolide | GnRH agonist > GnRH antagonist | Shore et al. (2020) [37] |
| Observational study Utilized database to search ICD-10 codes of patients with newly diagnosed PCa with CV events (death or hospitalization due to CVD) (n = 20 216) | ADTc (includes GnRH agonists and antagonists) use had a 30% increase of CV events (aHR, 1.3; 95% CI 1.2–1.4). This reflected increases in CV events associated with GnRH agonists (aHR, 1.3) and degarelix (aHR, 1.5), but not bicalutamide monotherapy (aHR, 1.0) | Degarelix and GnRH agonists both increased risk of CV events | Inconclusive | Cardwell et al. (2020) [35] |
| Prospective study (PRONOUNCE) Randomized, open-label, phase 3, assessor-blind, 12-month study in patients (n = 545) with advanced PCa and atherosclerotic CVD treated with ADT (degarelix or leuprolide) for 12 months. Primary endpoint was time to occurrence of MACE at 1 year | Primary endpoint: incidence of MACE was 15 (5.5%) for degarelix group versus 11 (4.1%) for leuprolide (HR, 1.28; P = 0.53). Composite endpoint of CV death, non-fatal MI, or non-fatal stroke occurred in 9 patients receiving degarelix and 7 receiving leuprolide (HR, 1.20; P = 0.71) | No significant differences were found in either treatment group. Both treatments have similar CV safety profiles | Inconclusive Study terminated owing to futility | Lopes et al. (2021) [12] |
| Retrospective, propensity-matched cohort study designed to emulate the PRONOUNCE study. Adult men with PCa and CVD who initiated degarelix or leuprolide using deidentified administrative claims data. Primary endpoint was time to first occurrence of MACE | Primary efficacy outcome: incidence of MACE was 10.18 per 100 person-years for degarelix group versus 8.6 per 100 person-years for leuprolide (HR, 1.18; P = 0.30). Secondary endpoints:
| No significant differences were found in either treatment group | No difference in MACE | Wallach et al. (2021) [13] |
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Klotz, L.; Van Komen, S.; Dragnic, S.; White, W.B. Impact of Androgen Deprivation Therapy on Cardiovascular Outcomes in Prostate Cancer. Soc. Int. Urol. J. 2022, 3, 259-275. https://doi.org/10.48083/VDNP9678
Klotz L, Van Komen S, Dragnic S, White WB. Impact of Androgen Deprivation Therapy on Cardiovascular Outcomes in Prostate Cancer. Société Internationale d’Urologie Journal. 2022; 3(4):259-275. https://doi.org/10.48083/VDNP9678
Chicago/Turabian StyleKlotz, Laurence, Stephen Van Komen, Sanja Dragnic, and William B. White. 2022. "Impact of Androgen Deprivation Therapy on Cardiovascular Outcomes in Prostate Cancer" Société Internationale d’Urologie Journal 3, no. 4: 259-275. https://doi.org/10.48083/VDNP9678
APA StyleKlotz, L., Van Komen, S., Dragnic, S., & White, W. B. (2022). Impact of Androgen Deprivation Therapy on Cardiovascular Outcomes in Prostate Cancer. Société Internationale d’Urologie Journal, 3(4), 259-275. https://doi.org/10.48083/VDNP9678



